Posttraumatic Stress Disorder and Cognitive Function: Findings From the Mind Your Heart Study
ABSTRACT
Objective: Prior studies have found that the patients with posttraumatic stress disorder (PTSD) have poorer performance on cognitive tests than patients without PTSD, but the underlying mechanisms remain unknown. We examined the association between PTSD and cognitive function in a large cohort and evaluated the role of potential biological and behavioral mediators.
Method: A cohort of 535 adult outpatients (≤ 65 years) without dementia, stroke, or other neurologic disorders was recruited from 2 Veterans Affairs medical centers between February 2008 and June 2010. PTSD was assessed with the Clinician Administered PTSD Scale (CAPS) using DSM-IV-TR criteria. Cognitive function tests included processing speed, Trails A and B, letter fluency, category fluency, and verbal learning and recognition. Linear regression was used to evaluate the association between PTSD and cognitive function test scores and to assess potential mediators of the association.
Results: For our analyses of PTSD and cognitive function, we combined 178 participants who met criteria for full PTSD and 18 who met criteria for partial PTSD and had a CAPS score > 40. After adjusting for demographics, these participants with PTSD scored significantly worse on processing speed (0.30 standard deviations [SDs], P ≤ .001), category fluency (0.23 SDs, P = .01), verbal learning (0.30 SDs, P = .001), and verbal recognition (0.18 SDs, P = .048) than those without PTSD. These associations were largely accounted for by health behaviors, vascular risk factors, and depression.
Conclusions: In this cohort of veterans under age 65 years without known neurologic disease, patients with versus without PTSD had significantly poorer performance in several domains of cognitive function, particularly in tests involving processing speed, executive function, and learning. These cognitive deficits were largely explained by modifiable risk factors. Interventions targeted at these risk factors might minimize the impact of PTSD on cognitive decline and dementia risk as patients age.
J Clin Psychiatry 2013;74(11):1063–1070
© Copyright 2013 Physicians Postgraduate Press, Inc.
Submitted: November 20, 2012; accepted June 14, 2013 (doi:10.4088/JCP.12m08291).
Corresponding author: Beth E. Cohen, MD, Box 111A1, San Francisco VA Medical Center, 4150 Clement St, San Francisco, CA 94121 ([email protected]).
The lifetime prevalence of posttraumatic stress disorder (PTSD) in the US general population is estimated at 7%,1 and the prevalence among veterans is considerably higher, ranging from 13% to 31%.2,3 Despite advances in treatment, PTSD is often a chronic condition, with studies in older veterans showing a prevalence of 12% up to 45 years after combat.4 Prior evidence demonstrates that patients with PTSD have an increased risk of cognitive dysfunction and nearly double the risk of dementia.5–9 However, the mechanisms underlying these impairments in cognition are not known, and therefore we have no targeted treatments to prevent cognitive decline in these patients. With the ongoing conflicts in Afghanistan and Iraq, the aging of veterans from prior wars, and the high frequency of noncombat traumatic events in the general population, a better understanding of how PTSD impacts cognition is urgently needed to prevent the disabling consequences of this chronic condition.10,11
Neuroimaging studies have demonstrated that patients with PTSD have reductions in the size of brain regions critical to memory and learning, such as the hippocampus, ventromedial prefrontal cortex, and anterior cingulate, as well as disruption of cortical white matter tracts.12–15 Yet, the mechanisms underlying these changes also remain unknown. Drawing from the literature on causes of cognitive decline and dementia, there are several risk factors that may be increased in patients with PTSD and therefore deserve further study as potential mechanisms.16 These include specific health behaviors, vascular risk factors, and depression.17,18 Regarding health behaviors, patients with PTSD have higher rates of substance use and sedentary behavior and poorer sleep quality than those without PTSD, and each of these behaviors has been linked to structural brain abnormalities and cognitive decline.19–25 Patients with PTSD are also more likely to have vascular risk factors, such as diabetes and hypertension, and evidence of atherosclerotic coronary artery disease.2,26,27 Each of these vascular risk factors has also been linked to cognitive impairment and dementia.17,28 Despite this theoretical evidence for their importance as mediators of the association of PTSD and neurologic deficits, such behavioral and vascular risk factors have typically gone unevaluated in studies of PTSD and cognitive impairment. Finally, depression is commonly comorbid with PTSD and also associated with cognitive decline, dementia, and neuroimaging changes, although the overlapping symptoms make understanding the unique contributions of these disorders challenging.29–32
Given these previous findings and remaining questions, we sought to examine the association of PTSD and multiple domains of cognitive function in a large outpatient cohort. We hypothesized that poorer cognitive performance in individuals with PTSD, compared to those without PTSD, would be partially mediated by several health behaviors, vascular risk factors, and comorbid conditions known to be important risk factors for cognitive decline.
METHOD
Participants
The Mind Your Heart Study is an ongoing cohort study designed to examine the association between PTSD and health outcomes. Patients were recruited between February 2008 and June 2010 from outpatient clinics affiliated with 2 Department of Veterans Affairs (VA) medical centers (San Francisco VA Medical Center, California, and the VA Palo Alto Health Care System, California). Potential participants were excluded if they planned on leaving the area in 3 years or did not have contact information for follow-up. As exercise treadmill testing was included in the study protocol, participants were also excluded if they were unable to walk 1 block or had a myocardial infarction in the prior 6 months. All patients provided written informed consent and the research protocol was approved by the University of California, San Francisco Committee on Human Research, and the San Francisco VA Medical Center Research and Development Committee.
