Prazosin for the Treatment of Nightmares Related to Posttraumatic Stress Disorder: A Review of the Literature

ABSTRACT
Background: Posttraumatic stress disorder (PTSD) is a psychiatric disorder with symptoms that include insomnia due to hyperarousal and recurring nightmares. These symptoms are believed to be due to a conditioned response that is regulated by norepinephrine. Prazosin, an α1 antagonist, can decrease levels of norepinephrine in the central nervous system, thereby reducing nightmares related to PTSD.
Data Sources: A literature search was conducted for all studies evaluating the effectiveness of prazosin as therapy for nightmare symptoms of PTSD. MEDLINE was utilized to identify all English-language studies published between 1966 and March 2011. Keywords searched included prazosin, PTSD, and nightmares.
Results: Eleven studies were identified, including 4 open-label trials, 4 retrospective chart reviews, and 3 placebo-controlled trials. Prazosin demonstrated favorable clinical efficacy and was found to be safe for relieving PTSD-associated nightmares.
Conclusions: Current data indicate that prazosin is an effective agent for the treatment of nightmares associated with PTSD. However, the data are limited by small study sizes, lack of diversified investigators, and lack of regional diversity.
Prim Care Companion CNS Disord 2012;14(2):doi:10.4088/PCC.11r01222
© Copyright 2012 Physicians Postgraduate Press, Inc.
Submitted: May 25, 2011; accepted August 15, 2011.
Published online: March 22, 2012.
Corresponding author: Kurt A. Wargo, PharmD, BCPS (AQ-ID), 301 Governors Dr SW, Ste 385C1, Huntsville, AL 35801 ([email protected]).
Posttraumatic stress disorder (PTSD) is a severe anxiety disorder. The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for diagnosing PTSD require exposure to a traumatic event (witnessed or experienced) that involves the threat of death or serious injury.1 This threat induces a feeling of intense fear or a sensation of helplessness in the afflicted individual.1,2 Traumatic events commonly include motor vehicle accidents and interpersonal trauma (ie, molestation and rape); however, victims of recent terror attacks and combat survivors are also subject to the consequences of these events.
It was estimated in the National Comorbidity Survey that 7.8% of the population will suffer from PTSD in their lifetime.3,4 It is also estimated that 50% of the US population are exposed to traumatic events in their lifetime that could lead to the development of PTSD.3,5 In addition, the incidence of PTSD is expected to increase due to the increasing number of people exposed to traumatic events such as the terror attacks of and following September 11, 2001, and the subsequent Middle Eastern military campaigns. Considering estimates that 31% of Vietnam veterans suffer from PTSD,6 the veterans who served in support of Operation Iraqi Freedom/Operation Enduring Freedom will produce a new generation of soldiers that will quite likely have similar rates of PTSD. In fact, of the soldiers returning from their campaigns in Iraq, data indicate that 17% are displaying symptoms of PTSD.7,8
Currently accepted pharmacotherapy for the treatment of symptoms related to PTSD focuses primarily on antidepressant agents including selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, and monoamine oxidase inhibitors.2,9-11 The only medications with a US Food and Drug Administration indication for the treatment of PTSD are sertraline and paroxetine.10,11 Unfortunately, these agents are not ideal because their effects on sleep are unpredictable, as they have the potential to worsen insomnia that patients may be experiencing as a result of PTSD.2,9-11
Sleep disruptions are common among sufferers of PTSD, with an estimated incidence of 70% to 80%.10,12 Typically, patients with PTSD develop recurrent nightmares and hyperarousal that in turn lead to insomnia.2,9,10,12-14 Therapies such as phenelzine, nefazodone, trazodone, mirtazapine, and prazosin have been utilized in the treatment of PTSD-related sleep disturbances.9,13
Prazosin is an α1-adrenergic antagonist that reduces the adrenergic response.2,10-13 In the central nervous system (CNS), α1 and α2 receptors are located in the locus ceruleus, cerebral cortex, and limbic region including the hippocampus and amygdala.10,13 Stimulation of α1 receptors in rats disrupts higher-order cognitive processing and induces the primitive fear response.12 Evidence exists demonstrating that α1-receptor stimulation disrupts rapid eye movement (REM) sleep and increases non-REM sleep.10 In addition, stimulation of α1 receptors leads to the release of corticotropin-releasing hormone, thereby enhancing the cortisol stress response.4,12 Prazosin is considered sufficiently lipophilic to cross the blood-brain barrier and antagonize the α1 receptors in the CNS, blocking these stress responses.10,12,13 Through this mechanism, prazosin can improve sleep and reduce nightmares associated with PTSD. The objective of this review is to evaluate the available data regarding the efficacy of prazosin to treat nightmares associated with PTSD.

