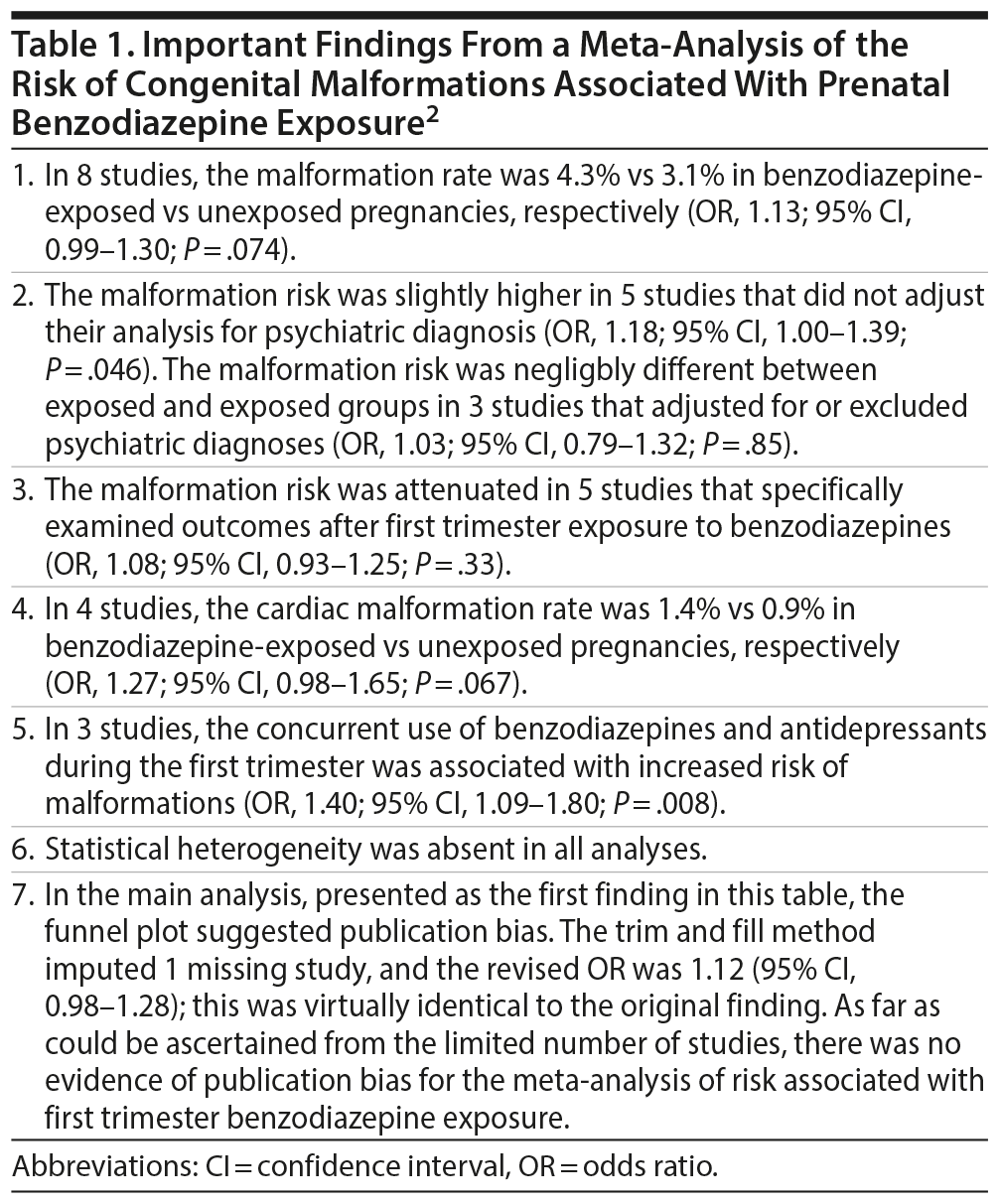
A recent meta-analysis of the major malformation risk after gestational exposure to benzodiazepines identified 8 prospective cohort studies with 5,195 exposed and 2,082,467 unexposed women. Benzodiazepine exposure was not associated with a statistically significant increase in the risk of total malformations (8 studies) or cardiac malformations (4 studies). The malformation risk was not significantly increased after specifically first trimester exposure, either (5 studies). However, there was a significant increase in risk associated with combined first trimester exposure to benzodiazepines and antidepressants (3 studies). The authors of the meta-analysis interpreted their findings based on the conventional P < .05 cutoff for statistical significance. However, when the confidence intervals (CIs) associated with the pooled odds ratios (ORs) were examined, it was evident that almost the entire range of values in the CIs for total malformations and for cardiac malformations was compatible with a population value for the OR that indicated increased risk (OR > 1.00). Importantly, the CI for the first trimester exposure analysis was somewhat better distributed around the null (OR = 1.00), suggesting a lower likelihood of increased risk. All ORs were very low to low in value, indicating a very small increase in the absolute risk. Besides explaining how CIs may be interpreted as compatibility intervals, this article reminds readers that associations identified even in meta-analyses of cohort studies do not indicate a causal effect because confounding by indication can never be ruled out in observational research designs. A reasonable conclusion, therefore, is that gestational exposure to benzodiazepines is a marker of risk for cardiac and total malformations, and, importantly, that first trimester exposure to benzodiazepines may not be associated with much increase in risk, if at all.