Overall, 1,020 patients were assessed for eligibility. One hundred four patients (10.2%) were found to be ineligible, primarily due to lack of contact information for follow-up
or plans to leave the study area (n = 82). Of the remaining 916 eligible patients, 170 (18.6%) declined to participate or did not complete the baseline interview, leaving 746 participants ultimately enrolled in the study. To focus on individuals with minimal risk of preclinical dementia, we restricted our analyses to the 603 subjects who were 65 years of age or younger and excluded an additional 26 participants who reported a history of stroke and 25 who reported having a neurologic disorder. Nine participants were excluded from these analyses because the validity of their Clinician Administered PTSD Scale (CAPS) data was questionable (eg, participants struggled to report their current symptomatology), and 8 were excluded for incomplete cognitive function testing, leaving 535 participants for our analyses.
PTSD
We evaluated PTSD with the CAPS using criteria from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR).33 The CAPS is the most widely used structured interview for diagnosing PTSD34,35 and has excellent test-retest reliability (r = 0.92–0.99) and internal consistency (α = 0.80–0.90).35 The diagnostic interviews were conducted in the San Francisco VA Stress and Health Research Program, which has performed thousands of CAPS interviews and established in an earlier study an interrater reliability intraclass correlation coefficient of 0.984.36 Interviews were conducted by masters-level clinicians and supervised by a licensed clinical psychologist (K.W.S.) with expertise in the CAPS and PTSD diagnosis. All interviewers were observed by the supervising psychologist until they had complete agreement on PTSD diagnostic status. Interviews were also reviewed in weekly case conferences with the supervising study psychologist.
We used the “1, 2” CAPS rule of at least a score of 1 for frequency and 2 for intensity to establish positivity for a specific symptom. We selected this rule because of its previously demonstrated high sensitivity and its use in prior studies of PTSD and neuropsychological outcomes.36,37 The PTSD group consisted of subjects with either full (n = 178) or partial PTSD, as partial PTSD is associated with significant impairment in health and functioning.38,39 Partial PTSD was defined as meeting diagnostic criteria for the reexperiencing cluster and either avoidance or hyperarousal clusters, in addition to the other CAPS criteria.40 We also required this group to exhibit symptoms meeting a total CAPS score > 40, as defined by the authors of the CAPS as the lower threshold for PTSD.35 Eighteen participants in this study met these criteria. In sensitivity analyses, excluding these participants or combining them with the group without PTSD did not substantially change our findings.
Cognitive Function
We used a neuropsychological test battery with measures that were selected a priori to assess multiple domains of cognition that have been associated with functional disability in prior studies.41–45 Measures included the Digit Symbol Substitution Test, Trail Making Test (Trails) A and B, letter fluency, category fluency, and the Hopkins Verbal Learning Test.46–52 All tests were performed and scored by trained staff blinded to the PTSD status of participants.
The Digit Symbol Substitution Test is a validated measure of processing speed, working memory, and executive function.46 Participants match symbols to numbers using a key, with the score representing the number of correct number-symbol pairs in a 120-second timed trial. The Trails A and B involve processing speed, memory, mental flexibility, and executive function.47,48 Participants draw lines to sequentially connect encircled numbers (Trails A) or alternating numbers and letters (ie, 1-A-2-B, Trails B) on a sheet of paper, with the score being the number of seconds required to complete the test. We used the Controlled Oral Word Association Test to measure letter fluency by asking the participant to name as many words as possible beginning with the letter L and then F during 60-second trials.49,50 The score was calculated as the mean number of correct answers on the 2 trials. To measure category fluency, participants named as many animals, followed by fruits and vegetables, as possible in 60-second trials, with scores calculated as the mean number of valid answers.51 These verbal fluency tests assess language abilities, processing speed, and executive function.53 For the Hopkins Verbal Learning Test, participants were read a list of 12 words and asked to name as many of the words as they could remember in a total of 3 trials, with the score calculated as the mean number of correct answers (range, 0–12).52 Next, verbal recognition was tested by asking participants to identify whether specific words were among the 12 previously presented words. Scores were calculated as the number of true positives (words from the original list that are correctly identified) minus errors (range, 0–12).
Covariates
We administered a self-report questionnaire to all patients to determine age, sex, race/ethnicity, education, pack years of tobacco use, and illicit substance use.54 Medical history was assessed by a standardized questionnaire asking, “Has a doctor or nurse ever diagnosed you with the following?” with a list of conditions that included dementia, Parkinson’s disease, stroke or other neurologic disorders, and standard vascular risk factors and events (heart attack, diabetes, high blood pressure, elevated cholesterol). We administered the Alcohol Use Disorders Identification Test consumption questions (AUDIT-C),55 a validated screening questionnaire that uses 3 questions to assess frequency and amount of alcohol use and yields a total score of 0–12.
To evaluate overall physical activity, participants were asked how often in the last month they performed 15–20 minutes of exercise. Participants chose 1 of the following 6 categories: not at all active, a little active (1–2 times per month), fairly active (3–4 times per month), quite active (1–2 times per week), very active (3–4 times per week), and extremely active (5 or more times per week). Those who reported being not at all active or a little active were considered “inactive,” while those who were fairly active, quite active, very active, or extremely active were coded as “active.”54 Self-report has been shown to be a reliable method of assessing physical activity, and this dichotomized single item measure was a strong predictor of cardiovascular events and mortality in a prior study.54
Sleep quality was assessed with a question from the Pittsburgh Sleep Quality Index,56 “During the last month, how would you rate your sleep quality overall?” Response options included very good, fairly good, good, fairly bad, and very bad. Similar to prior studies and based on sample distributions, we coded good, fairly good, and good as “good” sleep quality and fairly bad or very bad as “poor” sleep quality.57 Single-item sleep quality measures have been shown to have good test-retest reliability and correlation with more extensive questionnaires and to predict multiple negative health outcomes.58,59
We used the 9-item Patient Health Questionnaire (PHQ-9)60 to evaluate depressive symptoms. This self-report instrument measures the frequency of depressive symptoms corresponding to the 9 symptom criteria in the DSM-IV. A standard cut point of ≥ 10 is used to define depression and has demonstrated excellent validity when compared with a mental health interview, with a sensitivity of 88% and a specificity of 88%.
