Patients with schizophrenia have increased prevalence rates for many cardiac risk factors. As an example, the metabolic syndrome is common in schizophrenia, with elevated rates for the syndrome and its components evident in first-episode schizophrenia patients, itself. These rates are further elevated in multiepisode patients. Weight gain is a clinical marker of cardiometabolic risk. Antipsychotic drug treatment may drive at least part of the increased cardiometabolic risk; the effects are externally evident in the form of weight gain, with different drugs having different effects on weight and metabolic parameters. Finally, sedentariness and smoking are 2 common behaviors that increase cardiometabolic risks in schizophrenia. It is important for psychiatrists who treat schizophrenia to evaluate the cardiometabolic risks in their patients so that appropriate lifestyle and pharmacologic interventions can be planned.
- The metabolic syndrome and its components are common in schizophrenia, with prevalences increased from early during the course of the illness itself (even before antipsychotic drug exposure).
- Antipsychotic drug exposure is associated with the risk of weight gain and metabolic dysregulation; the risks vary with different antipsychotic drugs.
- Patients with schizophrenia smoke, are sedentary, and may have still other cardiometabolic risk factors.
- Psychiatrists who treat schizophrenia need to evaluate the cardiometabolic risks in their patients so that appropriate lifestyle and pharmacologic interventions can be planned.

ABSTRACT
Patients with schizophrenia have increased prevalence rates for many cardiac risk factors. As an example, the metabolic syndrome is common in schizophrenia, with elevated rates for the syndrome and its components evident in first-episode schizophrenia patients, itself. These rates are further elevated in multiepisode patients. Weight gain is a clinical marker of cardiometabolic risk. Antipsychotic drug treatment may drive at least part of the increased cardiometabolic risk; the effects are externally evident in the form of weight gain, with different drugs having different effects on weight and metabolic parameters. Finally, sedentariness and smoking are 2 common behaviors that increase cardiometabolic risks in schizophrenia. It is important for psychiatrists who treat schizophrenia to evaluate the cardiometabolic risks in their patients so that appropriate lifestyle and pharmacologic interventions can be planned.
J Clin Psychiatry 2016;77(7):e844-e847
dx.doi.org/10.4088/JCP.16f10997
© Copyright 2016 Physicians Postgraduate Press, Inc.
Clinical Question
Overweight, obesity, and the metabolic syndrome (MetS) are common in schizophrenia, particularly in association with antipsychotic drug treatment.1,2 Patients with schizophrenia are therefore at increased risk of adverse cardiovascular and cerebrovascular events and mortality associated therewith.3-6 What interventions should be considered for patients at increased risk? This initial article presents a statement of the magnitude and moderators of the problem; potential interventions will be examined in a subsequent article.
Introduction
There are many different definitions of MetS2,7; these essentially require endorsement of 3 of the following 5 criteria: increased waist circumference, increased triglycerides level, low HDL cholesterol level, high blood pressure (BP), and high fasting blood sugar level. Cutoff values are specified for each criterion, at least one of which (waist circumference) requires adjustment for ethnicity. A normal-value criterion is considered endorsed if the patient is receiving an appropriate treatment for it, such as an antihypertensive medication for hypertension.
Persons with MetS may be symptom free; however, MetS increases the risk of several adverse health outcomes, notably type 2 diabetes mellitus, cardiovascular and cerebrovascular events, and complications thereof.8,9 Overweight/obesity, the commonest component of MetS, is itself associated with adverse health consequences; these include MetS and its consequences, osteoarthritis, obstructive sleep apnea, nonalcoholic fatty liver disease, and even cancer.10
Brief Historical Notes
Weight gain with antipsychotic drugs was, in an earlier era, considered to represent improvement in physical health resulting from an improvement in mental health. However, it has long been recognized that weight gain with antipsychotic drugs is also a health concern.11-13 Stray reports on diabetes in patients receiving neuroleptic drugs were published as early as in the 1950s and 1960s; by the late 1990s, it became clear that atypical antipsychotic drugs such as clozapine, olanzapine, and quetiapine were associated with dysregulation of glucose and lipid metabolism, and new-onset diabetes mellitus.14-17 Presently, all antipsychotic drugs are labeled with a warning that their use may be associated with metabolic risks.