Gestational Exposure to Benzodiazepines, 2:
ABSTRACT
A recent meta-analysis of the major malformation risk after gestational exposure to benzodiazepines identified 8 prospective cohort studies with 5,195 exposed and 2,082,467 unexposed women. Benzodiazepine exposure was not associated with a statistically significant increase in the risk of total malformations (8 studies) or cardiac malformations (4 studies). The malformation risk was not significantly increased after specifically first trimester exposure, either (5 studies). However, there was a significant increase in risk associated with combined first trimester exposure to benzodiazepines and antidepressants (3 studies). The authors of the meta-analysis interpreted their findings based on the conventional P < .05 cutoff for statistical significance. However, when the confidence intervals (CIs) associated with the pooled odds ratios (ORs) were examined, it was evident that almost the entire range of values in the CIs for total malformations and for cardiac malformations was compatible with a population value for the OR that indicated increased risk (OR > 1.00). Importantly, the CI for the first trimester exposure analysis was somewhat better distributed around the null (OR = 1.00), suggesting a lower likelihood of increased risk. All ORs were very low to low in value, indicating a very small increase in the absolute risk. Besides explaining how CIs may be interpreted as compatibility intervals, this article reminds readers that associations identified even in meta-analyses of cohort studies do not indicate a causal effect because confounding by indication can never be ruled out in observational research designs. A reasonable conclusion, therefore, is that gestational exposure to benzodiazepines is a marker of risk for cardiac and total malformations, and, importantly, that first trimester exposure to benzodiazepines may not be associated with much increase in risk, if at all.
J Clin Psychiatry 2019;80(5):19f13081
To cite: Andrade C. Gestational exposure to benzodiazepines, 2: the risk of congenital malformations examined through the prism of compatibility intervals. J Clin Psychiatry. 2019;80(5):19f13081.
To share: https://doi.org/10.4088/JCP.19f13081
© Copyright 2019 Physicians Postgraduate Press, Inc.
Women may fall ill during pregnancy and may need medications to treat their illness; this is particularly likely when symptoms are severe and when the risks associated with the illness outweigh the risks associated with medication use. So, when pregnant women suffer from severe anxiety or depression, they may need to use anxiolytic or antidepressant medication. Whereas a large number of studies have examined adverse gestational, neonatal, and neurodevelopmental correlates of gestational antidepressant exposure, there is less literature available about the risks associated with gestational exposure to benzodiazepines.
The previous article in this column had critically examined the risk of spontaneous abortion associated with early gestational exposure to benzodiazepines1; the focus of the appraisal was on research design. This article critically examines a recent meta-analysis of studies on the risk of major congenital malformations associated with prenatal exposure to benzodiazepines2; the focus of this article is on the interpretation of the findings. Specifically, it is suggested that the main findings indicate conclusions that differ from those drawn by the authors of the study2 but that, after a few twists and turns, the final conclusions are reassuring.