Statistical Analysis
We compared differences in characteristics between participants with and without PTSD using t tests for continuous variables and χ2 tests for categorical variables. We evaluated the association of PTSD and each cognitive function test using linear regression models. Scores for each cognitive function test were normally distributed but were in different units (ie, number of seconds for Trails, number of words named in 1 minute for verbal fluency) with different possible ranges. Therefore, to allow better comparison among the different tests, we created standardized z scores by subtracting the sample mean for a particular test from the individual raw score then dividing the difference by the sample standard deviation. Using multivariate linear regression, we adjusted for patient characteristics from Table 1 that we hypothesized could affect cognitive function and that were associated with PTSD at P < .20. We developed staged models first adjusting for potential confounders (age, sex, race), then examining the effects of potential mediators by adding health behaviors (tobacco use, alcohol use, illicit drug use, physical activity, sleep quality), vascular risk factors (myocardial infarction, hypertension, diabetes, elevated cholesterol), and depressive symptoms.
We also evaluated the association of PTSD symptom severity with cognitive function by repeating these models using the same outcomes and covariates but substituting current CAPS score (entered per standard deviation) as the predictor. All statistical tests were 2-sided with α = .05. We used Stata version 11 (StataCorp; College Station, Texas) to perform all analyses.
RESULTS
Patient Characteristics
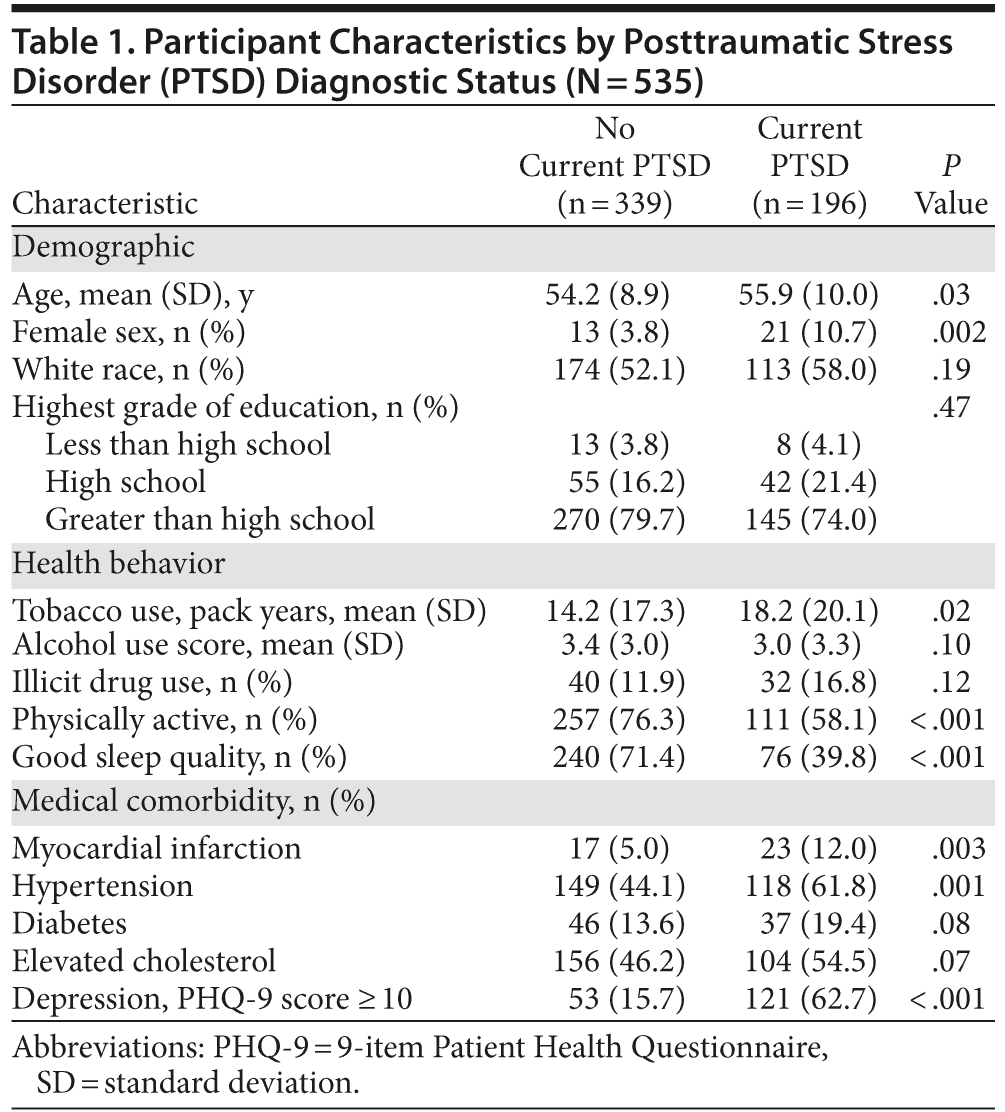
Of the 535 participants, 196 (37%) were included in the PTSD group, as described. Characteristics of participants with and without PTSD are shown in Table 1. Individuals with PTSD were, on average, 1.7 years older, were more likely to be female, and were more likely to have a number of poor health behaviors and vascular risk factors.