- Posttraumatic stress disorder (PTSD) is a severe anxiety disorder that impacts a large percentage of the US population, in particular, veterans.
- Current FDA-approved and unapproved treatments for PTSD are less than ideal due to their potential to produce insomnia.
- Prazosin has been shown, in several published studies, to significantly reduce nightmares and improve sleep in patients suffering from PTSD.
SEARCH AND DATA ANALYSIS METHODS
A literature search was conducted for all studies evaluating the effectiveness of prazosin as a treatment for nightmares associated with PTSD. MEDLINE was utilized to identify all studies published between 1966 and March 2011. Keywords searched included prazosin, PTSD, and nightmares. Articles included in this review were those limited to human subjects, case studies, and clinical studies. Articles excluded in this review were those not published in the English language, ethical discussions, and guidelines. Each article identified was specifically reviewed for, but not limited to, study type, location, authors, year published, subjects, interventions, outcomes, appropriateness of data analysis, bias, overall quality of study, and potential application to patient populations.
In the majority of studies evaluated, investigators used a validated measure of PTSD improvement called the Clinician-Administered PTSD Scale (CAPS).15 The CAPS was developed to aid in the evaluation of patients with PTSD. Although the CAPS was originally designed to assess patients who had been involved in combat situations, it has been utilized to evaluate PTSD in motor vehicle accident victims, rape victims, and cancer patients. By using a scale of 0 to 4 to identify the frequency and severity of 17 PTSD symptoms, health care professionals can assess the progression of symptoms and the efficacy of medical interventions.
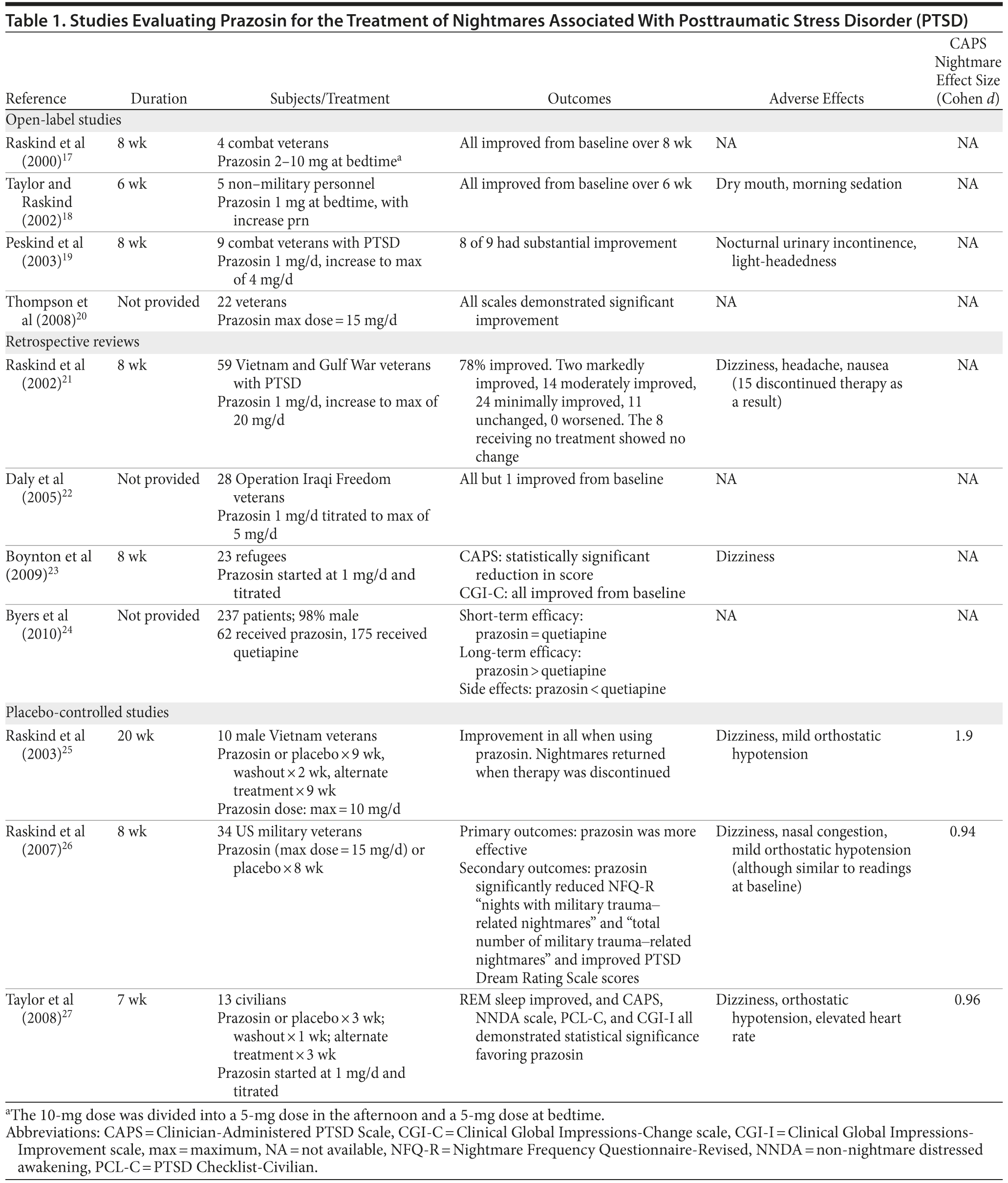