Major Malformations in Pregnancies Exposed to Benzodiazepines
About 1%-5% of women have been reported to receive/fill prescriptions for benzodiazepines or other sedative/hypnotic drugs during the course of pregnancy.3-5 A previous review6 qualitatively examined benzodiazepine exposure in pregnancy and the risk of major malformations. Data were available for alprazolam, clonazepam, chlordiazepoxide, diazepam, medazepam, nitrazepam, bromazepam, and lorazepam. After considering the role of confounding factors and other study limitations, the authors concluded that benzodiazepine exposure during pregnancy seems not to be associated with an increased risk of congenital malformations. They suggested that diazepam and chlordiazepoxide may have the best safety profile and should be considered drugs of first choice when a benzodiazepine needs to be prescribed in early pregnancy. They recommended caution with the prescription of clonazepam and lorazepam. They opined that the data are insufficient to draw firm conclusions about the risks associated with nitrazepam and medazepam.
Gestational Exposure to Benzodiazepines and Major Malformations: Meta-Analysis
Grigoriadis et al2 described a systematic review and meta-analysis of the risk of congenital malformations following benzodiazepine use, alone or in combination with an antidepressant, during pregnancy. These authors searched several databases and reference lists specifically for published cohort studies with prospectively collected data in benzodiazepine-exposed and unexposed groups. They identified 8 relevant studies; these were considerably heterogeneous in terms of inclusion/exclusion criteria, trimester of benzodiazepine exposure, nature of the control group, adjustment for confounds, and other study-specific characteristics.
There were altogether 5,195 exposed and 2,082,467 unexposed pregnancies in the main analysis. Important findings from the meta-analysis are presented in Table 1. In summary, there was no statistically significant increase in the malformation risk in the main analysis in which data from all 8 studies were pooled, in the secondary analysis in which data from 5 studies of first trimester exposure were pooled, and in the secondary analysis of 4 studies in which cardiac malformation data were examined. There was a statistically significant increase in malformation risk only in the pooled analysis of 5 studies that did not adjust their analyses for the presence of psychiatric illness and in the pooled analysis of 3 studies that examined the risk of combined first trimester exposure to benzodiazepines and antidepressants. The odds ratios (ORs) for these analyses were all very low to low, in the 1.08-1.40 range, indicating that the findings, even if statistically significant, were probably of small clinical significance (this is because a small increase in the odds of an infrequent event will result in a very small increase in the absolute risk; so the number needed to harm will be very large). The confidence intervals (CIs) for these ORs were mostly narrow, indicating precision of the pooled estimate (the ORs).
Significant or Not?
The authors of the meta-analysis2 viewed the statistical findings through the prism of statistical significance. The OR for the main analysis (Table 1, first finding) was not statistically significant (P > .05). However, the 95% CI for this OR was 0.99-1.30. That is, almost the entire range of values in the CI indicated compatibility with a population OR value that is > 1.00, indicating an increased risk.
There is a growing movement toward abandoning the use of P thresholds (eg, P < .05) to interpret research results as significant vs nonsignificant and to interpret results using more meaningful yardsticks, such as the 95% compatibility interval,7,8 as described in the preceding paragraph. As already stated, the compatibility interval under discussion suggests that benzodiazepine exposure during pregnancy is associated with an increased risk of malformations. The use of 95% CIs as compatibility intervals was explained in greater detail in an earlier article in this column in the context of intellectual disability in the offspring after gestational exposure to antidepressant drugs.9
The same interpretation, with the 95% CI considered as a compatibility interval, applies to the result presented for cardiac malformations (OR, 1.27; 95% CI, 0.98-1.65). That is, this finding is compatible with an increased risk of cardiac malformations, as well, because almost the entire range of values in the CI is compatible with a population OR that is > 1.00.
Thus, with regard to both findings, newer statistical approaches to the interpretation of research findings contradict the interpretations offered by the authors who drew the categorical conclusion that, because the P value was > .05, the risk was not elevated.
Association Does Not Mean Cause
The association of benzodiazepine exposure with increased cardiac and total malformation risk does not necessarily mean that the exposure is the cause of the increased risk. This also applies to the association between malformations and first trimester exposure to benzodiazepines and antidepressants, combined. The reason for the uncertainty is that, in the cohort studies that were subjected to meta-analysis, patients were not randomized to benzodiazepine and comparison groups. It is virtually certain, therefore, that patients who had a psychiatric diagnosis or who were more severely symptomatic were more likely to receive benzodiazepine medication. Such patients could consequently be more likely to have other risk factors for adverse gestational outcomes; for example, they might have been more likely to use alcohol, tobacco, cannabis, or other drugs of abuse in ways that might not have been captured in the records from which the data were extracted. Simply stated, the higher malformation risk associated with benzodiazepine exposure may merely have been due to confounding by indication; therefore, the benzodiazepine exposure may just be a marker for the increased risk, and not a cause of the increased risk.
