Objective: Auditory hallucinations are an important symptom for diagnosing dementia with Lewy bodies (DLB), yet they have received less attention than visual hallucinations. We investigated the clinical features of auditory hallucinations and the possible mechanisms by which they arise in patients with DLB.
Methods: We recruited 124 consecutive patients with probable DLB (diagnosis based on the DLB International Workshop 2005 criteria; study period: June 2007-January 2015) from the dementia referral center of Kumamoto University Hospital. We used the Neuropsychiatric Inventory to assess the presence of auditory hallucinations, visual hallucinations, and other neuropsychiatric symptoms. We reviewed all available clinical records of patients with auditory hallucinations to assess their clinical features. We performed multiple logistic regression analysis to identify significant independent predictors of auditory hallucinations.
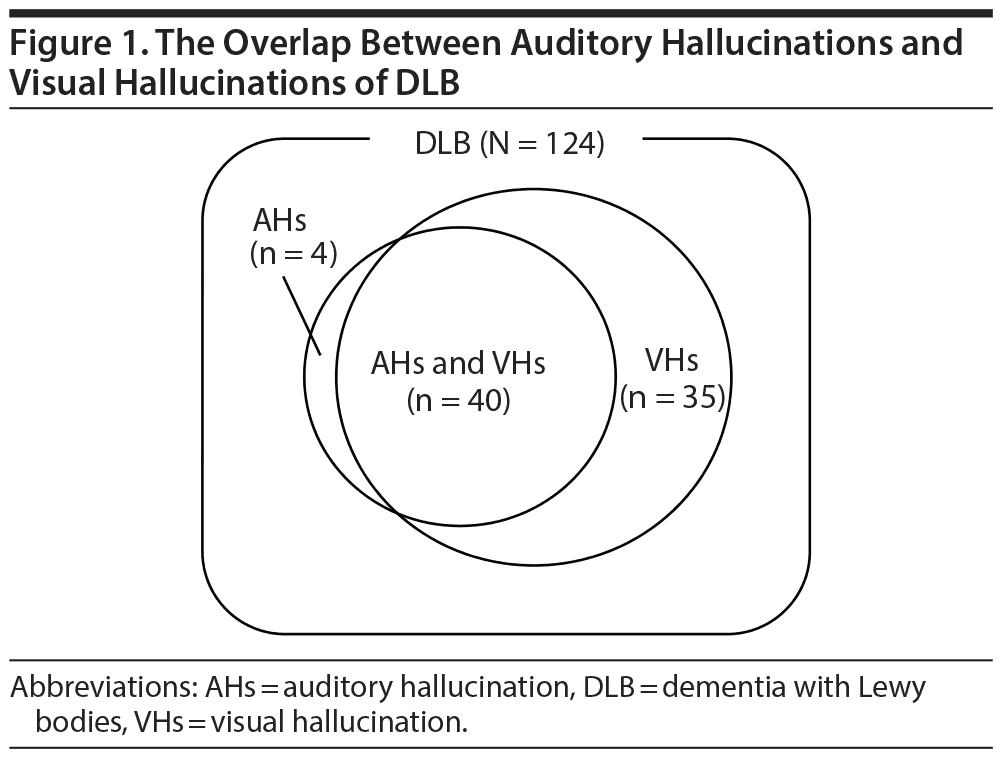
Results: Of the 124 patients, 44 (35.5%) had auditory hallucinations and 75 (60.5%) had visual hallucinations. The majority of patients (90.9%) with auditory hallucinations also had visual hallucinations. Auditory hallucinations consisted mostly of human voices, and 90% of patients described them as like hearing a soundtrack of the scene. Multiple logistic regression showed that the presence of auditory hallucinations was significantly associated with female sex (P = .04) and hearing impairment (P = .004). The analysis also revealed independent correlations between the presence of auditory hallucinations and visual hallucinations (P < .001), phantom boarder delusions (P = .001), and depression (P = .038).
Conclusions: Auditory hallucinations are common neuropsychiatric symptoms in DLB and usually appear as a background soundtrack accompanying visual hallucinations. Auditory hallucinations in patients with DLB are more likely to occur in women and those with impaired hearing, depression, delusions, or visual hallucinations.
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.

Clinical Features of Auditory Hallucinations in Patients With Dementia With Lewy Bodies:
A Soundtrack of Visual Hallucinations

ABSTRACT
Objective: Auditory hallucinations are an important symptom for diagnosing dementia with Lewy bodies (DLB), yet they have received less attention than visual hallucinations. We investigated the clinical features of auditory hallucinations and the possible mechanisms by which they arise in patients with DLB.
Methods: We recruited 124 consecutive patients with probable DLB (diagnosis based on the DLB International Workshop 2005 criteria; study period: June 2007-January 2015) from the dementia referral center of Kumamoto University Hospital. We used the Neuropsychiatric Inventory to assess the presence of auditory hallucinations, visual hallucinations, and other neuropsychiatric symptoms. We reviewed all available clinical records of patients with auditory hallucinations to assess their clinical features. We performed multiple logistic regression analysis to identify significant independent predictors of auditory hallucinations.
