Ondansetron is a 5-HT3 receptor antagonist that has been approved for the prevention of nausea and vomiting associated with cancer chemotherapy, radiotherapy, and surgery. Ondansetron has also been studied in the treatment of many neuropsychiatric and medical conditions. The drug is commonly used off-label to treat nausea and vomiting of pregnancy (NVP) and hyperemesis gravidarum (HG). Ondansetron crosses the placental barrier, and concerns have been expressed that using ondansetron for NVP/HG during the first trimester of pregnancy may increase the risk of major congenital malformations (MCMs) in the offspring. In this context, findings from a meta-analysis of 6 cohort and 2 case-control studies, read along with the results of subsequently published cohort (n = 3) and case-control (n = 1) studies, suggest that a signal does exist to associate early gestational exposure to ondansetron with an increased risk of heart defects and orofacial defects. Arguments both for and against confounding by indication have been proposed to explain these findings. Nevertheless, even if ondansetron is causally implicated in MCM risk, the absolute increase in risk, such as for orofacial clefts (by 0.03%) and ventricular septal defect (by 0.3%), is small. These small risks should be balanced against the risks associated with inadequately treated NVP/HG, and decision-making must be shared between clinician and patient. Repeated fetal scanning during the second trimester can help in the early detection of malformations, if present.

ABSTRACT
Ondansetron is a 5-HT3 receptor antagonist that has been approved for the prevention of nausea and vomiting associated with cancer chemotherapy, radiotherapy, and surgery. Ondansetron has also been studied in the treatment of many neuropsychiatric and medical conditions. The drug is commonly used off-label to treat nausea and vomiting of pregnancy (NVP) and hyperemesis gravidarum (HG). Ondansetron crosses the placental barrier, and concerns have been expressed that using ondansetron for NVP/HG during the first trimester of pregnancy may increase the risk of major congenital malformations (MCMs) in the offspring. In this context, findings from a meta-analysis of 6 cohort and 2 case-control studies, read along with the results of subsequently published cohort (n = 3) and case-control (n = 1) studies, suggest that a signal does exist to associate early gestational exposure to ondansetron with an increased risk of heart defects and orofacial defects. Arguments both for and against confounding by indication have been proposed to explain these findings. Nevertheless, even if ondansetron is causally implicated in MCM risk, the absolute increase in risk, such as for orofacial clefts (by 0.03%) and ventricular septal defect (by 0.3%), is small. These small risks should be balanced against the risks associated with inadequately treated NVP/HG, and decision-making must be shared between clinician and patient. Repeated fetal scanning during the second trimester can help in the early detection of malformations, if present.
J Clin Psychiatry 2020;81(3):20f13472
To cite: Andrade C. Major congenital malformation risk after first trimester gestational exposure to oral or intravenous ondansetron. J Clin Psychiatry. 2020;81(3):20f13472.
To share: https://doi.org/10.4088/JCP.20f13472
© Copyright 2020 Physicians Postgraduate Press, Inc.
Ondansetron is a selective serotonin 5-HT3 receptor antagonist that is approved for the prevention of nausea and vomiting associated with cancer chemotherapy, radiotherapy, and surgery.1 Ondansetron has been trialled or is used for many off-label indications. For example, because the drug has a weak inhibitory effect on dopaminergic neurotransmission,2-4 it has been examined as a potential treatment for schizophrenia,5,6 psychosis associated with Parkinson’s disease,7,8 obsessive-compulsive disorder,9 tic disorders,10 tardive dyskinesia,11 and alcoholism.12,13 Ondansetron has also been used to treat tinnitus,14 fatigue related to chronic hepatitis,15 and pruritus related to opiate use.16 It was found ineffective in benzodiazepine withdrawal.17 Other off-label uses have also been described.1
Use of Ondansetron for Nausea and Vomiting of Pregnancy
The efficacy of ondansetron in the treatment of nausea and vomiting has led to its general use for these symptoms. In this context, nausea and vomiting of pregnancy (NVP), especially when severe, appears to have become an important indication for this drug. For example, in an insurance database study conducted in the US, the use of ondansetron increased from < 1% of pregnancies during the year 2001 to 22.2% during the year 2014. Overall, across the entire period, in over 2.3 million pregnancies, the use of ondansetron anytime during pregnancy was 15.2%; the corresponding numbers were lower at 10.3%, 4.0%, and 0.4% for promethazine, metoclopramine, and doxylamine/pyridoxine.18
NVP is a first trimester syndrome that affects 70%-80% of pregnancies.19 The most severe form of NVP, hyperemesis gravidarum (HG), occurs in 0.3%-3.0% of pregnancies.20 Ondansetron is listed as an intervention for NVP and HG in treatment guidelines endorsed by societies in different countries, including the US, Canada, the UK, Ireland, Australia, and New Zealand; however, ondansetron is not listed as a first line treatment because of concerns about the risk of birth defects.21-25
Are the concerns valid? Ondansetron crosses the placental barrier and has been found in fetal tissue after surgical termination of pregnancy.26 It has also been found in the blood of neonates whose mothers received it before undergoing cesarean section.27 Some but not all cohort and case control studies have reported an increased risk of heart defects and orofacial defects after gestational exposure to ondansetron. An animal study suggested that cardiac birth defects may be mediated by heart rhythm disturbances in the embryo, caused by ondansetron-related blockade of hERG channels.28 The present article therefore discusses the risk of major congenital malformations (MCMs) following first trimester exposure to ondansetron.