Table 1 demonstrates overall results of the analysis. Effect sizes were calculated as mean posttreatment prazosin score minus mean posttreatment control score divided by the standard deviation of the mean posttreatment control score, using the Cohen d method.16 In the open-label and retrospective analyses, data used for the control group were pre-prazosin treatment data (individual patients served as their own controls). Significance of the effect size was defined as follows: less than 0.5 is a small difference, 0.5-0.8 is a moderate difference, and greater than 0.8 is a large difference.
RESULTS
Open-Label Trials
Raskind and colleagues17 evaluated 4 combat veterans with chronic nightmares prior to and during 8 weeks of treatment with prazosin. Three patients were initially treated with prazosin 1 mg at bedtime for 1 week, after which the dose was increased to 2 mg for the second week. If the adverse effects were tolerable and the patients were still experiencing nightmares, the dose was increased to 5 mg at bedtime for weeks 3 and 4. The prazosin dose was subsequently increased to 10 mg/d, divided into a 5-mg dose in the afternoon and a 5-mg dose at bedtime, for weeks 5 through 8 if the patients did not have a complete response. The CAPS was used to assess response to the medication at pretreatment and weeks 2, 4, and 8, while the Clinical Global Impressions-Change scale (CGI-C) was also utilized at week 8. Patients were asked 2 questions from the CAPS, and scores were summed to determine a final score (out of 8). The questions were, “In the past week, have you had any unpleasant dreams about [event]? (0 = never, 4 = daily/almost every day)” and “How much stress or discomfort did this cause you? (0 = no distress, 4 = severe distress causing the patient not to be able to return to sleep).”17
All of the patients had a CAPS score of 7 or 8 at baseline, and by the end of the 8-week trial it was found that nightmares were completely eliminated in 2 of the patients (CAPS score = 0).17 These 2 patients had also reached a daily dose of at least 5 mg. The other 2 patients had scores of 4 and 3, respectively. After week 8, CGI-C evaluations found that 2 of the patients had “markedly improved symptoms,” while the other 2 had “moderately improved symptoms.” It was concluded that there is promise in using prazosin for the treatment of PTSD-associated nightmares and that placebo-controlled trials would be necessary in order to further evaluate this option.