Results: Of the 124 patients, 44 (35.5%) had auditory hallucinations and 75 (60.5%) had visual hallucinations. The majority of patients (90.9%) with auditory hallucinations also had visual hallucinations. Auditory hallucinations consisted mostly of human voices, and 90% of patients described them as like hearing a soundtrack of the scene. Multiple logistic regression showed that the presence of auditory hallucinations was significantly associated with female sex (P = .04) and hearing impairment (P = .004). The analysis also revealed independent correlations between the presence of auditory hallucinations and visual hallucinations (P < .001), phantom boarder delusions (P = .001), and depression (P = .038).
Conclusions: Auditory hallucinations are common neuropsychiatric symptoms in DLB and usually appear as a background soundtrack accompanying visual hallucinations. Auditory hallucinations in patients with DLB are more likely to occur in women and those with impaired hearing, depression, delusions, or visual hallucinations.
J Clin Psychiatry 2018;79(3):17m11623
To cite: Tsunoda N, Hashimoto M, Ishikawa T, et al. Clinical features of auditory hallucinations in patients with dementia with Lewy bodies: a soundtrack of visual hallucinations. J Clin Psychiatry. 2018;79(3):17m11623.
To share: https://doi.org/10.4088/JCP.17m11623
© Copyright 2018 Physicians Postgraduate Press, Inc.
aDepartment of Neuropsychiatry, Graduate School of Medical Science, Kumamoto University, Kumamoto, Japan
bDepartment of Geriatric Psychiatry, Mitsugumachi Clinic, Kumamoto, Japan
cDepartment of Neuropsychiatry, Faculty of Life Sciences, Kumamoto University, Kumamoto, Japan
dDepartment of Psychiatry, Heisei Hospital, Yatsushiro, Kumamoto, Japan
eDepartment of Psychiatry, Osaka University Graduate School of Medicine, Suita, Osaka, Japan
*Corresponding author: Mamoru Hashimoto, MD, PhD, Department of Neuropsychiatry, Faculty of Life Sciences, Kumamoto University, 1-1-1 Honjo, Chuo-ku, Kumamoto 860-8556, Japan ([email protected]).
Dementia with Lewy bodies (DLB) is the second most common type of degenerative dementia among the elderly after Alzheimer’s disease. The core clinical features of DLB include neuropsychiatric symptoms, the motor symptoms of parkinsonism, and cognitive impairments characterized by deficits of memory, attention, executive function, and visual perception.1 A range of neuropsychiatric symptoms such as hallucinations, delusions, and sleep disturbances occur more frequently in DLB patients than in Alzheimer’s disease patients from the early stages of the disease.2-5 It has been suggested that caring for patients with DLB leads to a heavier caregiver burden than caring for those with Alzheimer’s disease because of the involvement of neuropsychiatric symptoms such as delusions and hallucinations.6-9 Therefore, the management of neuropsychiatric symptoms is a significant issue for maintaining the quality of life of patients with DLB and their caregivers.
Visual hallucinations are reported to occur in 60%-76% of pathologically confirmed DLB patients10-12; they are among the most common neuropsychiatric symptoms and have been identified as one of the core features in the clinical diagnostic criteria of DLB.1,13 Visual hallucinations frequently consist of formed images, predominantly of people, animals, and objects. Auditory hallucinations are also considered to be important features for the diagnosis of DLB and are listed as one of the supportive features in the consensus criteria.1 However, little is known about the clinical features of auditory hallucinations in DLB, as studies have paid less attention to them than to visual hallucinations. In the present study, we focused on auditory hallucinations in patients with DLB and addressed the following questions. What percentage of patients with DLB suffers from auditory hallucinations? What are the contents? What factors are associated with the development of auditory hallucinations?
METHODS
Subjects
All procedures followed the Clinical Study Guidelines of the Ethics Committee of Kumamoto University, Kumamoto, Japan, and were approved by the Internal Review Board. Informed written consent was obtained from patients and their caregivers in compliance with the research standards for human research for all participating institutions and in accordance with the Declaration of Helsinki.
According to the following criteria, 124 patients with probable DLB were consecutively selected from those who attended the dementia referral center of Kumamoto University Hospital between June 2007 and January 2015. All patients were examined comprehensively by experienced senior neuropsychiatrists (M.I., M.H., and T.I.) and were given routine laboratory testing, including vitamin B1, vitamin B12, and thyroid function, and standardized neuropsychological and neuropsychiatric examinations including the Mini-Mental State Examination (MMSE)14 and the Neuropsychiatric Inventory (NPI).15 Structural neuroimaging of the brain was performed using magnetic resonance imaging (MRI) or computed tomography and single-photon emission computed tomography for cerebral perfusion. All results were considered in the diagnosis. The inclusion criteria were the Consortium on DLB International Workshop 2005 criteria for probable DLB.1 The following patients were excluded from the study: (1) those with serious psychiatric diseases such as schizophrenia, major depression, or substance abuse before the onset of dementia; (2) those with evidence of focal brain lesions on MRI; (3) those without a reliable informant; and (4) those who were unable to provide informed consent. The recruited patients included 70 women and 54 men, and the mean ± SD age at the first examination was 78.3 ± 5.6 years. The mean educational attainment was 10.5 ± 2.7 years, and the mean MMSE score was 19.1 ± 5.7.