Materials Reviewed
A PubMed search for original articles and meta-analyses was conducted on May 11, 2020, using the search terms ondansetron and pregnancy. The search identified 197 records. The study of Kaplan et al29 emerged as the most recent and possibly only meta-analysis. This meta-analysis and 4 original articles30-33 (published around the same time as or after the meta-analysis, and hence not included in it) were taken up for scrutiny.
The study by Parker et al,34 not included in the Kaplan et al meta-analysis,29 is not examined in this article because its data very substantially overlap with the studies of Anderka et al35 and Van Bennekom et al,36 both of which were included in the meta-analysis.29 The study by Berard et al37 found no significant association between ondansetron exposure and the risk of MCM, but the analysis was underpowered; the study sample included only 31 pregnancies exposed to ondansetron. Therefore, this study is also not examined in the present article.
Meta-Analysis: Ondansetron and Malformation Risk
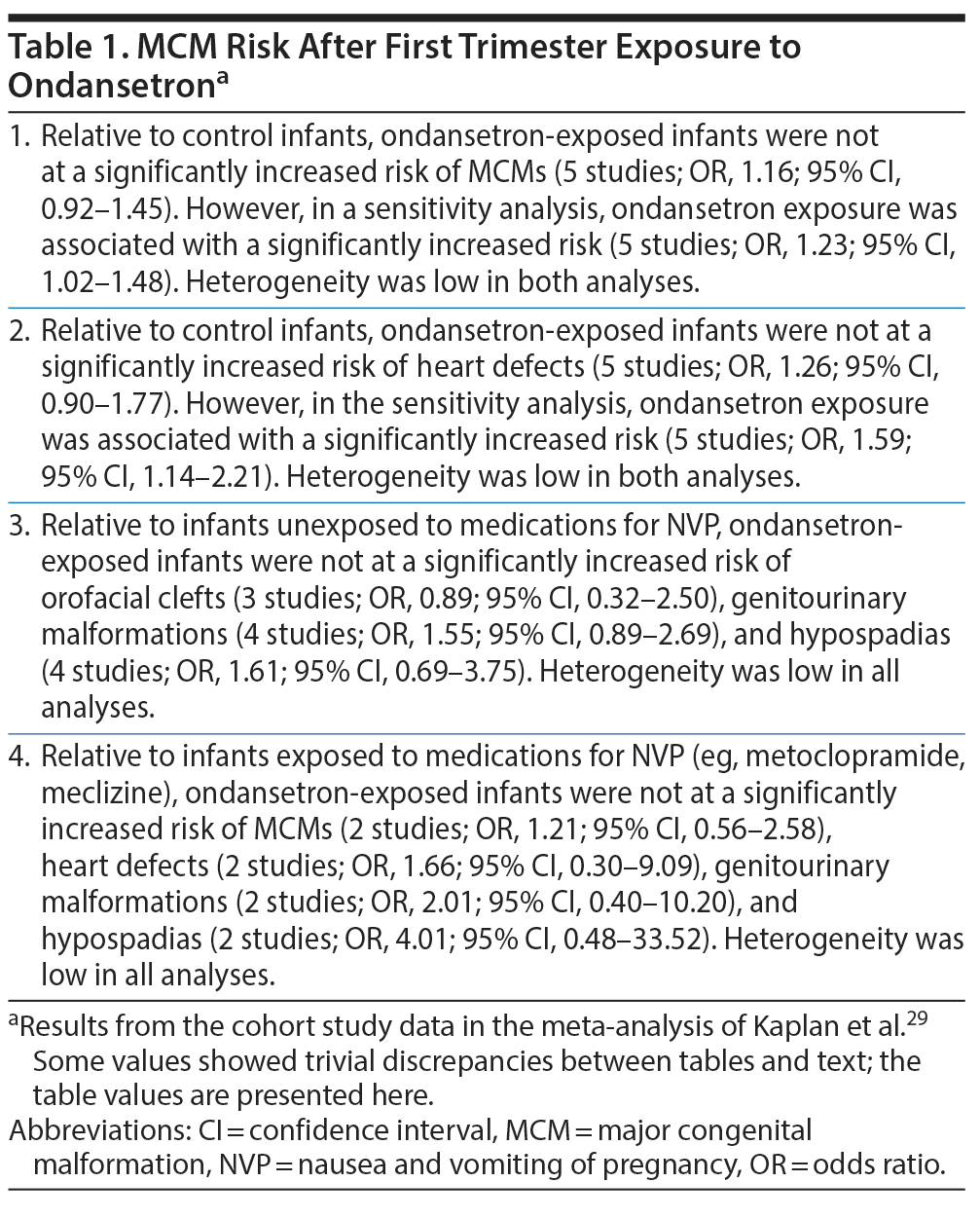
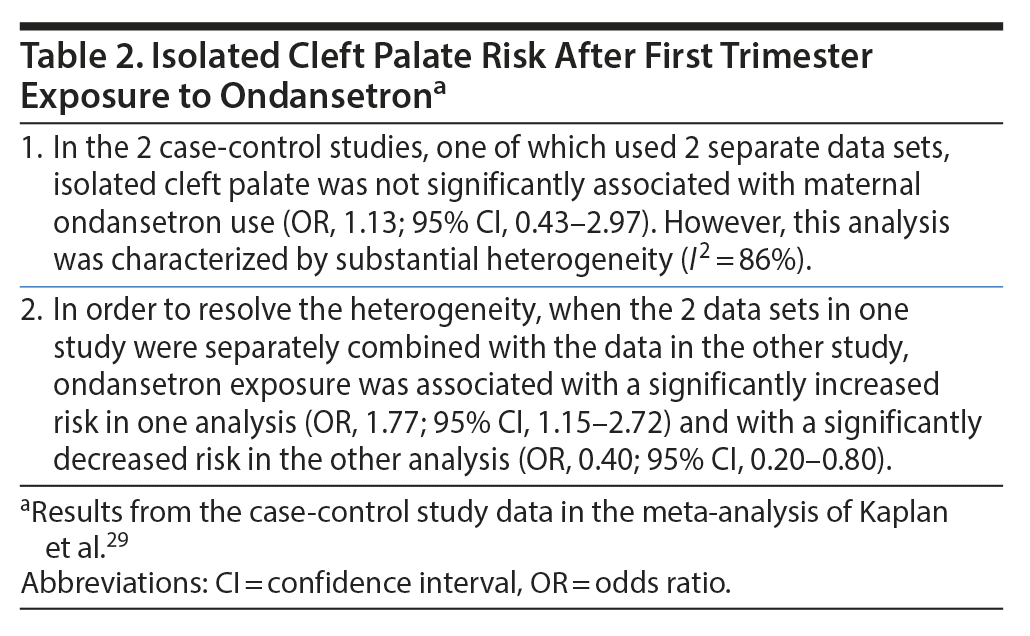
Kaplan et al29 described a PRISMA- and MOOSE-compliant systematic review and meta-analysis of MCMs in liveborn infants of women who were prescribed ondansetron during the first trimester of pregnancy. These authors searched electronic databases and reference lists and identified 6 cohort and 2 case-control studies that met their search criteria. Among the cohort studies (with 5,148 infants exposed to ondansetron and 2,459,053 control infants), 2 studies, with substantially overlapping data, presented data from Denmark, and 1, each, presented data from Canada and Australia, Australia, Sweden, and the US. Both case-control studies presented data from the US. Because one of the Danish studies was presented as an abstract, the other study was used in the main analysis, and data from the abstract substituted for the other study only in a sensitivity analysis. Important findings from the meta-analysis are presented in Table 1 and Table 2.
Other Studies of Ondansetron and Malformation Risk
Several studies were published around the time of or after the meta-analysis (Kaplan 2019) and were not included in the meta-analysis. These studies are summarized here.
