ABSTRACT
Delusions and hallucinations are common in Alzheimer disease (AD) and Parkinson disease (PD), especially in the later stages of illness. Antipsychotic drugs are effective in treating these psychotic symptoms but are associated with an increased risk of serious adverse events, including mortality. There is therefore a need to explore other treatment approaches. In this context, a recent individual patient data meta-analysis of 17 randomized controlled trials (RCTs) conducted in AD (12 RCTs) and PD (5 RCTs) found that the cholinesterase inhibitor (ChEI) drugs donepezil, rivastigmine, and galantamine attenuated the severity of both delusions and hallucinations in both AD and PD. Most of these trials were 24 weeks in duration. The effect sizes, expressed as standardized mean differences (SMDs), were, however, small, lying in the −0.08 to −0.14 range. These values are so small as to be perhaps clinically insignificant. When analyses were restricted to data from patients who actually had delusions and hallucinations at baseline, all effect sizes became larger, lying in the −0.13 to −0.39 range; however, after correcting for multiple hypothesis testing, only the finding for delusions in PD remained statistically significant. The meta-analysis did not provide information on what the best doses were, how long it took for improvement to become evident, and what proportion of patients showed remission from psychotic symptoms. Whereas the signal identified in this meta-analysis merits examination in appropriately designed RCTs, the findings of the meta-analysis may not much change current treatment strategies because patients with dementia would probably anyway receive a ChEI. Therefore, if psychotic symptoms persist for 24 weeks despite optimally dosed ChEI treatment, and if behavioral and psychosocial interventions do not help, clinicians may need to consider the potential benefits vs risks of other drugs, such as atypical antipsychotics and pimavanserin, in a shared decision-making process.
J Clin Psychiatry 2023;84(4):23f15009
Author affiliations are listed at the end of this article.
Medical services are gradually improving across the world, resulting in people living longer, and in an increased prevalence of age-related medical conditions such as dementia. It is estimated that the global prevalence of dementia will rise from 57.4 million cases in 2019 to 152.8 million cases in 2050.1
As neurodegeneration in dementia progresses, the capacity for reality testing erodes, and delusions and hallucinations emerge; however, it is now recognized that psychotic symptoms may be present in the early stages of illness, too.2 In Alzheimer disease (AD), a systematic review and meta-analysis3 found that the pooled prevalence of delusions was 31% (95% confidence interval [CI], 27%–35%; 34 studies; pooled N = 10,526) and that of hallucinations was 16% (95% CI, 13%–18%; 31 studies; pooled N = 10,123). Meta-analysis has found a high prevalence of psychotic symptoms in Parkinson disease (PD), as well4; that for psychotic symptoms was 20.7% (95% CI, 14.5%–28.6%; 15 studies; N = 2,919) and that for hallucinations, specifically, was 21.6% (95% CI, 14.7%–30.6%; 18 studies; N = 3,161). In a meta-analysis of studies on dementia with Lewy bodies (DLB), the pooled prevalence of auditory hallucinations was 30.8% and that of visual hallucinations was 61.8%.5
As a digression, hallucinations are a subgroup among psychotic symptoms; so, in PD, how can the pooled prevalence of hallucinations be greater than that of psychotic symptoms? The answer is that in the meta-analysis that obtained this finding,4 the group of studies which contributed data for the pooled prevalence for psychotic symptoms was not the same as that which contributed data for the pooled prevalence for hallucinations.
As patients live longer, the prevalence of psychotic symptoms in dementia could be expected to increase. Psychotic symptoms would increase patient distress and also caregiver burden.6 Effective treatments are therefore necessary for psychotic symptoms in dementia.
Treatments for Delusions and Hallucinations in Dementia
No drugs have been approved for the treatment of psychotic symptoms in dementia7 other than pimavanserin for psychosis in PD.8 Whereas antipsychotic drugs are effective against delusions and hallucinations in dementia,2,9 they are associated with an increased risk of serious adverse events, including mortality. The mortality risk was identified in randomized controlled trials (RCTs), has been known for decades,10 and was further confirmed in a recent meta-analysis of RCTs.11 The US Food and Drug Administration consequently has a boxed warning about the risk.7 Alternatives to antipsychotic drugs are therefore necessary.