- Auditory hallucinations are common neuropsychiatric symptoms in DLB, and the majority of patients with auditory hallucinations also had visual hallucinations.
- Auditory hallucinations consist mostly of human voices and usually appear as a background soundtrack accompanying their visual hallucinations.
- Auditory hallucinations in patients with DLB are more likely to occur in women and those with impaired hearing, depression, delusions, or visual hallucinations.
Assessment of Demographic Variables and Neuropsychiatric Symptoms
We collected patients’ demographic information, including previous disease and drug history, through clinical interviews with their caregivers. Hearing impairment was defined as “present” if the patient had difficulty hearing to an extent that interfered with daily living.
We assessed the patients’ comprehensive neuropsychiatric symptoms, including auditory hallucinations, through a semiquantitative interview with their primary caregivers using a Japanese version of the NPI.16 Using the NPI, the following 10 symptoms were rated on the basis of the patients’ condition during the month before the interview: delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritability, and aberrant motor behavior. In the NPI, caregivers are first asked a screening question to determine whether or not the symptom is present. If present, symptom frequency is rated from 1 (< once per week) to 4 (at least once per day), and severity is rated from 1 (mild, present but not causing distress) to 3 (severe, very disruptive), with a maximum total (frequency ×— severity) score of 12 for each domain. The NPI assessments were performed within a month of the first visit. The NPI total score, total score excluding the hallucinations domain, total score excluding the hallucinations and delusions domain, and the presence or absence of each of the 10 items were assessed. In addition, the presence or absence of the following 8 types of delusional thoughts was assessed using the items from the delusion subdomain of the NPI: delusion of persecution, delusion of theft, delusional jealousy, phantom boarder delusion, Capgras delusion, misidentification of one’s house as another place, delusion of abandonment, and the TV phenomenon.
We assessed the presence of auditory hallucinations using the items from the hallucinations subdomain of the NPI. Patients who answered “yes” to the question “Does the patient claim he or she can hear voices and sounds or act as if he or she hears voices and sounds?” were assigned to the auditory hallucination group. We excluded patients who only answered “yes” to the auditory hallucination subdomain question “Does he or she talk to people who are not there?” because even patients who experience only visual hallucinations can meet this condition. The remaining patients were assigned to the non-auditory hallucination group. We regarded visual hallucinations as present if patients answered “yes” to the hallucinations subscale question “Does the patient describe seeing things not seen by others or behave as if he/she is seeing things not seen by others (people, animals, lights, etc)?”
For each patient who experienced hallucinations, we routinely obtained their consent from the primary caregiver using the NPI, and senior neuropsychiatrists routinely questioned each patient about the details of their hallucinations. One of the authors (N.T.) reviewed all available clinical records of patients with auditory and/or visual hallucinations to investigate their clinical features.
Statistical Analysis
Student t tests or χ2 tests were used to compare the auditory hallucination group and the non-auditory hallucination group in terms of their demographic and clinical characteristics, total NPI score, total NPI score excluding the hallucinations domain, total NPI score excluding the hallucinations and delusions domain, the frequency of individual NPI domains, and the frequency of each type of delusion. Multiple logistic regression analysis was performed to identify significant independent predictors of auditory hallucinations. The following variables were entered: age, sex, duration of education, MMSE score, presence or absence of hearing impairment, use of cholinesterase inhibitors, antiparkinsonian and antipsychotic drugs, and total NPI scores excluding the hallucinations domain. Multiple regression analysis was used to test the association between auditory hallucinations and other neuropsychiatric symptoms. The significance level was set at P < .05, 2-tailed, for all analyses. Statistical operations were performed with SPSS for Windows, version 21 (IBM Japan, Tokyo, Japan).
RESULTS
Of the 124 patients, 79 (63.7%) reported having experienced hallucinations within the previous month; of these, 44 (35.5%) had auditory hallucinations, 75 (60.5%) had visual hallucinations, and 40 (32.3%) had auditory plus visual hallucinations. The majority of patients with auditory hallucinations also had visual hallucinations (90.9%), whereas only 4 patients reported auditory hallucinations unaccompanied by visual hallucinations. Figure 1 shows the overlap between auditory hallucinations and visual hallucinations.