Huybrechts et al30 described a retrospective cohort study using data from the 2000-2013 Medicaid Analytic eXtract in the US. After propensity score stratification, the sample comprised 88,446 pregnancies with first trimester exposure to ondansetron and 1,727,546 pregnancies that were not exposed to the drug. In a high-dimensional propensity score stratified analysis, ondansetron exposure was associated with a slightly but significantly increased risk of oral clefts (relative risk [RR], 1.25; 95% confidence interval [CI], 1.04-1.50); the increase in absolute (adjusted) risk was 0.03%. Of note, this increased risk was attributable to the occurrence of cleft palate and not cleft lip or cleft lip with cleft palate. Ondansetron exposure was not associated with an increased risk of cardiac malformations (RR, 0.98; 95% CI, 0.92-1.05) and overall malformations (RR, 1.02; 95% CI, 0.98-1.05).
The results were closely similar in secondary analyses in which ondansetron exposure was compared with exposure to other antiemetics; such an analysis reduces the effect of confounding by indication but does not eliminate it because ondansetron may have been preferentially prescribed for women with more severe NVP. The findings were also similar in various sensitivity analyses; however, the findings were not stronger in women who filled 2 or more prescriptions for the drug. Ondansetron exposure that was limited to late pregnancy was expectedly unassociated with risks.
Zambelli-Weiner et al31 described a nested case-control study within an administrative health care database, the Truven Health MarketScan Commercial Database, in the US. The sample comprised 32,100 infants with cardiovascular (CVS) defects, 1,590 infants with orofacial defects, and 802,252 infants with no birth defects. In adjusted analyses, medically supervised administration of ondansetron during the first trimester was significantly associated with CVS defects (odds ratio [OR], 1.43; 95% CI, 1.28-1.61) but not with orofacial defects (OR, 1.30; 95% CI, 0.75-2.25). Medically administered ondansetron was also significantly associated with atrial, ventricular, and atrioventricular septal defects; with other circulatory defects but not hypoplastic left heart syndrome; and with diaphragmatic hernia, but not with craniosynostosis, renal collecting system anomalies, and limb reduction defects. The pattern of findings was closely similar in analyses of data for prescribed and medically administered ondansetron combined.
In an attempt to control for confounding by indication, the authors31 compared women exposed to ondansetron with women exposed to NVP/HG but not to first trimester antiemetics. In this analysis, medically administered ondansetron was associated with an increased risk of cardiac defects (OR, 1.48; 95% CI, 1.14-1.92) but not orofacial defects (OR, 1.43; 95% CI, 0.78-2.64).
Lemon et al32 described a retrospective cohort study of the relationship between first trimester gestational exposure to ondansetron and ventricular septal defect (VSD), specifically, as an adverse outcome. These authors extracted data on 33,677 liveborn, singleton deliveries at the Magee-Womens Hospital of the University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania. There were 3,171 infants who had been exposed to ondansetron during the first trimester of pregnancy. Based on hospital records and prescription claims, the median cumulative dose of ondansetron was found to be 72 mg (range, 2.4 to 1,008 mg). In this context, dosing was reckoned in intravenous equivalents, with oral dose multiplied by 0.6 to provide an intravenous equivalent.
In adjusted log-binomial analysis and analyses based on propensity scores, the risk of VSD was found to be significantly higher in ondansetron-exposed relative to unexposed pregnancies; the ORs were in the 1.7 to 2.1 range and the number needed to harm (NNH) values were in the 263 to 385 range. ORs and significances attenuated when the testing of the relationship between ondansetron exposure and birth defects was expanded to include all septal defects, all heart defects, and all birth defects. In all of these expanded analyses, the adjusted ORs were not statistically significant.
Three groups of cumulative doses of ondansetron were formed, with medians at 4 mg, 144 mg, and 360 mg. The adjusted risk of VSD increased with increasing median dose and was thrice as high in the 360 mg group relative to the 4 mg group. An important limitation of this analysis is that there is no assurance that medications issued for a span of time were actually taken for the entire period. However, if the medications were not taken, it begs the question of why the VSD rate was higher in association with higher dispensed doses.
Intravenous Ondansetron and Malformation Risk
Health record databases contain information about prescribed and/or dispensed medications. Pregnant women do not necessarily take medications that have been prescribed and dispensed. In contrast, a claim for intravenous medication is a reasonable assurance that the medication was indeed used. In this context, Huybrechts et al33 examined the MCM risk after first trimester exposure specifically to intravenous ondansetron. The authors used data from the 2000-2014 Medicaid Analytic eXtract; the 2000-2013 data in this study are almost certainly a subset of the data in the study by the same authors30 that was summarized in the previous section. The reference group comprised women unexposed to either oral or intravenous ondansetron.