Metabolic Disturbances in Early Schizophrenia
Patients with psychosis are known to be at increased risk of MetS, or at least of its components, even before exposure to antipsychotic drugs. For example, data from the European First-Episode Schizophrenia Trial18 showed that the prevalence of MetS was 5.7% in antipsychotic-naive first-episode schizophrenia (FES) patients (n = 157) and 6.1% in FES patients with brief antipsychotic exposure (n = 326); these figures were similar to the prevalence in age-matched persons in the general population. However, 58.5% of the patients had one or more elevated metabolic risks at baseline: 28.5% had low HDL levels, 24.2% had elevated blood pressure, 17.7% had elevated triglycerides levels, 8.2% had abdominal obesity, and 7.3% had elevated blood sugar levels. A case-control study19 also reported increased prevalence of metabolic disturbances and MetS in 122 drug-naive or drug-free patients with schizophrenia.
A systematic review and meta-analysis of 19 studies of unmedicated FES patients (pooled N = 1,325) found that the overall prevalence of MetS was 9.8%. Individual risk factors were 40.2% for smoking, 26.6% for overweight, 24.3% for high BP, 20.4% for low HDL, 16.9% for high triglycerides, 6.4% for high blood sugar, and 2.1% for diabetes.1
In 394 patients with FES in the Recovery After an Initial Schizophrenia Episode (RAISE) study, after a mean of 47.3 days of lifetime antipsychotic exposure it was observed that 13.2% of patients had MetS; 15.4% were prediabetic, 2.9% had diabetes, 48.3% were obese/overweight, 56.5% had dyslipidemia, 39.9% had prehypertension, and 10.0% had hypertension. Greater illness duration predicted increased body mass index (BMI), waist circumference, fat mass, and fat percentage, but not metabolic parameters; greater duration of antipsychotic use predicted lower HDL, increased non-HDL cholesterol, and increased triglycerides.20
Interestingly, a review of data from 5 studies conducted in persons at ultrahigh risk of psychosis found that the BMI was similar to that of healthy controls and was in the normal range for the population.21
Metabolic Disturbances in Multiepisode Schizophrenia
The risk of MetS and the components thereof are sharply elevated in patients who have experienced repeated episodes of schizophrenia. In a systematic review and meta-analysis of cardiometabolic abnormalities in patients with multiple episodes of schizophrenia, Vancampfort et al22 reported that, relative to population controls, multiepisode patients had an increased risk of MetS (4 studies; N = 868; odds ratio [OR] = 2.35; 95% confidence interval [CI], 1.68-3.29), diabetes (15 studies; N = 106,720; OR = 1.99; 95% CI, 1.55-2.54), abdominal obesity (5 studies; N = 6,632; OR = 4.43; 95% CI, 2.52-7.82), hypertension (4 studies; N = 2,410; OR = 1.36; 95% CI, 1.21-1.53), low HDL (2 studies; N = 647; OR = 2.35; 95% CI, 1.78-3.10), and high triglycerides (2 studies; N = 647; OR = 2.73; 95% CI, 1.95-3.83).
In this meta-analysis, risks were significantly higher for multiepisode patients relative to drug-naive patients for abdominal obesity (50.0% vs 16.6%), elevated triglycerides (39.0% vs 23.3%), low HDL (41.7% vs 24.2%), and MetS (34.2% vs 10.0%), but not hypertension (37.3% vs 31.6%). There were no significant differences between drug-naive and FES patients.22
A very recent meta-analysis of 57 studies reported an 11.5% (95% CI, 9.8%-13.5%) prevalence of type 2 diabetes mellitus in schizophrenia. The prevalence was significantly higher in multiepisode patients relative to FES patients (13.1% vs 4.0%).23
Weight Gain as a Clinical Marker of Cardiometabolic Risks
Weight gain is a signal that is commonly used as a marker of the cardiometabolic risks associated with schizophrenia and its treatment. This is because weight gain is a change that is obvious to patients, their families, and their doctors, and it is easily measured in the clinic. Weight gain is also an early marker; changes are evident within weeks of treatment onset,24 and these early changes predict more significant long-term weight gain.25 Additionally, a validated criterion exists for clinically significant change in body weight (7% or greater). It goes without saying that weight gain is reflected in an increased waist circumference, which is an important criterion defining MetS.