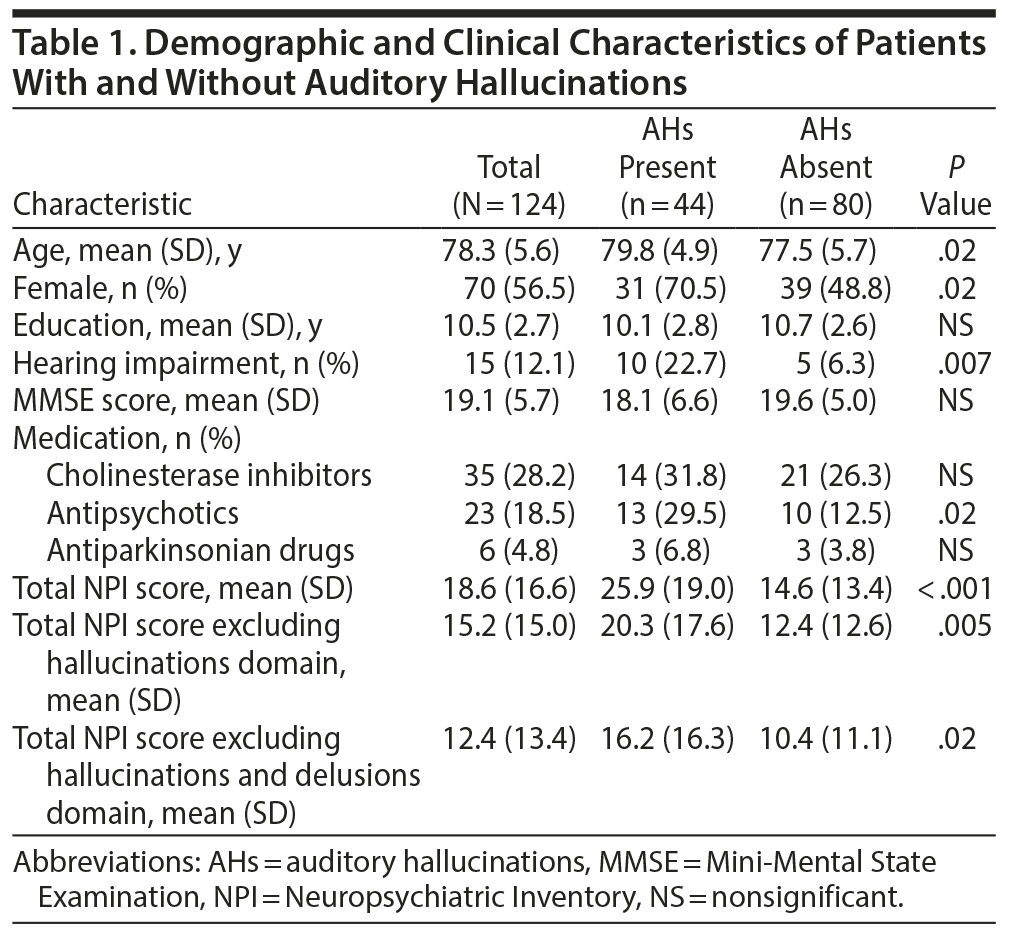
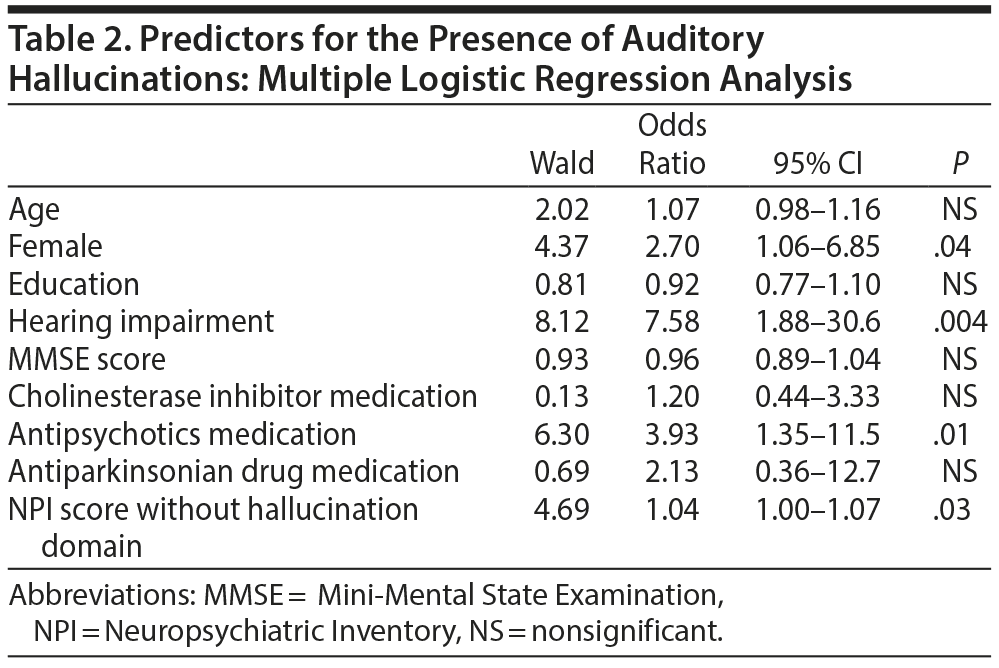
The demographic variables of the auditory hallucination group and the non-auditory hallucination group are shown in Table 1. Patients with auditory hallucinations were significantly older than those without (P = .02). The auditory hallucination group had significantly higher proportions of women (P = .02), patients with hearing impairment (P = .007), and patients taking antipsychotic medications (P = .02) and significantly higher total NPI scores (P < .001), total NPI scores excluding the hallucinations domain (P = .005), and total NPI scores excluding the hallucinations and delusions domain (P = .02) than the non-auditory hallucination group. We found no significant differences between the 2 groups with regard to educational attainment, MMSE scores, and the frequency of other drug usage. Multiple logistic regression analysis showed that the presence of auditory hallucinations was significantly associated with being female (P = .04), the presence of hearing impairment (P = .004), and the use of antipsychotics (P = .01) (Table 2).