After propensity score stratification, the sample included 23,866 ondansetron-exposed pregnancies and 1,762,018 pregnancies that were not exposed to ondansetron. Intravenous ondansetron was not associated with risk of cardiac malformations (OR, 0.97; 95% CI, 0.86-1.10), oral clefts (OR, 0.95; 95% CI, 0.63-1.43), or overall MCMs (OR, 1.02; 95% CI, 0.96-1.08).
Summary and Discussion of the Main Findings
Summarizing the main findings of the meta-analysis (Table 1 and Table 2),29 in primary analyses, infants exposed to ondansetron during the first trimester of pregnancy were not at increased risk of overall MCMs or of specific malformations such as heart defects and orofacial defects. However, in many of the main analyses, the 95% confidence intervals were compatible with increased risk,38,39 and in many of the sensitivity and secondary analyses, the risks were either significantly elevated (for overall MCMs, heart defects, and isolated cleft palate) or were compatible with elevated risk (for genitourinary defects and hypospadias). The findings for isolated cleft palate were contradictory, with both significantly increased and significantly decreased risks identified.
The retrospective cohort study of Huybrechts et al30 had far more ondansetron-exposed subjects than did the entire meta-analysis of Kaplan et al29; this study, but not the subset study of intravenous ondansetron by the same authors,33 found that first trimester ondansetron exposure was associated with an increased risk of orofacial defects (but not heart defects). The retrospective cohort study of Lemon et al32 found that ondansetron exposure was associated with an increased risk of VSD and that there was a dose-dependent effect. The case-control study by Zambelli-Weiner et al,31 in which medically confirmed administration of ondansetron was the exposure examined in the primary analyses, found that ondansetron exposure was associated with an increased risk of many different heart defects.
Women who fill prescriptions for a drug may not take the drug during pregnancy; so, if a drug truly increases the risk and women who are advised the drug do not take it, the results of studies that are based on records of prescribed or dispensed drugs would be biased toward null findings. The results of studies based on insurance claims could also be biased toward null findings because intravenous ondansetron administration may not be picked up as a filled prescription.32 Therefore, studies on medical administration of ondansetron ought to yield more trustworthy conclusions. There were 2 such studies; a case-control study31 that found an increased MCM risk associated with first trimester ondansetron exposure and a cohort study33 that found no increase in risk. It is unclear why the results differed in these 2 studies. Of note, the data in the negative study33 were substantially a subset of the data in a larger study30 that did find evidence of an increased risk of orofacial defects.
Stepping back and looking at the findings, it seems reasonable to conclude that first trimester ondansetron exposure is probably (but not definitely) associated with an increased risk of heart defects and/or orofacial defects, and possibly associated with other MCMs, as well.
Confounding by Indication: Arguments for and Against
Does ondansetron play a causal role in the MCM risk? All the reviewed studies were observational in design; there were no randomized controlled trials from which inferences regarding causality could reasonably be drawn. This means that it is possible and even likely that pregnancies that were exposed to ondansetron differed systematically from pregnancies that were unexposed, such as in ways related to the indication for the use of the drug. Rephrasing the question, one may ask whether the association between MCMs and first trimester gestational exposure to ondansetron is causal or whether it is due to confounding by indication, that is, related to dehydration, electrolyte imbalance, nutritional deficiencies, weight loss, and other changes and complications that characterize NVP and HG.
On the one hand, no amount of adjustment for covariates, propensity score matching, comparison with ondansetron-unexposed NVP and HG pregnancies, and comparison with pregnancies exposed to other antiemetics (as reported in the reviewed studies) can eliminate residual confounding, that is, confounding by inadequately measured, unmeasured, and unknown variables. On the other hand, if confounding by indication is truly responsible for the adverse outcomes, it is not clear why vomiting, dehydration, electrolyte imbalance, or other variables associated with NVP and HG should produce structural birth defects as opposed to, say, fetal growth restriction.40 In fact, one review suggested that NVP may be protective with regard to the risk of miscarriage, premature birth, and even MCMs.41 If this is correct, then confounding by indication becomes unlikely as an explanation for ondansetron-associated MCMs.