The Importance of Secondary Analysis
The authors of the meta-analysis2 concluded that gestational exposure to benzodiazepines was not associated with an increased cardiac or total malformation risk. In a preceding section, we learnt that this reassurance was misplaced. However, a secondary analysis provided better reassurance. In this analysis, the malformation risk was examined after first trimester exposure, specifically; the OR was observed to be attenuated (OR = 1.08) and the confidence interval (95% CI, 0.93-1.25) was somewhat better spread around the null value of 1.00. Thus, these findings are compatible with both increased and decreased risk, and not (mostly) with increased risk alone.
Drug-induced malformations usually occur as a consequence of first trimester exposure, so the question that the reader really wants answered is whether first trimester exposure to benzodiazepines is associated with an increased malformation risk. The results of this secondary analysis indicate that it is unlikely that the risk is much increased, if at all.
Readers are usually advised to focus on the primary analysis, and the results of secondary analyses are usually considered exploratory.10 Nevertheless, subgroup analysis in meta-analyses can often yield important insights.11 It is suggested that secondary analyses can likewise, sometimes, be important, as in the case of the present meta-analysis.2
In this context, one wonders whether the analysis of first-trimester exposure was a worthier primary outcome (that could have been stated a priori) than the pooled analysis of data from all the identified studies. After all, research should address clinically relevant questions.
Take-Home Message
Specific first trimester gestational exposure to benzodiazepines may not be associated with an increased risk of major malformations in the offspring; however, a more general use of benzodiazepines during pregnancy may be a marker for cardiac and total malformation risk. These conclusions need to be explained to patients and their families in language that they understand so that a shared decision can be taken to treat or not to treat with benzodiazepines during pregnancy.
Published online: October 1, 2019.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1.Andrade C. Gestational exposure to benzodiazepines, 1: the risk of spontaneous abortion examined through the prism of research design. J Clin Psychiatry. 2019;80(0).
2.Grigoriadis S, Graves L, Peer M, et al. Benzodiazepine use during pregnancy alone or in combination with an antidepressant and congenital malformations: systematic review and meta-analysis. J Clin Psychiatry. 2019;80(4):18r12412. PubMed CrossRef
3.Hanley GE, Mintzes B. Patterns of psychotropic medicine use in pregnancy in the United States from 2006 to 2011 among women with private insurance. BMC Pregnancy Childbirth. 2014;14(1):242. PubMed CrossRef
4.Leong C, Raymond C, Ch×¢teau D, et al. Psychotropic drug use before, during, and after pregnancy: a population-based study in a Canadian cohort (2001-2013). Can J Psychiatry. 2017;62(8):543-550. PubMed CrossRef
5.Sheehy O, Zhao JP, Bérard A. Association between incident exposure to benzodiazepines in early pregnancy and risk of spontaneous abortion. JAMA Psychiatry. 2019;76(9):948-957. PubMed CrossRef
6.Bellantuono C, Tofani S, Di Sciascio G, et al. Benzodiazepine exposure in pregnancy and risk of major malformations: a critical overview. Gen Hosp Psychiatry. 2013;35(1):3-8. PubMed CrossRef
7.Andrade C. The P value and statistical significance: misunderstandings, explanations, challenges, and alternatives. Indian J Psychol Med. 2019;41(3):210-215. PubMed CrossRef
8.Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567(7748):305-307. PubMed CrossRef
9.Andrade C. Intellectual disability after gestational exposure to antidepressant drugs: the confidence interval as a compatibility interval. J Clin Psychiatry. 2019;80(3):19f12912. PubMed CrossRef
10.Andrade C. The primary outcome measure and its importance in clinical trials. J Clin Psychiatry. 2015;76(10):e1320-e1323. PubMed CrossRef
11.Hemilפ H. Random-effects assumption in meta-analyses. JAMA. 2019;322(1):81. PubMed CrossRef
This PDF is free for all visitors!