The contents of auditory hallucinations consisted mostly of human voices (41 patients), the sound of running water (1 patient), and unspecified sounds (2 patients). There were close phenomenological relationships between auditory and visual hallucinations. Of the 41 patients with verbal auditory hallucinations, 37 patients (90%) heard the visual hallucinations speak. In 25 patients (61%), the visual hallucinations spoke to them, and 4 patients reported that the voices gave commands such as “Get up early.” With respect to the subjective affective aspects of auditory hallucinations, 29 (71%) patients whose auditory hallucinations consisted of voices experienced bad or unpleasant voices. For example, some patients reported that they heard several people speak ill of them, and others reported that they heard someone discussing plans to frame them. Only 4 patients (10%) reported good or pleasant voices telling them, for instance, that they intended to prepare tea and food for imaginary guests. The rest reported neither good nor bad voices.
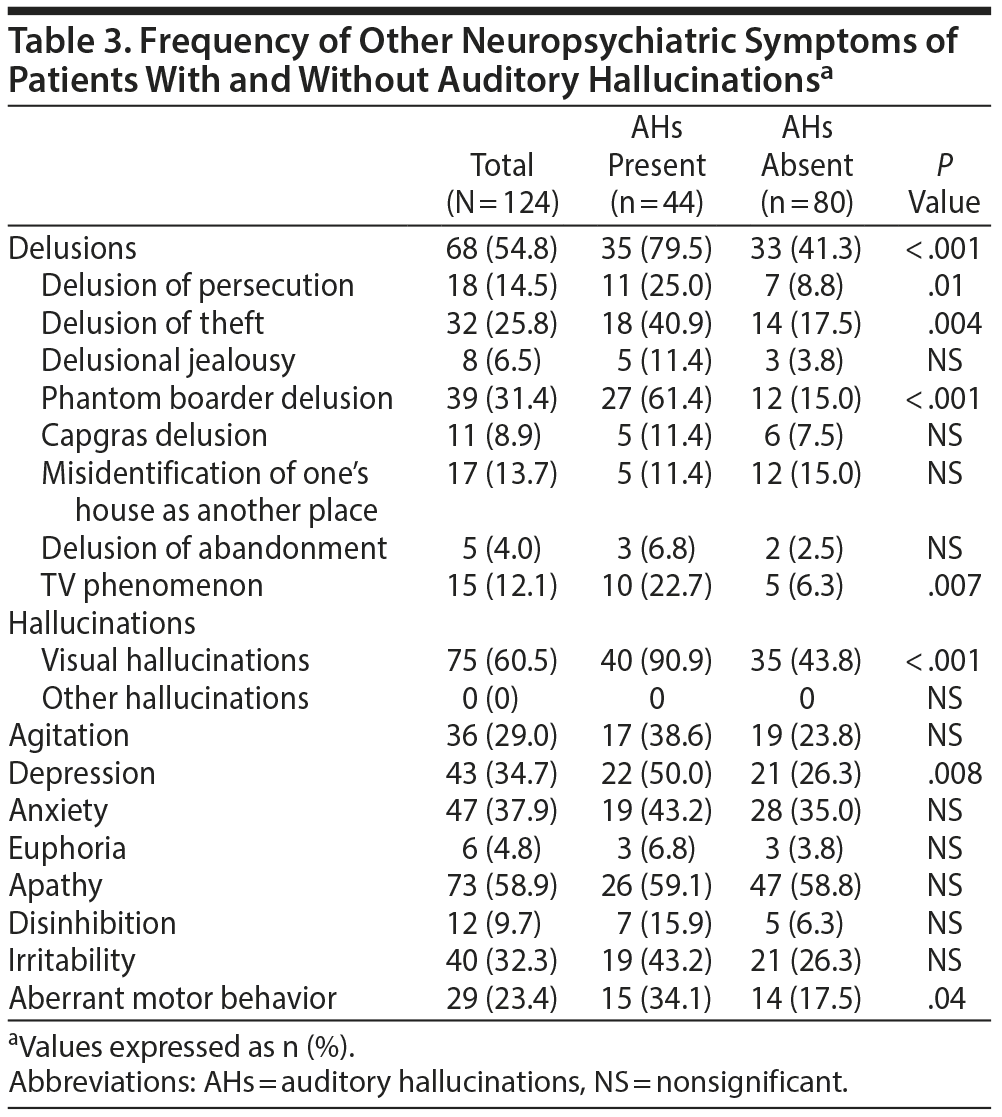
The frequencies of the individual NPI domains and of each delusional thought in the 2 groups are shown in Table 3. Three of the NPI individual domains were associated with the presence of auditory hallucinations: higher frequencies of delusions, depression, and aberrant motor behavior. The frequencies of delusions of persecution, delusions of theft, phantom boarder delusions, and the TV phenomenon were significantly higher in the auditory hallucination than in the non-auditory hallucination group. Multiple regression analysis revealed that the presence of visual hallucinations (P < .001), phantom boarder delusions (P = .001), and depression (P = .038) was independently correlated with the presence of auditory hallucinations.