The Magnitude of the Risk
The birth prevalence of congenital heart disease is about 0.9%, and that of VSD is about 0.3%.42,43 If the risk of VSD is doubled as in a worst case scenario,32 the absolute increase in risk associated with ondansetron exposure would be 0.3%, and the corresponding NNH would be 333. This is similar to the NNH values of 263-385 estimated by Lemon et al.32 In a review of 13 studies published between 1969 and 1998, the median rate was 44% for spontaneous closure of VSD.44 In a recent study45 from China, 113 neonates with VSD were followed for up to 7 years. Among these, 18 of 35 (51%) perimembranous VSDs closed spontaneously, as did 70 of 72 (97%) muscular VSDs. Thus, on the one hand, the absolute risk associated with ondansetron exposure is small and the number of cases that persist beyond a year after birth would be even smaller; on the other hand, whereas surgical correction of persisting or severe VSD is possible, long-term complications are also possible, and so lifelong surveillance of surgically corrected cases may be necessary.46
The prevalence of orofacial clefts is about 1 in 600.47 If the risk related to ondansetron exposure is increased by 25%,30 the absolute risk would increase by 0.04%, which is similar to the adjusted value of 0.03% estimated in the study by Huybrechts et al.30 On the one hand, the absolute risk associated with ondansetron exposure appears very small. On the other hand, whereas orofacial clefts can be surgically corrected, complications are possible, repeated surgeries may be necessary, and ongoing follow-up with a multidisciplinary team is inevitable.48
In summary, the absolute risk of MCMs in an ondansetron-exposed pregnancy is increased by a fraction of a percentage point, but the MCM could be of considerable concern to some children who are born with the MCM.
Critical Appraisal: Other Issues
In future studies, it could be useful to examine the relationship between the timing (during pregnancy) of ondansetron exposure and the risk of specific MCMs. This is because the risk of specific MCMs depends on when during the first trimester the pregnancy was exposed to a teratogen. Future studies could also examine other pregnancy outcomes, including neurodevelopmental outcomes in early childhood.
Take-Home Message
It is possible that first trimester exposure to ondansetron is associated with an increased risk of MCMs. The increase in absolute risk is very small; for example, it may be just 0.03% for orofacial defects and just 0.3% for VSD. This risk should be balanced against the risks associated with untreated or inadequately treated NVP/HG, and individualized, shared decisions regarding treatment must be made. In all cases in which ondansetron exposure occurs, repeat fetal scans should be offered at the latest week of gestation in which termination of pregnancy is possible, should there be an adverse scan finding, and depending on patient preference and the prevailing laws. For management of NVP/HG, it is suggested that readers follow the guidelines prevalent in their respective countries.
Parting Notes
It may be noted that ondansetron, particularly intravenous ondansetron, increases the QTc interval49,50; this can be of concern in women who have electrolyte imbalance associated with NVP/HG. US FDA guidance on the subject is available here: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-new-information-regarding-qt-prolongation-ondansetron-zofran (accessed May 14, 2020).
Published online: June 2, 2020.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1.Wilde MI, Markham A. Ondansetron: a review of its pharmacology and preliminary clinical findings in novel applications. Drugs. 1996;52(5):773-794. PubMed CrossRef
2.Ye JH, Ponnudurai R, Schaefer R. Ondansetron: a selective 5-HT(3) receptor antagonist and its applications in CNS-related disorders. CNS Drug Rev. 2001;7(2):199-213. PubMed CrossRef
3.Thompson AJ, Lummis SC. 5-HT3 receptors. Curr Pharm Des. 2006;12(28):3615-3630. PubMed CrossRef
4.Thompson AJ, Lummis SC. The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets. 2007;11(4):527-540. PubMed CrossRef
5.Bennett AC, Vila TM. The role of ondansetron in the treatment of schizophrenia. Ann Pharmacother. 2010;44(7-8):1301-1306. PubMed CrossRef