PTSD and Cognitive Function
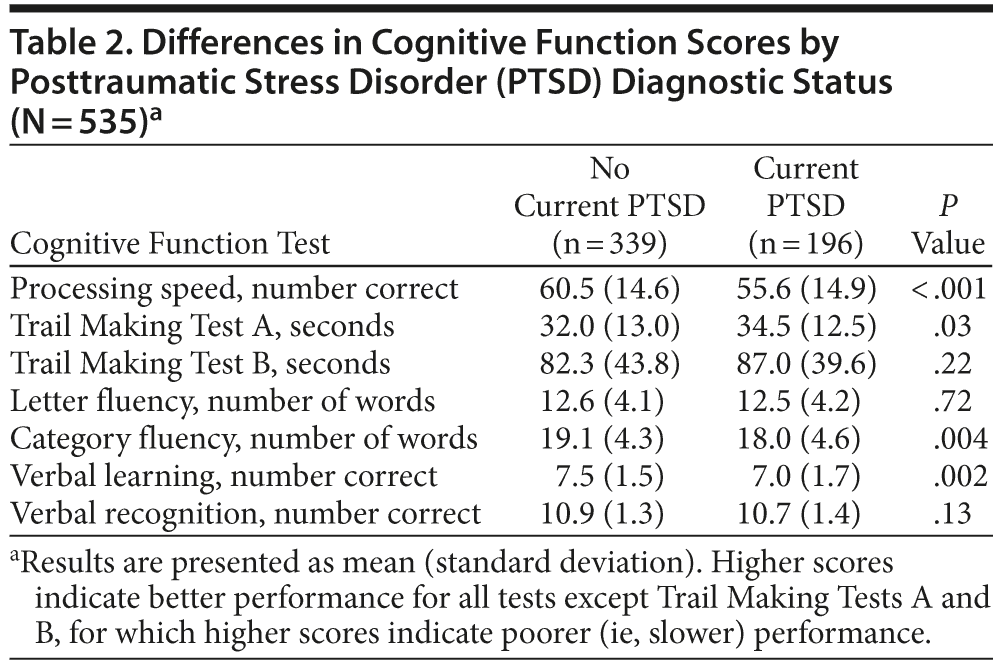
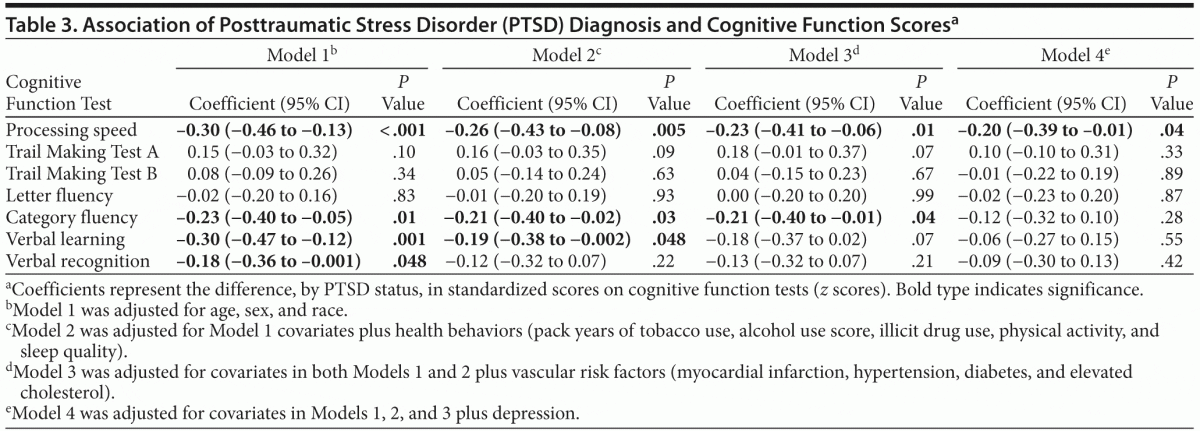
As shown in Table 2, in unadjusted analyses, individuals with PTSD scored significantly worse than individuals without PTSD on several cognitive tests. For example, participants with PTSD completed 5 fewer items on the processing speed test. In models adjusting for age, sex, and race, PTSD was associated with significantly worse performance on processing speed, category fluency, verbal recall, and verbal recognition, with scores 0.18 to 0.30 standard deviations [SDs] lower in those with PTSD than those without PTSD (Table 3). Adjustment for health behaviors reduced the association with processing speed by 13% (ie, reduced from −0.30 to −0.26 SDs), category fluency by 9%, and verbal learning by 37%, and verbal recognition was no longer significant. Additional adjustment for vascular risk factors further reduced the coefficient for processing speed by an additional 12%, did not change the coefficient for category fluency, and eliminated the significance of the association with verbal learning. Adjustment for depression reduced the coefficient for processing speed by an additional 13%, and the association with category fluency was no longer significant.