DISCUSSION
Clinical studies on DLB have paid less attention to auditory hallucinations than visual hallucinations. In the limited number of studies that have reported the prevalence of auditory hallucinations, the figures vary from 6.7% to 45%.2,4,17-19 In the present study, the prevalence of auditory hallucinations was 35.5%, and that of visual hallucinations was 60%. We excluded patients from the auditory hallucination group if they gave a positive answer only to the NPI question “Does he or she talk to people who are not there?” This procedure might mean that the actual prevalence was underestimated. Nevertheless, the finding that more than one-third of the patients with DLB developed auditory hallucinations suggests that they are common neuropsychiatric symptoms in patients with DLB, along with visual hallucinations.
In the few studies that have focused on the characteristics of auditory hallucinations in DLB, the most frequently reported contents were described as human voices,17,20 a doorbell ringing,17 people’s footsteps,17 music,21 and people speaking. Studies on hallucinations in Parkinson’s disease have also reported that auditory hallucinations consisted of human voices and, less often, music and other noises, such as the sound of steps.22-24 As with the previous studies, the contents of auditory hallucinations in this study consisted mostly of human voices and rarely other sounds. Interestingly, most of the patients heard the visual hallucinations talk to each other or to the patient. In the abovementioned studies, visual hallucinations were accompanied by auditory hallucinations, described as being like a soundtrack to the scene. The phenomenon of visual hallucinations speaking and the rarity of pure auditory hallucinations in our patients suggest that auditory hallucinations usually occur on the basis of visual hallucinations in DLB. Nevertheless, nearly half of the patients with visual hallucinations did not experience auditory hallucinations. Therefore, additional factors might be involved in those who develop auditory hallucinations in addition to visual hallucinations in DLB.
Multiple logistic regression analysis revealed that being female, the presence of hearing impairment, the use of antipsychotics, and total NPI score without the hallucination domain were significant predictors of auditory hallucinations. To the best of our knowledge, no studies have determined a possible link between gender and neuropsychiatric symptoms in patients with DLB. However, it has been reported that women in the early stage of vascular dementia are more likely to exhibit hallucinations.25 In addition, some previous studies have indicated that most of the individuals experiencing musical hallucinations were women.20,26,27 It remains unclear why women are more prone to developing auditory hallucinations in DLB; however, the cause is possibly related to some underlying structural or functional differences between genders.
Regarding the relationship between auditory hallucinations and hearing impairment, it has been hypothesized that auditory hallucinations might occur as a functional compensation for sensory impairment. When auditory input to the primary auditory cortex is decreased in patients with hearing impairment, the reduced basal inhibition of the auditory association cortex might allow spontaneous activity. This mechanism is used to explain visual hallucinations in Charles Bonnet syndrome.28,29 We were unable to find any previous studies that have investigated the association between auditory hallucinations and hearing impairment in DLB. However, Cole et al30 reported that auditory hallucinations are frequent in elderly people with hearing impairment, and Golden and Josephs21 reported that formed auditory hallucinations such as musical hallucinations can occur in patients with hearing loss. The exact mechanism by which hearing impairment can predispose an individual to auditory hallucinations is unclear, but hearing impairment may be a possible risk factor for developing auditory hallucinations in DLB. This finding suggests that improving the hearing ability could be a potential treatment option for auditory hallucinations in patients with DLB.
We found the total NPI score without the hallucination domain to be significantly higher in the auditory hallucination group than in the non-auditory hallucination group. This finding suggests that in patients with DLB, the presence of auditory hallucinations may be associated with the presence of other neuropsychiatric symptoms. In this study, auditory hallucinations were independently associated with 3 neuropsychiatric symptoms: phantom boarder delusions, visual hallucinations, and depression. Among the 8 types of delusional thoughts, only phantom boarder delusion was associated with auditory hallucinations. Considering that most of the auditory hallucinations observed in this study were human voices, the patients with auditory hallucinations had good reason to believe that someone uninvited was living in their home. It is noteworthy that only 17.1% of patients with pure visual hallucinations exhibited phantom boarder delusion, but as many as 61.4% of patients with both visual and auditory hallucinations developed the delusion. That is, even if DLB patients saw a vision of an unfamiliar person in their home, they considered it a visual hallucination unless the person spoke. Once the visual hallucination spoke, however, they were more likely to believe in its existence. We found that over two-thirds of patients with auditory hallucinations felt the voices were bad or unpleasant. The negative feelings toward the voices may also have contributed to the development of the delusion.
The biological mechanism of auditory hallucinations in DLB is unknown. However, considering that auditory hallucinations in Parkinson’s disease have been shown to disappear after decreasing or discontinuing l-dopa,24,31,32 it is possible that the dopamine neuron system is involved in the development of auditory hallucinations in DLB. However, auditory hallucinations in DLB nearly always coexist with visual hallucinations, which have been reported to be associated with the disturbance of cholinergic neurons in some clinical trials of cholinesterase inhibitors for DLB.33-36 In addition, the disturbance of other neuron systems such as serotonin or norepinephrine might be involved in auditory hallucinations because of the high comorbidity of depression. Given that auditory hallucinations were common symptoms and were strongly associated with other neuropsychiatric symptoms in patients with DLB in our study, more phenomenological and pathophysiologic studies should focus on auditory hallucinations in DLB.