6.Andrade C. Nonsteroidal anti-inflammatory drugs and 5-HT3 serotonin receptor antagonists as innovative antipsychotic augmentation treatments for schizophrenia. J Clin Psychiatry. 2014;75(7):e707-e709. PubMed CrossRef
7.Zoldan J, Friedberg G, Livneh M, et al. Psychosis in advanced Parkinson’s disease: treatment with ondansetron, a 5-HT3 receptor antagonist. Neurology. 1995;45(7):1305-1308. PubMed CrossRef
8.Tampi RR, Maksimowski M, Lingamchetty T, et al. Is ondansetron beneficial for psychosis associated with dementia? Ann Clin Psychiatry. 2018;30(3):200-206. PubMed
9.Andrade C. Ondansetron augmentation of serotonin reuptake inhibitors as a treatment strategy in obsessive-compulsive disorder. J Clin Psychiatry. 2015;76(1):e72-e75. PubMed CrossRef
10.Toren P, Weizman A, Ratner S, et al. Ondansetron treatment in Tourette’s disorder: a 3-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2005;66(4):499-503. PubMed CrossRef
11.Sirota P, Mosheva T, Shabtay H, et al. Use of the selective serotonin 3 receptor antagonist ondansetron in the treatment of neuroleptic-induced tardive dyskinesia. Am J Psychiatry. 2000;157(2):287-289. PubMed CrossRef
12.Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284(8):963-971. PubMed CrossRef
13.Johnson BA, Ait-Daoud N, Seneviratne C, et al. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am J Psychiatry. 2011;168(3):265-275. PubMed CrossRef
14.Taslimi S, Vahidi H, Pourvaziri A, et al. Ondansetron in patients with tinnitus: randomized double-blind placebo-controlled study. Eur Arch Otorhinolaryngol. 2013;270(5):1635-1641. PubMed CrossRef
15.Piche T, Vanbiervliet G, Cherikh F, et al. Effect of ondansetron, a 5-HT3 receptor antagonist, on fatigue in chronic hepatitis C: a randomised, double blind, placebo controlled study. Gut. 2005;54(8):1169-1173. PubMed CrossRef
16.Charuluxananan S, Somboonviboon W, Kyokong O, et al. Ondansetron for treatment of intrathecal morphine-induced pruritus after cesarean delivery. Reg Anesth Pain Med. 2000;25(5):535-539. PubMed CrossRef
17.Romach MK, Kaplan HL, Busto UE, et al. A controlled trial of ondansetron, a 5-HT3 antagonist, in benzodiazepine discontinuation. J Clin Psychopharmacol. 1998;18(2):121-131. PubMed CrossRef
18.Taylor LG, Bird ST, Sahin L, et al. Antiemetic use among pregnant women in the United States: the escalating use of ondansetron. Pharmacoepidemiol Drug Saf. 2017;26(5):592-596. PubMed CrossRef
19.Lee NM, Saha S. Nausea and vomiting of pregnancy. Gastroenterol Clin North Am. 2011;40(2):309-334, vii. PubMed CrossRef
20.London V, Grube S, Sherer DM, et al. Hyperemesis gravidarum: a review of recent literature. Pharmacology. 2017;100(3-4):161-171. PubMed CrossRef
21.Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin No. 189: Nausea And Vomiting Of Pregnancy. Obstet Gynecol. 2018;131(1):e15-e30. PubMed CrossRef
22.Campbell K, Rowe H, Azzam H, et al. The management of nausea and vomiting of pregnancy. J Obstet Gynaecol Can. 2016;38(12):1127-1137. PubMed CrossRef
23.Shehmar M, MacLean MA, Nelson-Piercy C, et al. The Management of Nausea and Vomiting of Pregnancy and Hyperemesis Gravidarum. Green-top Guideline No. 69. Royal College of Obstetricians and Gynecologists; 2016. https://www.evidence.nhs.uk/search?q=Hyperemesis+gravidarum. Accessed May 10, 2020.
24.Institute of Obstetricians and Gynaecologists, Royal College of Physicians of Ireland and the Clinical Strategy and Programmes Division, Health Service Executive. Clinical Practice Guideline: Hyperemesis and Nausea/Vomiting in Pregnancy. Guideline 12. Published 2015; revised 2018. https://www.hse.ie/eng/about/who/acute-hospitals-division/woman-infants/clinical-guidelines/hyperemesis-and-nausea-vomiting-in-pregnancy.pdf. Accessed May 11, 2020.
25.Lowe SA, Bowyer L, Beech A, et al. Guideline for the Management of Nausea and Vomiting in Pregnancy and Hyperemesis Gravidarum. Society of Obstetric Medicine of Australia and New Zealand; 2019. https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=
19&ved=2ahUKEwjoi5K47KnpAhUVXisKHXICC0AQFjASegQICRAB&url=https%3A%2F%2Fwww.somanz.org%2Fdownloads%2FNVPGUIDELINEFinal.pdf&usg=
AOvVaw3V27x5px37UustHiMvBdUB. Accessed May 10, 2020.