Medication (and Choice Thereof) as a Moderator of the Cardiometabolic Risk
Relative to placebo, in short-term randomized controlled trials (RCTs), patients with FES gain a mean of 3.22 kg and a mean of 1.4 BMI points; in long-term studies, the weight gain is a mean of 5.30 kg and the increase in BMI is by a mean of 1.86 points.26 Overall, however, data from meta-analysis suggest that there is no clear signal that a longer duration of antipsychotic treatment is associated with greater weight gain; possible exceptions are when drugs strongly associated with weight gain (eg, olanzapine, clozapine) are used and when clinically significant weight gain is the outcome criterion.27 Clinically significant weight gain and dysregulation of metabolic parameters are observed after antipsychotic exposure in pediatric samples, as well.28
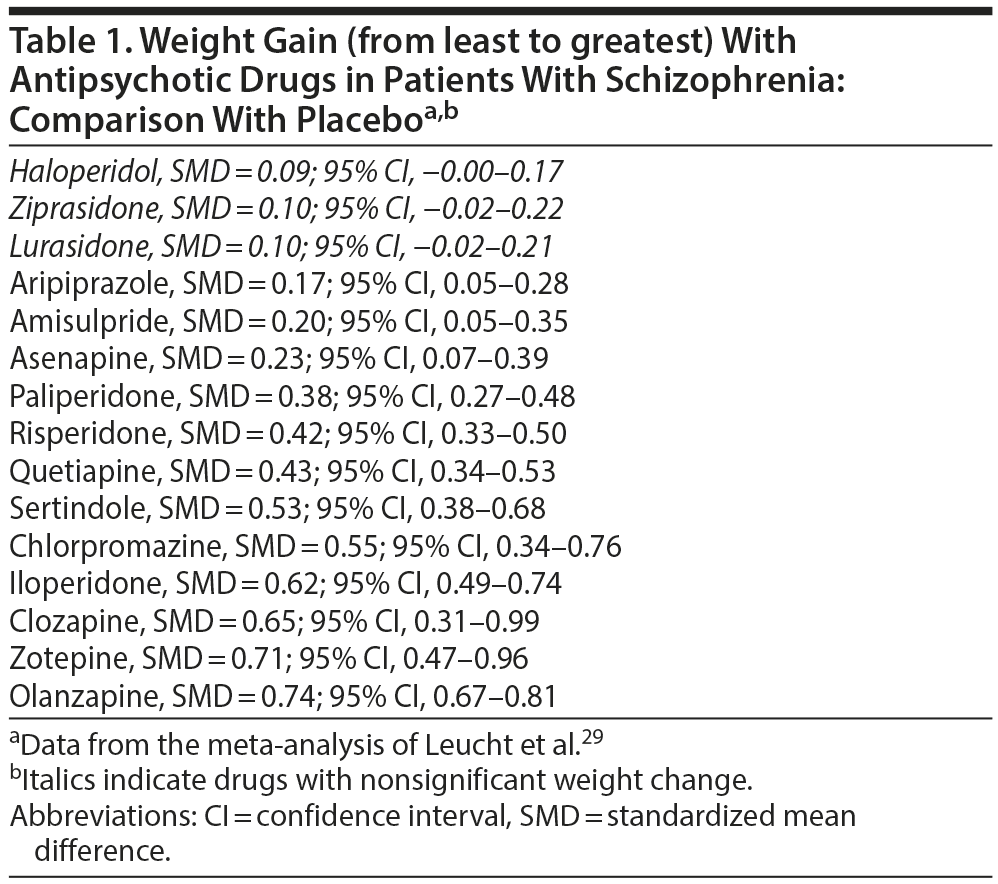
In a systematic review and network meta-analysis of 212 RCTs (pooled N = 42,049), Leucht et al29 found that only haloperidol, ziprasidone, and lurasidone were not associated with an increased risk of weight gain relative to placebo; the greatest weight gain was observed with clozapine, zotepine, and olanzapine (Table 1).