Various methodological issues should be taken into consideration when interpreting our results. First, in patients with dementia, internal psychiatric symptoms such as auditory hallucinations cannot be directly studied or quantified because of patients’ incomplete recollection. Consequently, the contents of auditory hallucinations tend to be biased toward those that are most memorable to the patient or those associated with behavioral disorders reported by the caregiver. This methodological problem might mean that the proportion of unpleasant human voice hallucinations is not quite as high as reported. Second, patients were diagnosed using the clinical diagnostic criteria for DLB,1 which might have introduced a selection bias. According to the criteria, demented patients with pure visual hallucinations may be diagnosed as having DLB with either parkinsonism or cognitive fluctuation, whereas in those with pure auditory hallucinations, both parkinsonism and cognitive fluctuation are required for a diagnosis of DLB. This selection bias may make the prevalence of DLB patients with pure auditory hallucinations seem lower than it is and may affect the association between auditory hallucinations and visual hallucinations. Third, repeated comparisons in the present study raise the likelihood of an experimental type I error. Corrections for repeated comparisons were not made in this study because of the exploratory goals of this study. However, we finally avoided this possibility by applying multiple logistic regression analysis to identify independent predictors of auditory hallucinations. Fourth, as the present study was performed at a dementia referral center, some patients had already received drug treatment, including cholinesterase inhibitors and antipsychotics, even at the first visit. Thus, our results may have been partially modified by medication.
In conclusion, auditory hallucinations are common neuropsychiatric symptoms that frequently accompany visual hallucinations in patients with DLB. Auditory hallucinations consist mostly of human voices and usually accompany patients’ visual hallucinations, similar to a “soundtrack.” Additional factors, such as being female, having a hearing impairment, depression, and delusions about visual hallucinations might induce auditory hallucinations in patients with DLB.
Submitted: April 2, 2017; accepted October 31, 2017.
Published online: May 8, 2018.
Author contributions: Dr Tsunoda collected and analyzed the data and drafted the article. Dr Hashimoto designed this study, collected the data, and was responsible for the statistical design of the study. Drs Ishikawa, Fukuhara, Yuki, Tanaka, Hatada, and Miyagawa helped to collect and interpret the data. Dr Ikeda collected the data and supervised the study.
Potential conflicts of interest: None.
Funding/support: Grants were provided by the Ministry of Health, Labour and Welfare, Tokyo, Japan (Research on Dementia; H27-Dementia-General-004) and by the Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan (Grant No.26461750) for Dr Ikeda.
Role of the sponsor: The study funders and sponsors had no role in the design, conduct, or publication of the study.
Acknowledgments: The authors gratefully acknowledge the assistance of the Department of Neuropsychiatry, Kumamoto University Hospital.
REFERENCES
1. McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863-1872. PubMed CrossRef
2. Ballard C, Holmes C, McKeith I, et al. Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer’s disease. Am J Psychiatry. 1999;156(7):1039-1045. PubMed
3. Hashimoto M, Yatabe Y, Ishikawa T, et al. Relationship between dementia severity and behavioral and psychological symptoms of dementia in dementia with Lewy bodies and Alzheimer’s disease patients. Dement Geriatr Cogn Dis Extra. 2015;5(2):244-252. PubMed CrossRef
4. Hirono N, Cummings JL. Neuropsychiatric aspects of dementia with Lewy bodies. Curr Psychiatry Rep. 1999;1(1):85-92. PubMed CrossRef
5. Ballard C, McKeith I, Harrison R, et al. A detailed phenomenological comparison of complex visual hallucinations in dementia with Lewy bodies and Alzheimer’s disease. Int Psychogeriatr. 1997;9(4):381-388. PubMed CrossRef
6. Leggett AN, Zarit S, Taylor A, et al. Stress and burden among caregivers of patients with Lewy body dementia. Gerontologist. 2011;51(1):76-85. PubMed CrossRef
7. Ricci M, Guidoni SV, Sepe-Monti M, et al. Clinical findings, functional abilities and caregiver distress in the early stage of dementia with Lewy bodies (DLB) and Alzheimer’s disease (AD). Arch Gerontol Geriatr. 2009;49(2):e101-e104. PubMed CrossRef
8. Lee DR, McKeith I, Mosimann U, et al. Examining carer stress in dementia: the role of subtype diagnosis and neuropsychiatric symptoms. Int J Geriatr Psychiatry. 2013;28(2):135-141. PubMed CrossRef
9. Svendsboe E, Terum T, Testad I, et al. Caregiver burden in family carers of people with dementia with Lewy bodies and Alzheimer’s disease. Int J Geriatr Psychiatry. 2016;31(9):1075-1083. PubMed CrossRef