PTSD Symptom Severity and Cognitive Function
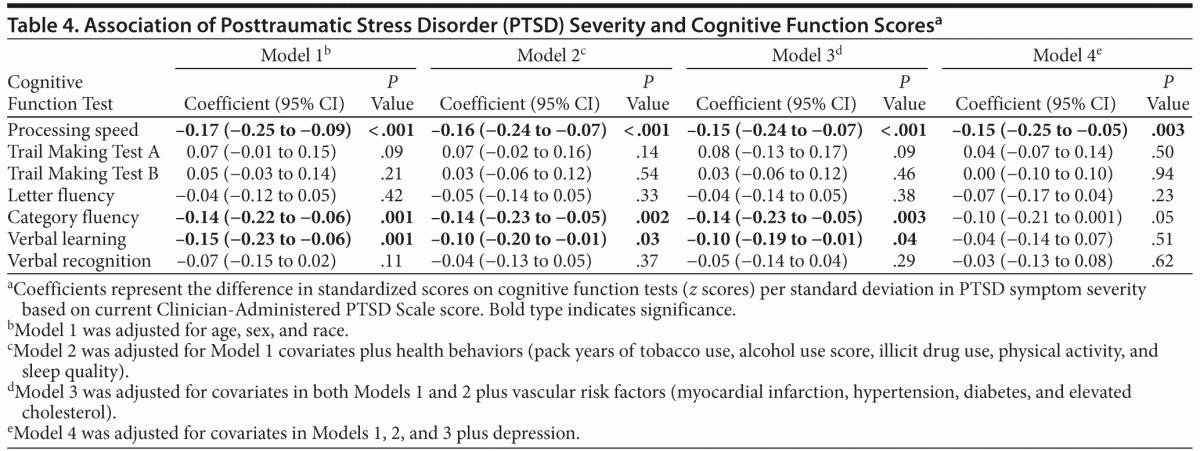
In similar models using PTSD symptom severity score rather than PTSD diagnosis as a predictor, greater PTSD symptom severity was significantly associated with poorer performance on processing speed, category fluency, and verbal learning after adjusting for demographics (Table 4). These associations remained significant after adjustment for health behaviors and vascular risk factors, with the coefficient for processing speed reduced by 12%, category fluency unchanged, and verbal learning reduced by 33%. After further adjustment for depression, PTSD symptom severity remained significantly associated only with processing speed.
DISCUSSION
In this large cohort of VA patients under age 65 years without reported dementia or other neurologic disorders, we found that PTSD diagnosis and symptom severity were associated with significantly worse performance in a variety of cognitive domains, including processing speed and learning, independent of demographics. These differences were largely accounted for by a combination of poor health behaviors and vascular risk factors, highlighting the role of these factors as potential targets to prevent cognitive decline following psychological trauma.
Our work extends prior studies of PTSD and cognitive function by evaluating a relatively large clinical sample using a gold standard diagnostic measure of PTSD and by exploring the role of multiple potential mediators. Several important prior studies have evaluated the effects of war-zone deployment and PTSD on cognitive function.5–9,36,61 Although many studies have had small sample sizes (N < 50), most have found that patients with PTSD have poorer performance than controls without PTSD, with deficits in attention and memory being most common.36,62,63 While several prior studies have adjusted for demographics and health behaviors such as alcohol use, to our knowledge, no prior studies have comprehensively evaluated potential mechanisms linking PTSD to cognitive impairment. In our study, adjustment for a variety of health behaviors, vascular risk factors, and depression largely explained poorer performance on cognitive tests among those with PTSD or greater PTSD symptom severity. Even though interpretation of adjustment for depression may be complicated by the overlap of symptoms with PTSD, these findings highlight the important role of potentially modifiable behaviors and comorbid conditions in cognitive impairment. Given that we focused on a nonelderly population with no known dementia or neurologic disorders, these risk factors could be targets for preventive efforts to reduce dementia and cognitive decline as patients with PTSD age.
To understand the neurologic changes that may underlie these cognitive deficits, neuroimaging studies have examined structural and functional brain abnormalities in patients with PTSD, and some have also included cognitive assessments.12 Patients with PTSD have decreases in the size of the hippocampus, an area critical for episodic memory, and the frontal lobes, which control higher level processing and executive function. In our study, the most notable deficits in cognitive tasks correlated with the brain regions shown to be affected in prior neuroimaging work. For example, the Digit Symbol Substitution Test involves processing speed, working memory, and executive function, which depend on frontal lobe activation.64 Patients with versus without PTSD also had poorer performance on category fluency but not letter fluency. Category fluency involves language and memory and is linked to the hippocampus. Interestingly, a more profound deficit in category versus verbal fluency has also been seen in patients with Alzheimer’s disease.51 It has been hypothesized that, although both tasks involve memory and the hippocampus, only category fluency requires retrieval from language memory stores in the temporal lobe.51 Finally, we found deficits in verbal learning and recognition, processes also dependent on the hippocampus and frontal lobes.9
PTSD may be linked to cognitive dysfunction and underlying structural brain changes through a variety of biological and behavioral mechanisms. Traumatic stress leads to activation of the hypothalamic-pituitary-adrenal axis and sympathetic nervous system, causing increased catecholamine production65 and heightened inflammatory activity.66 Inflammatory cytokines can affect the central nervous system by crossing the blood-brain barrier or by promoting endogenous secretion within the brain.67,68 Although increases in inflammatory cytokines can be adaptive in the setting of repair of acute injury, chronic inflammation causes neurodegeneration and neuronal death and impairs neurogenesis.68 In addition to its direct effect on neurons, inflammation accelerates atherosclerosis, which could cause cerebral ischemia and further neurodegeneration. It is well established that PTSD increases ischemic heart disease via accelerated coronary atherosclerosis, yet the analogous process has not been fully examined in the brain.26 Adjustment for vascular risk factors explained a small portion of the association of PTSD and cognitive decline, but further evaluation using biologic measures of inflammation and atherosclerosis is warranted. Prior studies have also demonstrated that patients with PTSD have increases in smoking, alcohol use, sedentary lifestyles, poor sleep, and depression. Each of these factors has been linked to cognitive decline, and indeed they contributed to the associations of PTSD and cognitive function in our study.19,20,22 Through further study, we may be able to identify the specific neurologic structures and functions affected by the risk factors that explained the association of PTSD and cognitive performance.