Two patients had significant decreases in blood pressure with the addition of prazosin, with baseline measures of 160/85 mm Hg and 140/100 mm Hg dropping to 140/75 mm Hg and 115/70 mm Hg, respectively.17 One patient had a slight drop in blood pressure from 150/110 mm Hg to 145/95 mm Hg. The final patient showed persistent hypertension, hypervigilance, and a low anger threshold with the addition of prazosin, with a blood pressure of 150/95 mm Hg. Along with this therapy, it was decided to start propranolol, even though it was noted that the patient experienced nightmares with on a previous trial. It was theorized that the combination would reduce behavioral symptoms without exacerbating nightmares. After 2 days of this combination, the patient’s blood pressure had dropped to 130/80 mm Hg, his irritability was reduced, and he did not experience any nightmares.
Five patients with PTSD symptoms were selected to undergo treatment with prazosin for 6 weeks in a trial conducted by Taylor and Raskind.18 Doses were initiated at 1 mg at bedtime, and if patients failed to have a response after 2 weeks, the dose was increased to 2 mg at bedtime. A morning dose was added as needed if the patient experienced daytime symptoms. All patients were assessed using the Clinical Impression of Change-Nightmares scale, CAPS, and CGI-C. At the end of the 6 weeks, 3 of the patients had CGI-C scores that indicated “marked improvement.” The clinicians found that patients experienced moderate improvement according to the Clinical Impression of Change-Nightmares scale. All patients also self-reported moderate improvement in symptoms. CAPS score decreased by at least 4 points for each patient by the end of the trial. The mean daily dose of prazosin used was 1.8 ± 1.3 mg (range, 1-4 mg). Prazosin was tolerated well, and dry mouth and morning sedation for the first few days of treatment were the only side effects reported. Changes in blood pressure were not discussed in this study. The patients continued to see response for months after the trial while they were taking prazosin. These findings again suggested that placebo-controlled trials were needed in order to fully evaluate the function of prazosin in eliminating PTSD-related nightmares.18
Peskind and colleagues19 assessed 9 combat veterans ranging from 67 to 83 years of age in an 8-week trial to determine the efficacy of prazosin in eliminating trauma-related nightmares. Prazosin was started at 1 mg 1 hour before bedtime for 1 week. If no response was seen and the patient’s blood pressure remained stable, the dose was increased by 1 mg on a weekly basis to a maximum dose of 4 mg 1 hour prior to bedtime. CAPS and CGI-C scores were used to assess the efficacy of prazosin throughout the trial. The frequency of recurrent distressing dreams was scored 2 weeks before initiation of prazosin and after prazosin treatment, at the end of the trial. The maximum mean dose of prazosin was 2.3 ± 0.7 mg (range, 2-4 mg) daily. After 8 weeks of treatment, 8 of the patients had greater than 50% reduction in nightmares (P < .001). CGI-C scores indicated that 8 of the patients experienced moderate to marked improvement in PTSD symptoms. The mean CGI-C score was 1.8 ± 0.7, and the CAPS nightmare scale indicated a mean change from 6.6 ± 1.1 at baseline to 0.9 ± 1.5 after 8 weeks. All patients saw a reduction in CAPS score by at least 2 points. Prazosin was tolerated well, with nocturnal urinary incontinence and light-headedness being the only reported side effects (occurring in 1 and 2 patients, respectively). Blood pressures decreased significantly: baseline average blood pressure was 144/74 ± 19/4 mm Hg, and postanalysis 8-week average blood pressure was 134/72 ± 16/4 mm Hg. It was concluded that improvement in PTSD symptom severity was probably due to improved sleep and secondary to improvement in daytime symptoms.19
A study conducted by Thompson and colleagues20 evaluated 22 veterans with PTSD-related nightmares and sleep disturbances. Seventeen of the patients participating in the study had PTSD secondary to combat. Patients were administered prazosin beginning at 1 mg 1 hour prior to bedtime for 3 nights, and then the dose was increased to 2 mg before bedtime through day 7. If nightmares were not at least moderately reduced and adverse effects were tolerable, prazosin was increased in weekly intervals of 2 mg up to a maximum dose of 10 mg at bedtime. If patients did not demonstrate moderate improvement in symptoms 1 week after starting 10 mg, an extra 5 mg could be added.20 One item of the CAPS, “recurrent distressing