10. Klatka LA, Louis ED, Schiffer RB. Psychiatric features in diffuse Lewy body disease: a clinicopathologic study using Alzheimer’s disease and Parkinson’s disease comparison groups. Neurology. 1996;47(5):1148-1152. PubMed CrossRef
11. Aarsland D, Ballard C, Larsen JP, et al. A comparative study of psychiatric symptoms in dementia with Lewy bodies and Parkinson’s disease with and without dementia. Int J Geriatr Psychiatry. 2001;16(5):528-536. PubMed CrossRef
12. Heitz C, Noblet V, Blanc F, et al. Neural correlates of visual hallucinations in dementia with Lewy bodies. Alzheimers Res Ther. 2015;7(1):6. PubMed CrossRef
13. McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113-1124. PubMed CrossRef
14. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed CrossRef
15. Cummings JL, Mega M, Gray K, et al. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308-2314. PubMed CrossRef
16. Hirono N, Mori E, Ikejiri Y, et al. Japanese version of the Neuropsychiatric Inventory: a scoring system for neuropsychiatric disturbance in dementia patients [in Japanese]. No To Shinkei. 1997;49(3):266-271. PubMed
17. Suárez-González A, Serrano-Pozo A, Arroyo-Anlló EM, et al. Utility of neuropsychiatric tools in the differential diagnosis of dementia with Lewy bodies and Alzheimer’s disease: quantitative and qualitative findings. Int Psychogeriatr. 2014;26(3):453-461. PubMed CrossRef
18. McKeith IG, Fairbrian AF, Bothwell RA, et al. An evaluation of the predictive validity and inter-rater reliability of clinical diagnostic criteria for senile dementia of Lewy body type. Neurology. 1994;44(5):872-877. PubMed CrossRef
19. Nagahama Y, Okina T, Suzuki N, et al. Classification of psychotic symptoms in dementia with Lewy bodies. Am J Geriatr Psychiatry. 2007;15(11):961-967. PubMed CrossRef
20. Bjoerke-Bertheussen J, Ehrt U, Aarsland D, et al. Neuropsychiatric symptoms in mild dementia with Lewy bodies and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2012;34(1):1-6. PubMed CrossRef
21. Golden EC, Josephs KA. Minds on replay: musical hallucinations and their relationship to neurological disease. Brain. 2015;138(pt 12):3793-3802. PubMed CrossRef
22. Fénelon G, Mahieux F, Huon R, et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(pt 4):733-745. PubMed CrossRef
23. Inzelberg R, Kipervasser S, Korczyn AD. Auditory hallucinations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64(4):533-535. PubMed CrossRef
24. Amar BR, Yadav R. Janardhan Reddy: a clinical profile of patients with Parkinson’s disease and psychosis. Ann Indian Acad Neurol. 2014;17(2):187-192. PubMed CrossRef
25. Xing Y, Wei C, Chu C, et al. Stage-specific gender differences in cognitive and neuropsychiatric manifestations of vascular dementia. Am J Alzheimers Dis Other Demen. 2012;27(6):433-438. PubMed CrossRef
26. Evers S, Ellger T. The clinical spectrum of musical hallucinations. J Neurol Sci. 2004;227(1):55-65. PubMed CrossRef
27. Berrios GE. Musical hallucinations: a historical and clinical study. Br J Psychiatry. 1990;156(2):188-194. PubMed CrossRef
28. Cogan DG. Visual hallucinations as release phenomena. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1973;188(2):139-150. PubMed CrossRef
29. Ffytche DH. Visual hallucinations and the Charles Bonnet syndrome. Curr Psychiatry Rep. 2005;7(3):168-179. PubMed CrossRef
30. Cole MG, Dowson L, Dendukuri N, et al. The prevalence and phenomenology of auditory hallucinations among elderly subjects attending an audiology clinic. Int J Geriatr Psychiatry. 2002;17(5):444-452. PubMed CrossRef
31. Fénelon G. Psychosis in Parkinson’s disease: phenomenology, frequency, risk factors, and current understanding of pathophysiologic mechanisms. CNS Spectr. 2008;13(suppl 4):18-25. PubMed CrossRef
32. Ballard C. Definition and diagnosis of dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2004;17(suppl 1):15-24. PubMed CrossRef
33. Ikeda M, Mori E, Iseki E, et al. Adequacy of using consensus guidelines for diagnosis of dementia with Lewy bodies in clinical trials for drug development. Dement Geriatr Cogn Disord. 2016;41(1-2):55-67. PubMed CrossRef
34. Mori E, Ikeda M, Kosaka K Donepezil-DLB Study Investigators. Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial. Ann Neurol. 2012;72(1):41-52. PubMed CrossRef
35. Thomas AJ, Burn DJ, McKeith IG, et al. A comparison of the efficacy of donepezil in Parkinson’s disease with dementia and dementia with Lewy bodies. Int J Geriatr Psychiatry. 2005;20(10):938-944. PubMed CrossRef
36. McKeith I, Del Ser T, Spiegel R, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomized, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031-2036. PubMed CrossRef
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Geriatric Psychiatry section. Please contact Jordan F. Karp, MD, at [email protected], or Gary W. Small, MD, at [email protected].
This PDF is free for all visitors!