Our results should be interpreted in light of several limitations. Reflecting the demographics of our recruitment population, this sample is mostly male, and results may not generalize to women or nonveteran populations. Although we chose to examine potential mediators that had prior evidence for being on a causal pathway linking PTSD and cognitive performance, we cannot evaluate causality with these cross-sectional analyses, and it will be important to confirm our findings in prospective analyses with repeated cognitive testing. In addition, we did not have data on all established risk factors for dementia and cognitive decline, and it would be important for future studies to examine the roles of additional processes, such as inflammation, elevated homocysteine, and genetic variation to more fully understand the association of PTSD and cognitive impairment. We selected a cognitive test battery that identified multiple domains of cognitive performance associated with a variety of brain regions; however, we lack a comprehensive neuropsychological assessment. Finally, adjusting for depressive symptoms in patients with PTSD is complex given the high comorbidity of these disorders and their overlapping symptoms. We did explore stratification of our data to separate those with PTSD with and without depression and other psychiatric comorbidities, but this led to relatively small subgroups, and we feel the differing contributions of these disorders would be better explored in larger studies.
Despite these limitations, our findings that nonelderly patients with PTSD have poorer performance than those without PTSD in a number of cognitive domains provide rationale for ongoing basic and epidemiologic research examining how PTSD impacts brain structure and function. While appropriate early identification and evidence-based treatment of PTSD are being pursued by the VA and other health care centers, our results suggest that poor health behaviors, vascular risk factors, and depressive symptoms may also be important targets for interventions to improve cognitive function and prevent subsequent disability in the large number of veterans and civilians with PTSD.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration–approved labeling has been presented in this activity.
Author affiliations: Department of Medicine (Dr Cohen), Department of Psychiatry (Drs Neylan, Samuelson, Yaffe, and Barnes), Department of Neurology (Dr Yaffe), and Department of Epidemiology and Biostatistics (Drs Yaffe and Barnes), University of California, San Francisco; General Internal Medicine (Drs Cohen and Li) and Mental Health Services (Drs Neylan, Yaffe, Samuelson, and Barnes),Veterans Affairs Medical Center, San Francisco, California; and Alliant International University, San Francisco, California (Dr Samuelson). Dr Li is currently affiliated with Genentech.
Financial disclosure: Dr Neylan has received study medication from Actelion for a study funded by the US Department of Defense and has received study medication from GlaxoSmithKline for a study funded by the US Department of Veterans Affairs. Dr Barnes has received funding support from UCB Pharma. Drs Cohen, Yaffe, Samuelson, and Li have no personal affiliations or financial relationships with any commercial interest to disclose relative to this article.
Funding/support: The Mind Your Heart Study was supported by the National Heart, Lung, and Blood Institute (K23 HL 094765-0), by the Irene Perstein Foundation, and by departmental funds from the University of California, San Francisco. Publication of this article was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through University of California, San Francisco, Clinical and Translational Science Institute grant UL1 RR024131.
Role of the sponsors: The funding organizations were not involved in the design or conduct of the study; data collection, management, or analysis; interpretation of the data; or the preparation, review, or approval of the manuscript.
Disclaimer: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of any of the funding agencies.
Previous presentation: An abstract of these findings was presented at the 28th Annual Meeting of the International Society for Traumatic Stress Studies; November 1–3, 2012; Los Angeles, California.
Acknowledgments: We thank the Mind Your Heart Study participants and gratefully acknowledge the contributions of the Mind Your Heart Study staff. We also thank Mind Your Heart Study Co-Investigator Mary Whooley, MD, of the University of California, San Francisco, and San Francisco Veterans Affairs Medical Center, for her support in establishing the study cohort and protocols. Dr Whooley has no personal affiliations or financial relationships with any commercial interest to disclose relative to this article.
REFERENCES
1. Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi:10.1001/archpsyc.62.6.617 PubMed
2. Boscarino JA. Posttraumatic stress disorder and mortality among US Army veterans 30 years after military service. Ann Epidemiol. 2006;16(4):248–256. doi:10.1016/j.annepidem.2005.03.009 PubMed
3. Dohrenwend BP, Turner JB, Turse NA, et al. The psychological risks of Vietnam for US veterans: a revisit with new data and methods. Science. 2006;313(5789):979–982. doi:10.1126/science.1128944 PubMed
4. Spiro A 3rd, Schnurr PP, Aldwin CM. Combat-related posttraumatic stress disorder symptoms in older men. Psychol Aging. 1994;9(1):17–26. doi:10.1037/0882-7974.9.1.17 PubMed
5. Brewin CR, Kleiner JS, Vasterling JJ, et al. Memory for emotionally neutral information in posttraumatic stress disorder: a meta-analytic investigation. J Abnorm Psychol. 2007;116(3):448–463. doi:10.1037/0021-843X.116.3.448 PubMed
6. Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67(6):608–613. doi:10.1001/archgenpsychiatry.2010.61 PubMed
7. Yehuda R, Tischler L, Golier JA, et al. Longitudinal assessment of cognitive performance in Holocaust survivors with and without PTSD. Biol Psychiatry. 2006;60(7):714–721. doi:10.1016/j.biopsych.2006.03.069 PubMed
8. Vasterling JJ, Proctor SP, Amoroso P, et al. Neuropsychological outcomes of army personnel following deployment to the Iraq war. JAMA. 2006;296(5):519–529. doi:10.1001/jama.296.5.519 PubMed
9. Johnsen GE, Asbjørnsen AE. Consistent impaired verbal memory in PTSD:
a meta-analysis. J Affect Disord. 2008;111(1):74–82. doi:10.1016/j.jad.2008.02.007 PubMed
10. Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(suppl 5):4–12, discussion 13–14. PubMed
11. Seal KH, Metzler T, Gima K, et al. Growing burden of mental disorders among Iraq and Afghanistan veterans: trends and risk factors for mental health diagnoses in new users of VA healthcare, 2002–2008. Am J Public Health. In press.