In patients with dementia, when important cognitive functions are severely impaired, the brain has limited resources to make sense of external and internal experiences, and the results can be delusions and hallucinations. If this is true, then improving cognitive functioning with drugs such as cholinesterase inhibitors (ChEIs) may attenuate the psychotic symptoms. The ChEIs donepezil, rivastigmine, and galantamine have been found to attenuate neuropsychiatric symptoms associated with dementia; so, might ChEIs specifically reduce delusions and hallucinations in dementia patients? This possibility was examined in a systematic review and individual patient data meta-analysis by d’Angremont et al.12
The Meta-Analysis12: Methods
D’Angremont et al12 searched electronic databases and reference lists for English-language, placebo-controlled RCTs of ChEIs in AD, PD, and DLB. They identified 34 relevant RCTs but could obtain individual participant data for only half of the trials (17 RCTs; pooled N = 6,649). There were 12 AD RCTs, 5 PD RCTs, but no DLB RCTs. All but 3 trials were industry RCTs.
Donepezil was the study drug in 7 AD trials; it was dosed at 10 mg/d in almost all of these. Galantamine (8–32 mg/d; 3 trials) and rivastigmine (oral, 9.5 and 17.4 mg/d; patch, 4.6 and 9.5 mg/d; 2 trials) were the other AD study drugs. The PD trials included 1 donepezil RCT (5–10 mg/d), 3 rivastigmine RCTs (3–12 mg/d), and 1 galantamine RCT (24 mg/d). Most of the RCTs were 24-week trials. The mean age of the pooled sample was 75 years. The pooled sample was 63% female. No RCT was considered to be at high risk of bias, though several were associated with concerns.