26.Siu SS, Chan MT, Lau TK. Placental transfer of ondansetron during early human pregnancy. Clin Pharmacokinet. 2006;45(4):419-423. PubMed CrossRef
27.Elkomy MH, Sultan P, Carvalho B, et al. Ondansetron pharmacokinetics in pregnant women and neonates: towards a new treatment for neonatal abstinence syndrome. Clin Pharmacol Ther. 2015;97(2):167-176. PubMed CrossRef
28.Danielsson B, Webster WS, Ritchie HE. Ondansetron and teratogenicity in rats: evidence for a mechanism mediated via embryonic hERG blockade. Reprod Toxicol. 2018;81:237-245. PubMed CrossRef
29.Kaplan YC, Richardson JL, Keskin-Arslan E, et al. Use of ondansetron during pregnancy and the risk of major congenital malformations: a systematic review and meta-analysis. Reprod Toxicol. 2019;86:1-13. PubMed CrossRef
30.Huybrechts KF, Hernández-D×az S, Straub L, et al. Association of maternal first-trimester ondansetron use with cardiac malformations and oral clefts in offspring. JAMA. 2018;320(23):2429-2437. PubMed CrossRef
31.Zambelli-Weiner A, Via C, Yuen M, et al. First trimester ondansetron exposure and risk of structural birth defects. Reprod Toxicol. 2019;83:14-20. PubMed CrossRe
32.Lemon LS, Bodnar LM, Garrard W, et al. Ondansetron use in the first trimester of pregnancy and the risk of neonatal ventricular septal defect. Int J Epidemiol. 2019;dyz255. PubMed CrossRef
33.Huybrechts KF, Hernandez-Diaz S, Straub L, et al. Intravenous ondansetron in pregnancy and risk of congenital malformations. JAMA. 2019;323(4):372-374. PubMed CrossRef
34.Parker SE, Van Bennekom C, Anderka M, et al; National Birth Defects Prevention Study. Ondansetron for treatment of nausea and vomiting of pregnancy and the risk of specific birth defects. Obstet Gynecol. 2018;132(2):385-394. PubMed CrossRef
35.Anderka M, Mitchell AA, Louik C, et al; National Birth Defects Prevention Study. Medications used to treat nausea and vomiting of pregnancy and the risk of selected birth defects. Birth Defects Res A Clin Mol Teratol. 2012;94(1):22-30. PubMed CrossRef
36.Van Bennekom CM, Park SE, Anderka M, et al. Ondansetron for the treatment of nausea and vomiting of pregnancy and the risk of birth defects. Pharmacoepidemiol Drug Saf. 2015;24(S1):401-402.
37.Bérard A, Sheehy O, Gorgui J, et al. New evidence for concern over the risk of birth defects from medications for nausea and vomiting of pregnancy. J Clin Epidemiol. 2019;116:39-48. PubMed CrossRef
38.Andrade C. The P value and statistical significance: misunderstandings, explanations, challenges, and alternatives. Indian J Psychol Med. 2019;41(3):210-215. PubMed CrossRef
39.Andrade C. Intellectual disability after gestational exposure to antidepressant drugs: the confidence interval as a compatibility interval. J Clin Psychiatry. 2019;80(3):19f12912. PubMed CrossRef
40.Veenendaal MV, van Abeelen AF, Painter RC, et al. Consequences of hyperemesis gravidarum for offspring: a systematic review and meta-analysis. BJOG. 2011;118(11):1302-1313. PubMed CrossRef
41.Koren G, Madjunkova S, Maltepe C. The protective effects of nausea and vomiting of pregnancy against adverse fetal outcome: a systematic review. Reprod Toxicol. 2014;47:77-80. PubMed CrossRef
42.van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241-2247. PubMed CrossRef
43.Liu Y, Chen S, Zühlke L, et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol. 2019;48(2):455-463. PubMed CrossRef
44.Zhang J, Ko JM, Guileyardo JM, et al. A review of spontaneous closure of ventricular septal defect. Proc Bayl Univ Med Cent. 2015;28(4):516-520. PubMed CrossRef
45.Zhao QM, Niu C, Liu F, et al. Spontaneous losure rates of ventricular septal defects (6,750 consecutive neonates). Am J Cardiol. 2019;124(4):613-617. PubMed CrossRef
46.Goldberg JF. Long-term follow-up of "simple" lesions: atrial septal defect, ventricular septal defect, and coarctation of the aorta. Congenit Heart Dis. 2015;10(5):466-474. PubMed CrossRef
47.Campbell A, Costello BJ, Ruiz RL. Cleft lip and palate surgery: an update of clinical outcomes for primary repair. Oral Maxillofac Surg Clin North Am. 2010;22(1):43-58. PubMed CrossRef
48.Worley ML, Patel KG, Kilpatrick LA. Cleft lip and palate. Clin Perinatol. 2018;45(4):661-678. PubMed CrossRef
49.Charbit B, Albaladejo P, Funck-Brentano C, et al. Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology. 2005;102(6):1094-1100. PubMed CrossRef
50.Li K, Vo K, Lee BK, et al. Effect of a single dose of IV ondansetron on QTc interval in emergency department patients. Am J Health Syst Pharm. 2018;75(5):276-282. PubMed CrossRef
This PDF is free for all visitors!