12. Francati V, Vermetten E, Bremner JD. Functional neuroimaging studies in posttraumatic stress disorder: review of current methods and findings. Depress Anxiety. 2007;24(3):202–218. doi:10.1002/da.20208 PubMed
13. Apfel BA, Ross J, Hlavin J, et al. Hippocampal volume differences in Gulf War veterans with current versus lifetime posttraumatic stress disorder symptoms. Biol Psychiatry. 2011;69(6):541–548. doi:10.1016/j.biopsych.2010.09.044 PubMed
14. Kasai K, Yamasue H, Gilbertson MW, et al. Evidence for acquired pregenual anterior cingulate gray matter loss from a twin study of combat-related posttraumatic stress disorder. Biol Psychiatry. 2008;63(6):550–556. doi:10.1016/j.biopsych.2007.06.022 PubMed
15. Kitayama N, Quinn S, Bremner JD. Smaller volume of anterior cingulate cortex in abuse-related posttraumatic stress disorder. J Affect Disord. 2006;90(2–3):171–174. doi:10.1016/j.jad.2005.11.006 PubMed
16. Kivipelto M, Ngandu T, Fratiglioni L, et al. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62(10):1556–1560. doi:10.1001/archneur.62.10.1556 PubMed
17. Dichgans M, Zietemann V. Prevention of vascular cognitive impairment. Stroke. 2012;43(11):3137–3146. doi:10.1161/STROKEAHA.112.651778 PubMed
18. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011;10(9):819–828. doi:10.1016/S1474-4422(11)70072-2 PubMed
19. Zen AL, Whooley MA, Zhao S, et al. Post-traumatic stress disorder is associated with poor health behaviors: findings from The Heart and Soul Study. Health Psychol. 2012;31(2):194–201. doi:10.1037/a0025989 PubMed
20. Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry. 2003;60(3):289–294. doi:10.1001/archpsyc.60.3.289 PubMed
21. Medina KL, Schweinsburg AD, Cohen-Zion M, et al. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29(1):141–152. doi:10.1016/j.ntt.2006.10.010 PubMed
22. Ferrie JE, Shipley MJ, Akbaraly TN, et al. Change in sleep duration and cognitive function: findings from the Whitehall II Study. Sleep. 2011;34(5):565–573. PubMed
23. Barnes DE, Yaffe K, Satariano WA, et al. A longitudinal study of cardiorespiratory fitness and cognitive function in healthy older adults.
J Am Geriatr Soc. 2003;51(4):459–465. doi:10.1046/j.1532-5415.2003.51153.x PubMed
24. Yaffe K, Barnes D, Nevitt M, et al. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–1708. doi:10.1001/archinte.161.14.1703 PubMed
25. Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306(6):613–619. doi:10.1001/jama.2011.1115 PubMed
26. Kubzansky LD, Koenen KC, Spiro A 3rd, et al. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch Gen Psychiatry. 2007;64(1):109–116. doi:10.1001/archpsyc.64.1.109 PubMed
27. Edmondson D, Cohen BE. Posttraumatic stress disorder and cardiovascular disease. Prog Cardiovasc Dis. 2013;55(6):548–556. doi:10.1016/j.pcad.2013.03.004 PubMed
28. Whitmer RA, Sidney S, Selby J, et al. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi:10.1212/01.WNL.0000149519.47454.F2 PubMed
29. McDermott LM, Ebmeier KP. A meta-analysis of depression severity and cognitive function. J Affect Disord. 2009;119(1–3):1–8. doi:10.1016/j.jad.2009.04.022 PubMed
30. Barnes DE, Alexopoulos GS, Lopez OL, et al. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63(3):273–279. doi:10.1001/archpsyc.63.3.273 PubMed
31. Byers AL, Covinsky KE, Barnes DE, et al. Dysthymia and depression increase risk of dementia and mortality among older veterans. Am J Geriatr Psychiatry. 2012;20(8):664–672. doi:10.1097/JGP.0b013e31822001c1 PubMed
32. Barnes DE, Yaffe K, Byers AL, et al. Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry. 2012;69(5):493–498. doi:10.1001/archgenpsychiatry.2011.1481 PubMed
33. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
34. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75–90. doi:10.1002/jts.2490080106 PubMed
35. Weathers FW, Keane TM, Davidson JR. Clinician-Administered PTSD Scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):132–156. doi:10.1002/da.1029 PubMed
36. Samuelson KW, Neylan TC, Metzler TJ, et al. Neuropsychological functioning in posttraumatic stress disorder and alcohol abuse. Neuropsychology. 2006;20(6):716–726. doi:10.1037/0894-4105.20.6.716 PubMed
37. Weathers FW, Ruscio AM, Keane TM. Psychometric properties of nine scoring rules for the Clinician-Administered Posttraumatic Stress Disorder Scale. Psychol Assess. 1999;11(2):124–133. doi:10.1037/1040-3590.11.2.124
38. Sayer NA, Noorbaloochi S, Frazier P, et al. Reintegration problems and treatment interests among Iraq and Afghanistan combat veterans receiving VA medical care. Psychiatr Serv. 2010;61(6):589–597. doi:10.1176/appi.ps.61.6.589 PubMed
39. Marshall RD, Olfson M, Hellman F, et al. Comorbidity, impairment, and suicidality in subthreshold PTSD. Am J Psychiatry. 2001;158(9):1467–1473. doi:10.1176/appi.ajp.158.9.1467 PubMed
40. Blanchard EB, Hickling EJ, Taylor AE, et al. Psychological morbidity associated with motor vehicle accidents. Behav Res Ther. 1994;32(3):283–290. doi:10.1016/0005-7967(94)90123-6 PubMed
41. Rosano C, Newman AB, Katz R, et al. Association between lower digit symbol substitution test score and slower gait and greater risk of mortality and of developing incident disability in well-functioning older adults. J Am Geriatr Soc. 2008;56(9):1618–1625. doi:10.1111/j.1532-5415.2008.01856.x PubMed
42. McGough EL, Kelly VE, Logsdon RG, et al. Associations between physical performance and executive function in older adults with mild cognitive impairment: gait speed and the timed “up & go” test. Phys Ther. 2011;91(8):1198–1207. doi:10.2522/ptj.20100372 PubMed
43. Razani J, Casas R, Wong JT, et al. Relationship between executive functioning and activities of daily living in patients with relatively mild dementia. Appl Neuropsychol. 2007;14(3):208–214. doi:10.1080/09084280701509125 PubMed
44. Mast BT, Allaire JC. Verbal learning and everyday functioning in dementia: an application of latent variable growth curve modeling. J Gerontol B Psychol Sci Soc Sci. 2006;61(3):167–173. doi:10.1093/geronb/61.3.P167 PubMed
45. Lonie JA, Parra-Rodriguez MA, Tierney KM, et al. Predicting outcome in mild cognitive impairment: 4-year follow-up study. Br J Psychiatry. 2010;197(2):135–140. doi:10.1192/bjp.bp.110.077958 PubMed