The Meta-Analysis12: Primary Outcomes
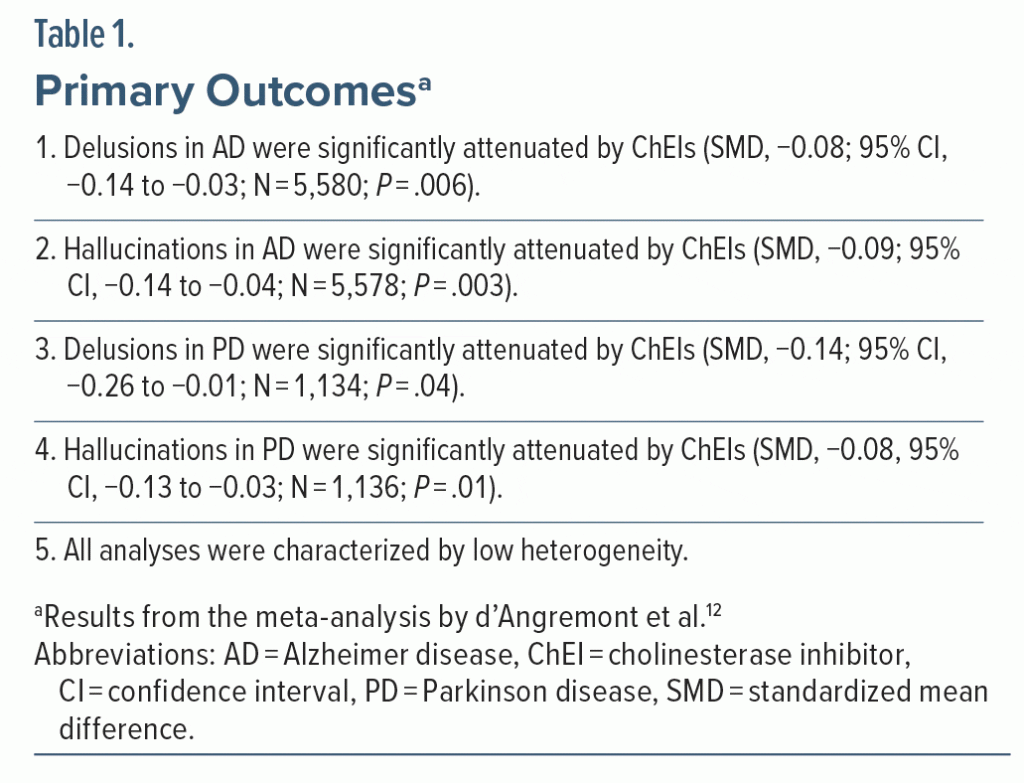
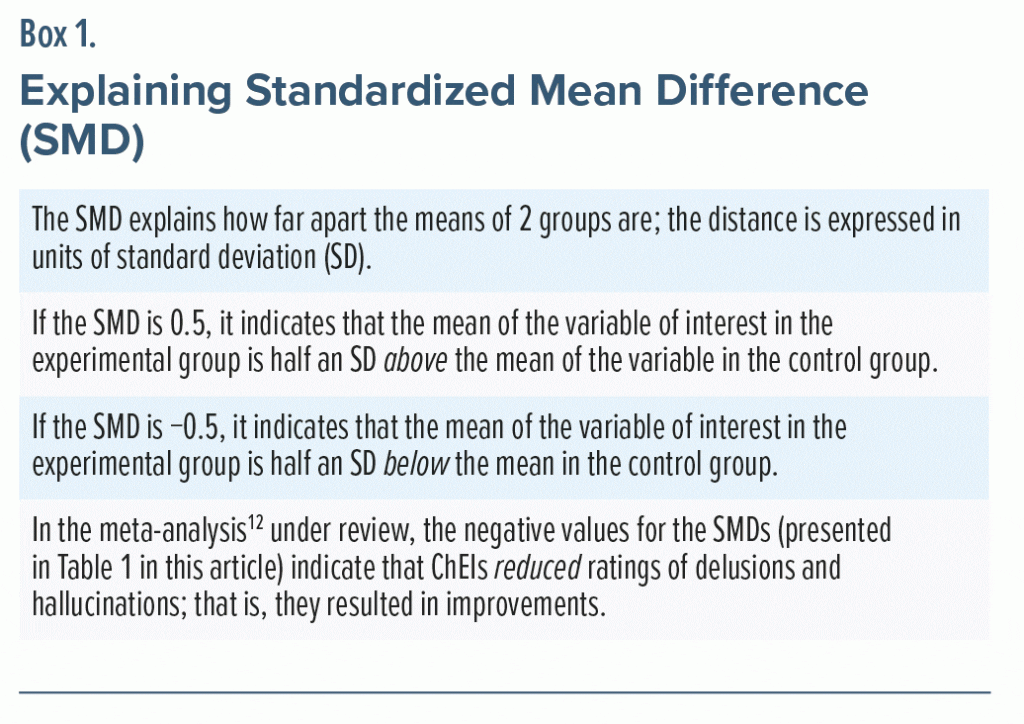
As primary outcomes, the meta-analysis12 examined whether ChEIs attenuate the severity of delusions and hallucinations in AD and PD. The findings are presented in Table 1. In summary, ChEIs were found to significantly reduce the severity of delusions as well as hallucinations in AD as well as PD; the effect sizes, expressed as standardized mean differences (SMDs), ranged from −0.08 to −0.14 (Box 1).
SMDs of 0.2, 0.5, and 0.8 (whether positive or negative) are conventionally considered to be small, medium, and large, respectively.13 So, the SMDs of −0.08 to −0.14 suggest that whereas ChEIs do attenuate psychotic symptoms in AD and PD, the effect sizes are so small as to be clinically insignificant. How this “smallness” is more easily understood will be explained in the next article in this column.
The Meta-Analysis12: Other Outcomes
In analyses of AD and PD separately and together, ChEIs did not significantly impact ratings of aberrant motor behavior, agitation/aggression, anxiety, depression/dysphoria, disinhibition, irritability/lability, and sleep. Whereas ChEIs significantly reduced apathy in PD but not AD, and reduced appetite in AD but not PD, the findings did not remain statistically significant after correcting for multiple hypothesis testing.
ChEIs significantly reduced the total neuropsychiatric symptom score in PD (SMD, −0.18; 95% CI, −0.25 to −0.11) but not in AD (SMD, −0.04; 95% CI, −0.09 to 0.01).