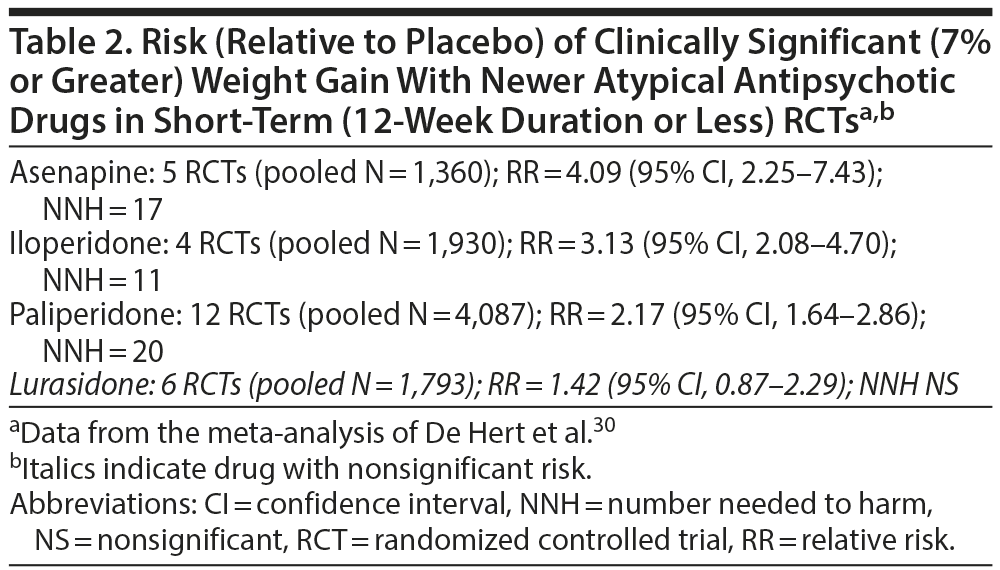
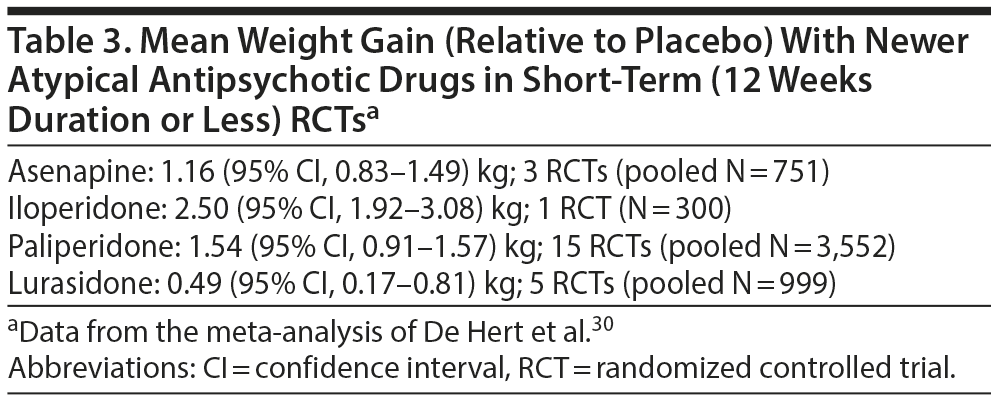
In another systematic review and meta-analysis (56 RCTs, pooled N = 21,691) that compared asenapine (9 RCTs), iloperidone (11 RCTs), lurasidone (8 RCTs), and paliperidone (28 RCTs) with placebo in patients with schizophrenia or bipolar disorder, De Hert et al30 found that, in short-term studies, only lurasidone was not associated with clinically significant weight gain (Table 2) and that absolute weight gain, relative to placebo, was least with lurasidone (Table 3). Mean weight gain with drugs such as chlorpromazine, olanzapine, and clozapine, in particular, can be quite substantial—in the region of 5 kg and above, particularly in the longer term.27
Antipsychotic-related weight gain is multifactorial in etiology and depends not only on the drug but also on, for example, genetic factors that may moderate their pharmacodynamic effects.31,32
Sedentary Behavior as a Moderator of the Cardiometabolic Risk
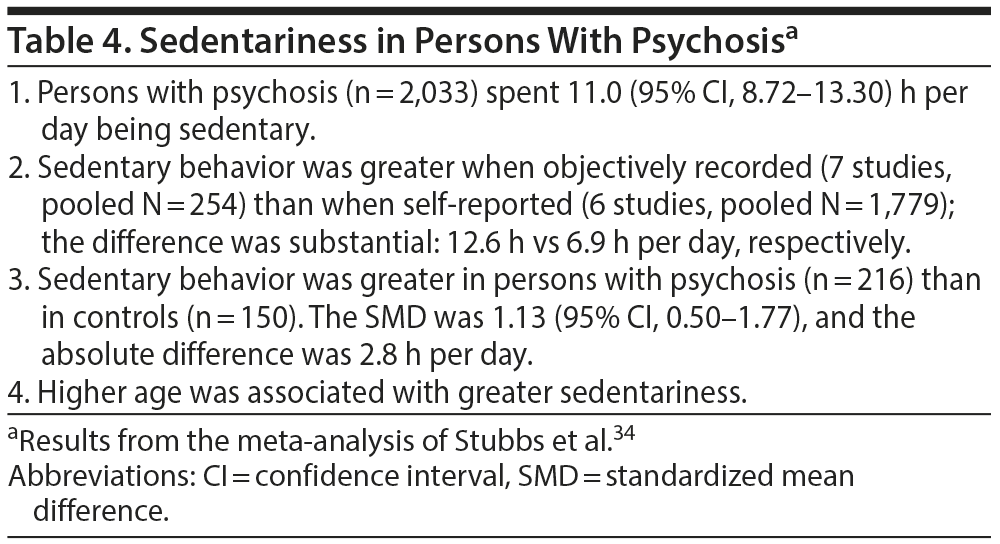
Lack of physical activity, lack of physical fitness, and sedentariness increase cardiovascular risks.33 In a systematic review and meta-analysis/meta-regression analysis, Stubbs et al34 identified 13 studies (pooled N for psychosis, 2,033) that measured sedentary behavior in persons with psychotic conditions, including schizophrenia spectrum and (1 sample of) bipolar disorder. Important results of the study are presented in Table 4; in summary, persons with psychosis were found to show considerable sedentary behavior; this was significantly greater in patients than in controls and was more apparent in objective assessments than from self-reports.
Patients with psychosis may display reduced activity levels, for example, for reasons related to diminished interests and motivation, or medication-related sedation, or weight gain due to any cause. In this meta-analysis,34 the mean BMI of the pooled sample was 29; that is, the average patient was overweight. Thus, the sample was biased toward sedentariness because being overweight is known to be associated with lower levels of physical activity. However, between overweight and sedentariness, it may not be easy to identify which one is the cause and which is the effect, and the two states are probably interdependent. Importantly, in this study,34 the meta-regression analysis found that the BMI was unrelated to sedentary time, implying that weight, per se, may not have driven the finding of sedentariness.
Other Moderators of Cardiometabolic Risk
All variables that moderate the cardiometabolic risk in healthy individuals have the potential to moderate the cardiometabolic risk in persons with schizophrenia; a family history of ischemic heart disease is a simple example of such a moderating variable. A further discussion on the subject is out of the scope of the present article. However, one important moderator, smoking, requires especial mention.