46. Wechsler DA. Manual for the Wechsler Adult Intelligence Scale-Revised.
New York, NY: Psychological Corporation; 1981.
47. Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi:10.1016/S0887-6177(03)00039-8 PubMed
48. Sánchez-Cubillo I, Periáñez JA, Adrover-Roig D, et al. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. 2009;15(3):438–450. doi:10.1017/S1355617709090626 PubMed
49. Ruff RM, Light RH, Parker SB, et al. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338. PubMed
50. Gladsjo JA, Schuman CC, Evans JD, et al. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment. 1999;6(2):147–178. doi:10.1177/107319119900600204 PubMed
51. Cerhan JH, Ivnik RJ, Smith GE, et al. Diagnostic utility of letter fluency, category fluency, and fluency difference scores in Alzheimer’s disease.
Clin Neuropsychol. 2002;16(1):35–42. doi:10.1076/clin.16.1.35.8326 PubMed
52. Rasmusson DX, Bylsma FW, Brandt J. Stability of performance on the Hopkins Verbal Learning Test. Arch Clin Neuropsychol. 1995;10(1):21–26. PubMed
53. McDowd J, Hoffman L, Rozek E, et al. Understanding verbal fluency in healthy aging, Alzheimer’s disease, and Parkinson’s disease. Neuropsychology. 2011;25(2):210–225. doi:10.1037/a0021531 PubMed
54. Whooley MA, de Jonge P, Vittinghoff E, et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379–2388. doi:10.1001/jama.2008.711 PubMed
55. Bush K, Kivlahan DR, McDonell MB, et al. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158(16):1789–1795. doi:10.1001/archinte.158.16.1789 PubMed
56. Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi:10.1016/0165-1781(89)90047-4 PubMed
57. Caska CM, Hendrickson BE, Wong MH, et al. Anger expression and sleep quality in patients with coronary heart disease: findings from The Heart and Soul Study. Psychosom Med. 2009;71(3):280–285. doi:10.1097/PSY.0b013e31819b6a08 PubMed
58. Cappelleri JC, Bushmakin AG, McDermott AM, et al. Psychometric properties of a single-item scale to assess sleep quality among individuals with fibromyalgia. Health Qual Life Outcomes. 2009;7(1):54. doi:10.1186/1477-7525-7-54 PubMed
59. Prather AA, Puterman E, Lin J, et al. Shorter leukocyte telomere length in midlife women with poor sleep quality. J Aging Res. 2011;2011:721390. doi:10.4061/2011/721390 PubMed
60. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–613. doi:10.1046/j.1525-1497.2001.016009606.x PubMed
61. Scott Mackin R, Lesselyong JA, Yaffe K. Pattern of cognitive impairment in older veterans with posttraumatic stress disorder evaluated at a memory disorders clinic. Int J Geriatr Psychiatry. 2012;27(6):637–642. doi:10.1002/gps.2763 PubMed
62. Horner MD, Hamner MB. Neurocognitive functioning in posttraumatic stress disorder. Neuropsychol Rev. 2002;12(1):15–30. doi:10.1023/A:1015439106231 PubMed
63. Isaac CL, Cushway D, Jones GV. Is posttraumatic stress disorder associated with specific deficits in episodic memory? Clin Psychol Rev. 2006;26(8):939–955. doi:10.1016/j.cpr.2005.12.004 PubMed
64. Nakahachi T, Ishii R, Iwase M, et al. Frontal activity during the digit symbol substitution test determined by multichannel near-infrared spectroscopy. Neuropsychobiology. 2008;57(4):151–158. doi:10.1159/000147467 PubMed
65. Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25(2):341–368. doi:10.1016/S0193-953X(02)00002-3 PubMed
66. Gill JM, Saligan L, Woods S, et al. PTSD is associated with an excess of inflammatory immune activities. Perspect Psychiatr Care. 2009;45(4):
262–277. doi:10.1111/j.1744-6163.2009.00229.x PubMed
67. Yarlagadda A, Alfson E, Clayton AH. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry (Edgmont). 2009;6(11):18–22. PubMed
68. Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120(3):277–286. doi:10.1007/s00401-010-0722-x PubMed