dreams,” was used to assess the severity of symptoms. Another item of the CAPS, “difficulty falling or staying asleep,” was utilized as well to assess sleep difficulty. The CGI-C was conducted at a follow-up maintenance dose visit. At the conclusion of the study, the mean dose of prazosin used was 9.6 ± 6 mg at bedtime.20 Significant changes from baseline to posttreatment were found for sleeping difficulties and nightmares (P < .05) in favor of prazosin. The changes in non-nightmare distressed awakenings (NNDAs) were also found to be significant (P < .05). Changes in blood pressure were not discussed in this study. It was concluded that NNDAs are prevalent among patients with PTSD who complain of sleep problems, and the CGI-C scores suggested that prazosin could be a viable therapy option for the treatment of NNDAs as well as trauma-related nightmares.20
Retrospective Chart Reviews
Raskind and colleagues21 analyzed data from 59 Vietnam and Gulf War veterans in a large, consecutive-sample study. Treatment began with 1 mg at bedtime for a few days and was titrated by 2-mg increments until the patient was symptom free, experienced intolerable adverse effects, or reached a maximum dose of 20 mg/d. If a dose larger than 10 mg was administered, dosing was split into an early afternoon dose and a bedtime dose. The CAPS and CGI-C scores were measured at baseline and 8 weeks after initiation of therapy.21 If a patient did not complete 8 weeks of treatment, the rating prior to discontinuation was reported. Eight patients decided not to take the prescribed prazosin, so they were in a designated “no treatment” comparison group because they were adherent to other medications. Fifty-one of the patients were included in the primary analysis, and 36 of these received complete titration of prazosin and 8 additional weeks of prazosin treatment after titration.21 A separate analysis was conducted for the 36 subjects who completed the study. A mean dose of 6.3 ± 0.8 mg was reached in all patients treated in the primary analysis, and a mean dose of 9.6 ± 0.9 was achieved in the patients who completed the study. CAPS scores were significantly decreased for both the primary analysis and the completer analysis (P < .0001 for both). Changes in CAPS scores from baseline were significant for both the primary analysis (7.1 ± 0.2 to 4.2 ± 0.3, P < .0001) and the patients who completed the study (7.0 ± 0.2 to 3.5 ± 0.3, P < .0001). In regard to severity of symptoms, 78% of the patients reported at least some improvement. The CGI-C scores were improved more in the 51 patients of the primary analysis group compared to those who did not take prazosin (P < .01). Dizziness, headache, and nausea were the most frequently reported side effects of taking prazosin, and 15 patients in the treatment group discontinued therapy due to these issues. The recurrent distressing dreams item on the CAPS was significantly improved in those 15 patients (P < .01). Changes in blood pressure were not discussed in this study. Analysis of the results led to the conclusion that in patients who have been previously treated for PTSD-associated nightmares, prazosin may provide substantial relief of symptoms.
In another study, by Daly and colleagues,22 28 Operation Iraqi Freedom veterans (27 males) who reported trauma-associated nightmares were assessed before and after therapy with prazosin. Consistent with other studies, prazosin was initiated at 1 mg at bedtime. Patients were monitored on a weekly basis, and the dose could be increased by 1 mg every week. There was no predetermined maximum dose of prazosin in this study. Demographics, combat history, and concomitant psychotropic medications were also reviewed for every patient. The CGI-C was used to monitor response to the drug in relation to nightmares. Long-term follow-up was not feasible for many of the patients because most had to return to their home units after therapy. Five of the patients could not be assessed because they did not follow up with the treatment team. Of the remaining 23 patients in the trial, 20 were rated by the CGI-C as markedly improved, 2 were moderately improved, and 1 was unchanged with a maximum dose of 6 mg. In 16 patients, only 1 mg at bedtime was needed to demonstrate moderate improvement in nightmares. One patient required a dose increase to 5 mg to have a response. Changes in blood pressure were not discussed in this study. It was concluded that in a high percentage of patients with combat-related nightmares, low-dose prazosin was effective in eliminating or drastically decreasing symptoms.