In conventional meta-analysis (individual participant data were unavailable), ChEIs did not significantly attenuate the severity of either delusions (SMD, −0.20; 95% CI, −4.65 to 4.24) or hallucinations (SMD, −0.22; 95% CI, −4.76 to 4.32) in 2 RCTs conducted in DLB.
The Meta-Analysis12: Special Outcomes
The 17 RCTs that had contributed individual patient data to the meta-analysis had been conducted in patients who had not been preselected for the presence of delusions and hallucinations. As a result, many patients in the results presented (Table 1) may have had no delusions or hallucinations at study baseline. These patients could not possibly “improve” with ChEIs; at best, the ChEIs could prevent the emergence of the target symptoms during the (median, 24 weeks) course of the RCTs.
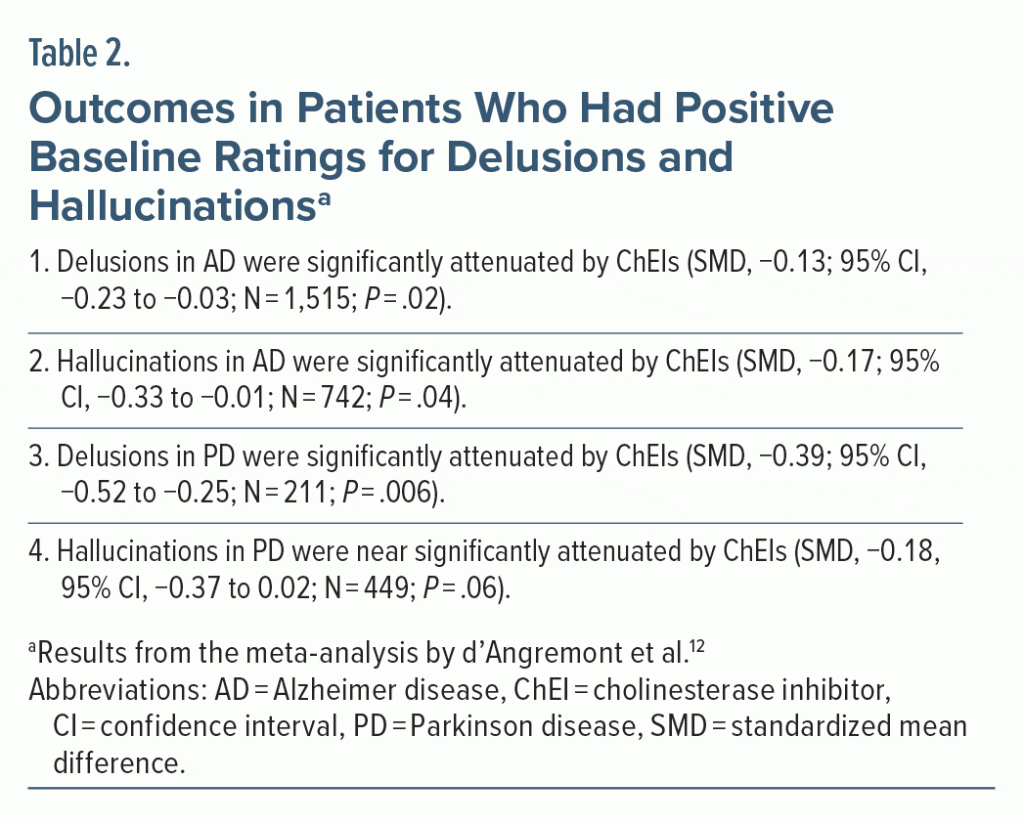
The data from such patients would thus be irrelevant to the objectives of the meta-analysis and would introduce statistical noise into the analyses. D’Angremont et al12 therefore examined outcomes specifically in patients in whom delusions and hallucinations were present at baseline.
Important findings from these analyses are presented in Table 2. In summary, all effect sizes became larger (SMDs, −0.13 to −0.39); however, whereas the other findings remained statistically significant, the finding for hallucinations in PD narrowly missed significance.
What Was the Primary Outcome of the Meta-Analysis12?