In a meta-analysis of 17 studies,21 smoking was identified in 33% (95% CI, 24%-42%) of persons at ultrahigh risk of psychosis; this figure was significantly higher than that in control subjects (OR = 2.26; 95% CI, 1.48-3.48). Another meta-analysis,35 which examined 31 studies in first-episode psychosis, estimated the prevalence of tobacco use at 58.9% (95% CI, 54.3%-63.4%); tobacco use was more common in patients than in healthy controls (OR = 6.04; 95% CI, 3.03-12.02). Similar, high, values for odds of smoking were obtained in a meta-analysis of 42 studies from across the world in patients with schizophrenia; the odds in male patients were double those in female patients.36
Summary
Patients with schizophrenia are at increased risk of MetS and of other cardiac risk factors, including sedentariness and smoking. Most antipsychotic drugs also increase the risk of weight gain and MetS. It is important for psychiatrists to be aware of cardiometabolic risks in their patients so that appropriate lifestyle and pharmacologic interventions can be planned.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Mitchell AJ, Vancampfort D, De Herdt A, et al. Is the prevalence of metabolic syndrome and metabolic abnormalities increased in early schizophrenia? a comparative meta-analysis of first episode, untreated and treated patients. Schizophr Bull. 2013;39(2):295-305. PubMed doi:10.1093/schbul/sbs082
2. Mitchell AJ, Vancampfort D, Sweers K, et al. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306-318. PubMed doi:10.1093/schbul/sbr148
3. Hennekens CH. Increasing global burden of cardiovascular disease in general populations and patients with schizophrenia. J Clin Psychiatry. 2007;68(suppl 4):4-7. PubMed
4. Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620-627. PubMed doi:10.1016/S0140-6736(09)60742-X
5. Brown S, Kim M, Mitchell C, et al. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry. 2010;196(2):116-121. PubMed doi:10.1192/bjp.bp.109.067512
6. Lahti M, Tiihonen J, Wildgust H, et al. Cardiovascular morbidity, mortality and pharmacotherapy in patients with schizophrenia. Psychol Med. 2012;42(11):2275-2285. PubMed doi:10.1017/S0033291712000396
7. Kassi E, Pervanidou P, Kaltsas G, et al. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9(1):48. PubMed doi:10.1186/1741-7015-9-48
8. Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008;31(9):1898-1904. PubMed doi:10.2337/dc08-0423
9. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113-1132. PubMed doi:10.1016/j.jacc.2010.05.034
10. Segula D. Complications of obesity in adults: a short review of the literature. Malawi Med J. 2014;26(1):20-24. PubMed
11. Anon. Editorial: drugs causing weight gain. BMJ. 1974;1(5900):168. PubMed doi:10.1136/bmj.1.5900.168
12. Doss FW. The effect of antipsychotic drugs on body weight: a retrospective review. J Clin Psychiatry. 1979;40(12):528-530. PubMed
13. Rockwell WJ, Ellinwood EH Jr, Trader DW. Psychotropic drugs promoting weight gain: health risks and treatment implications. South Med J. 1983;76(11):1407-1412. PubMed doi:10.1097/00007611-198311000-00021
14. Wirshing DA, Spellberg BJ, Erhart SM, et al. Novel antipsychotics and new onset diabetes. Biol Psychiatry. 1998;44(8):778-783. PubMed doi:10.1016/S0006-3223(98)00100-0
15. Hפgg S, Joelsson L, Mjörndal T, et al. Prevalence of diabetes and impaired glucose tolerance in patients treated with clozapine compared with patients treated with conventional depot neuroleptic medications. J Clin Psychiatry. 1998;59(6):294-299. PubMed doi:10.4088/JCP.v59n0604
16. Sobel M, Jaggers ED, Franz MA. New-onset diabetes mellitus associated with the initiation of quetiapine treatment. J Clin Psychiatry. 1999;60(8):556-557. PubMed doi:10.4088/JCP.v60n0809f
17. Melkersson KI, Hulting AL, Brismar KE. Elevated levels of insulin, leptin, and blood lipids in olanzapine-treated patients with schizophrenia or related psychoses. J Clin Psychiatry. 2000;61(10):742-749. PubMed doi:10.4088/JCP.v61n1006