Researchers, including those who undertake meta-analysis, are expected to state one primary outcome. This is to reduce the risk of a type I (false positive) statistical error associated with the testing of many different hypotheses.
In explanation, if I identify a single target in advance, throw a single stone at it, and hit it, the result suggests that my aim was good. Likewise, if I specify a single primary outcome in advance, test it in a study, and find that the result of the test is statistically significant, it suggests that my hypothesis was justified. However, if I identify many different targets and throw a handful of stones at them, one or more of the stones might hit one or more of the targets by chance, and not because my aim is good. Likewise, if I specify many different outcomes in a study and statistically examine all of them, and if some results are statistically significant, some of the significant findings may be chance findings. The importance of prespecifying a single primary outcome in research was discussed in greater detail in an earlier article in this column.14
The meta-analysis by d’Angremont et al12 appeared to have 4 primary outcomes (Table 1), judging from the manner in which the results were presented in the abstract as well as in the main text. Technically, if these had been prespecified as coprimary outcomes, one might have expected the authors to set a lower threshold for statistical significance, such as through Bonferroni correction, where the conventional P value for statistical significance is divided by the number of prespecified coprimary outcomes. This would mean that P for statistical significance for each outcome would be set at .05/4; that is, at .0125. Then, in Table 1, the finding for delusions in PD would lose statistical significance but the other 3 findings would remain statistically significant even after applying the Bonferroni correction.
What Should Have Been the Primary Outcome(s) of the Meta-Analysis12?
Antidepressant drugs are trialed in patients with depression. Drugs intended to improve cognition in dementia are trialed in patients with documented cognitive deficits. So, logically, if the effects of ChEIs on delusions and hallucinations in dementia are to be examined, they should be examined in dementia patients who do have delusions and hallucinations at baseline. In other words, regardless of what the authors of the meta-analysis examined as their primary outcomes, the real primary outcomes were those that are presented in Table 2. However, after applying a Bonferroni correction to these 4 coprimary outcomes, only the finding for delusions in PD remains statistically significant.
As a digression, although it may be reasonable for the reader to choose the outcome of interest based on the reader’s question of interest, it must be kept in mind that the study may not have been adequately powered for the reader’s question. This is relevant to studies such as RCTs14 but, of course, does not apply to meta-analysis, in which the authors must make do with whatever data they have.
The Many Clinical Unknowns
In about half of the RCTs in the meta-analysis the ChEIs were dosed at different levels; however, although d’Angremont et al12 had individual patient data, they did not examine outcomes as a function of dosing. If higher doses are better, some patients may have been suboptimally dosed, and the potential of ChEIs to treat delusions and hallucinations in dementia may have been underestimated by the meta-analysis.
The RCTs in the meta-analysis were 12–26 (median, 24) weeks in duration. Outcomes were presumably assessed at study endpoint. So, whereas benefits may have appeared earlier, we can only conclude that it could take as long as 24 weeks for ChEIs to attenuate the severity of delusions and hallucinations in patients with dementia.
Outcomes were assessed as reductions in symptom ratings and not as remission of symptoms. So, we cannot estimate a patient’s chance of being freed of delusions and hallucinations after initiating treatment with a ChEI.
Thus, we do not know what the best doses are, how long it takes for improvement to become evident, and what proportion of patients show remission from psychotic symptoms.
Summary
ChEIs significantly reduced the severity of delusions and hallucinations in patients with AD and PD (but not DLB). However, effect sizes were so small as to be clinically almost meaningless. Furthermore, even the (small) statistical significance was lost in many analyses after correcting for multiple hypothesis testing. The ideal drug doses, the time to improvement, and even the chances of improvement are unknown.
Parting Notes
D’Angremont et al12 observed that the RCTs examined in the meta-analysis had not been designed to examine how delusions and hallucinations respond to ChEI treatment; so, these symptoms had not been assessed using appropriate instruments. Their observation is correct, but it does not automatically follow that using more appropriate instruments would be more likely to discover an advantage for ChEIs. What it does mean is that the findings of their meta-analysis justify conducting an RCT that is designed to specifically examine whether ChEIs attenuate psychotic symptoms in dementia.