18. Fleischhacker WW, Siu CO, Bodén R, et al; EUFEST study group. Metabolic risk factors in first-episode schizophrenia: baseline prevalence and course analysed from the European First-Episode Schizophrenia Trial. Int J Neuropsychopharmacol. 2013;16(5):987-995. PubMed doi:10.1017/S1461145712001241
19. Enez Darcin A, Yalcin Cavus S, Dilbaz N, et al. Metabolic syndrome in drug-naׯve and drug-free patients with schizophrenia and in their siblings. Schizophr Res. 2015;166(1-3):201-206. PubMed doi:10.1016/j.schres.2015.05.004
20. Correll CU, Robinson DG, Schooler NR, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry. 2014;71(12):1350-1363. PubMed doi:10.1001/jamapsychiatry.2014.1314
21. Carney R, Cotter J, Bradshaw T, et al. Cardiometabolic risk factors in young people at ultra-high risk for psychosis: a systematic review and meta-analysis. Schizophr Res. 2016;170(2-3):290-300. PubMed doi:10.1016/j.schres.2016.01.010
22. Vancampfort D, Wampers M, Mitchell AJ, et al. A meta-analysis of cardio-metabolic abnormalities in drug naׯve, first-episode and multi-episode patients with schizophrenia versus general population controls. World Psychiatry. 2013;12(3):240-250. PubMed doi:10.1002/wps.20069
23. Vancampfort D, Correll CU, Galling B, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. 2016;15(2):166-174. doi:10.1002/wps.20309
24. Musil R, Obermeier M, Russ P, et al. Weight gain and antipsychotics: a drug safety review. Expert Opin Drug Saf. 2015;14(1):73-96. PubMed doi:10.1517/14740338.2015.974549
25. Vandenberghe F, Gholam-Rezaee M, Saig×-Morgui N, et al. Importance of early weight changes to predict long-term weight gain during psychotropic drug treatment. J Clin Psychiatry. 2015;76(11):e1417-e1423. PubMed doi:10.4088/JCP.14m09358
26. Tek C, Kucukgoncu S, Guloksuz S, et al. Antipsychotic-induced weight gain in first-episode psychosis patients: a meta-analysis of differential effects of antipsychotic medications. Early Interv Psychiatry. 2016;10(3):193-202. PubMed doi:10.1111/eip.12251
27. Bak M, Fransen A, Janssen J, et al. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS ONE. 2014;9(4):e94112. PubMed doi:10.1371/journal.pone.0094112
28. Almandil NB, Liu Y, Murray ML, et al. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs. 2013;15(2):139-150. PubMed doi:10.1007/s40272-013-0016-6
29. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. PubMed doi:10.1016/S0140-6736(13)60733-3
30. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759. PubMed doi:10.2165/11634500-000000000-00000
31. Brandl EJ, Tiwari AK, Zai CC, et al. Genome-wide association study on antipsychotic-induced weight gain in the CATIE sample [published online ahead of print September 1, 2015]. Pharmacogenomics J. PubMed doi:10.1038/tpj.2015.59
32. Zhang JP, Lencz T, Zhang RX, et al. Pharmacogenetic associations of antipsychotic drug-related weight gain: a systematic review and meta-analysis [published online ahead of print May 23, 2016]. Schizophr Bull. PubMed
33. Després JP. Physical activity, sedentary behaviours, and cardiovascular health: when will cardiorespiratory fitness become a vital sign? Can J Cardiol. 2016;32(4):505-513. PubMed doi:10.1016/j.cjca.2015.12.006
34. Stubbs B, Williams J, Gaughran F, et al. How sedentary are people with psychosis? a systematic review and meta-analysis. Schizophr Res. 2016;171(1-3):103-109. PubMed doi:10.1016/j.schres.2016.01.034
35. Myles N, Newall HD, Curtis J, et al. Tobacco use before, at, and after first-episode psychosis: a systematic meta-analysis. J Clin Psychiatry. 2012;73(4):468-475. PubMed doi:10.4088/JCP.11r07222
36. de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res. 2005;76(2-3):135-157. PubMed doi:10.1016/j.schres.2005.02.010
This PDF is free for all visitors!