This meta-analysis12 may not change current treatment strategies much because patients with dementia would probably anyway receive a ChEI. Novel approaches are currently under development and are not yet approved options.15 Therefore, if psychotic symptoms persist beyond 24 weeks, despite dose optimization, and if behavioral and psychosocial interventions do not help, clinicians may need to consider the potential benefits versus risks of other drugs, such as atypical antipsychotics and pimavanserin, in a shared decision-making process.
Finally, clinician readers would find it useful to know that, in May 2023, the US Food and Drug Administration approved brexpiprazole for the treatment of agitation associated with Alzheimer disease. This is the first approval for the stated indication. The announcement is available here: https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-treat-agitation-symptoms-associated-dementia-due-alzheimers-disease.
Article Information
Published Online: July 31, 2023. https://doi.org/10.4088/JCP.23f15009
© 2023 Physicians Postgraduate Press, Inc.
To Cite: Andrade C. Cholinesterase inhibitors for delusions and hallucinations in Alzheimer disease and Parkinson disease: questionably significant benefits. J Clin Psychiatry. 2023;84(4):23f15009.
Author Affiliations: Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
References (15)

- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):e105–e125. PubMed CrossRef
- Ismail Z, Creese B, Aarsland D, et al. Psychosis in Alzheimer disease - mechanisms, genetics and therapeutic opportunities. Nat Rev Neurol. 2022;18(3):131–144. PubMed CrossRef
- Zhao QF, Tan L, Wang HF, et al. The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: systematic review and meta-analysis. J Affect Disord. 2016;190:264–271. PubMed CrossRef
- Chendo I, Silva C, Duarte GS, et al. Frequency and characteristics of psychosis in Parkinson’s disease: a systematic review and meta-analysis. J Parkinsons Dis. 2022;12(1):85–94. PubMed CrossRef
- Eversfield CL, Orton LD. Auditory and visual hallucination prevalence in Parkinson’s disease and dementia with Lewy bodies: a systematic review and meta-analysis. Psychol Med. 2019;49(14):2342–2353. PubMed CrossRef
- Ballard C, Aarsland D. Relieving caregiver burden through evidence-based treatment for dementia-related psychosis. J Clin Psychiatry. 2021;82(4):AD19038AH5C. PubMed CrossRef
- Khanna R, White L, Bessey FY, et al. Barriers to treatment of hallucinations and delusions in people with dementia residing in long-term care. J Clin Psychiatry. 2022;83(2):21m14050. PubMed CrossRef
- Mathis MV, Muoio BM, Andreason P, et al. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J Clin Psychiatry. 2017;78(6):e668–e673. PubMed CrossRef
- Madhusoodanan S, Shah P, Brenner R, et al. Pharmacological treatment of the psychosis of Alzheimer’s disease: what is the best approach? CNS Drugs. 2007;21(2):101–115. PubMed CrossRef
- Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294(15):1934–1943. PubMed CrossRef
- Yeh TC, Tzeng NS, Li JC, et al. Mortality risk of atypical antipsychotics for behavioral and psychological symptoms of dementia: a meta-analysis, meta-regression, and trial sequential analysis of randomized controlled trials. J Clin Psychopharmacol. 2019;39(5):472–478. PubMed CrossRef
- d’Angremont E, Begemann MJH, van Laar T, et al. Cholinesterase inhibitors for treatment of psychotic symptoms in Alzheimer disease and Parkinson disease: a meta-analysis. JAMA Neurol. 2023:e231835. PubMed CrossRef
- Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J Clin Psychiatry. 2020;81(5):20f13681. PubMed CrossRef
- Andrade C. The primary outcome measure and its importance in clinical trials. J Clin Psychiatry. 2015;76(10):e1320–e1323. PubMed CrossRef
- Marcinkowska M, Śniecikowska J, Fajkis N, et al. Management of dementia-related psychosis, agitation and aggression: a review of the pharmacology and clinical effects of potential drug candidates. CNS Drugs. 2020;34(3):243–268. PubMed CrossRef
This PDF is free for all visitors!