Boynton and colleagues23 analyzed 23 cases of refugees treated with prazosin, who had previously been prescribed psychotropic medications for the treatment of PTSD-related nightmares. Prazosin was initiated at 1 mg at bedtime, and the dose was gradually increased until symptoms significantly improved or side effects could not be tolerated. Nightmare severity was assessed retrospectively according to data included in patient charts. The CAPS was used to establish a score at baseline and 8 weeks after the start of treatment. The CGI-C score was utilized to assess improvement of PTSD-associated symptoms at the end of the 8-week period. The mean daily dose of prazosin was 2.3 ± 1.4 mg (range, 1-6 mg). A significant reduction in the CAPS score was found after the 8-week treatment period (P < .0005). It was determined that changes in scores correlated with duration of treatment (P < .006) and maximum dose achieved (P < .034). The relationship between duration of trauma and treatment outcome was not significant. Severity of PTSD was assessed as markedly improved in 6 patients, moderately improved in 11 patients, and minimally improved in 6 patients. Dizziness was the most commonly reported side effect of treatment. Changes in blood pressure were not discussed in this study. The low cost of the drug along with its ease of use led to the conclusion that prazosin is a promising component in the treatment of PTSD-related nightmares.23
Byers and colleagues24 reviewed 237 medical records for drug effectiveness and long-term safety after treatment with quetiapine or prazosin for PTSD-associated nightmares. Patients included in the study had been on long-term therapy (range, 3-6 years). Sixty-two patients had received prazosin, while 175 received quetiapine. Doses for each drug were recorded but not included in the report. Overall, prazosin was found to be more effective than quetiapine (P = .03) and symptoms were better controlled with prazosin (P = .02) on the basis of an electronic survey. Discontinuation due to adverse events was higher in the patients who were prescribed quetiapine compared to prazosin (P = .008). Forty-four patients who initially started quetiapine treatment were switched to prazosin in the middle of therapy, and 21 remained on prazosin treatment until the end of the study. Six of the patients in the prazosin group were prescribed quetiapine in the middle of the study period, and 3 remained on quetiapine treatment until the end. Nonadherence, paradoxical reactions, and other reasons for discontinuation were not significantly different between the groups (P = .86, P = .72, and P = .56, respectively). Changes in blood pressure were not discussed in this study. It was concluded that short-term effectiveness was similar between the groups, but prazosin, at a mean daily dose of 6.5 mg, was favored as being a more effective and safer long-term therapy.
Placebo-Controlled Studies
In a double-blind, crossover trial, Raskind and colleagues25 assessed 10 Vietnam combat veterans who were given prazosin or placebo over a 20-week period. Seven of the patients were taking other medications for PTSD symptoms (SSRIs, trazodone, benzodiazepines, anticonvulsants, hydroxyzine, or risperidone), and these regimens were not discontinued while the trial was being conducted. Patients were split into 2 groups of 5, and each group was administered prazosin or placebo for 9 weeks, followed by a 2-week washout period, before receiving the other treatment for 9 weeks. Prazosin was started at 1 mg at bedtime for 3 days and then increased to 2 mg at bedtime for 4 days. The dose could be titrated to a maximum of 10 mg if patients failed to have a response and the adverse reactions were tolerable (mean daily dose = 9.5 mg). The CAPS and CGI-C were used to rate 3 primary outcome measures in this trial: recurrent distressing dreams, difficulty falling or staying asleep, and overall change in severity of PTSD status. All items assessed were found to be significantly improved from baseline: recurrent distressing dreams (P < .001), difficulty falling/staying asleep (P < .01), CGI-C score change (P < .01), and total CAPS score (P < .01). The effect size of prazosin on the CAPS nightmare scale for this study was large, at 1.9. Although overall changes in blood pressure were not discussed in this study, the mean pressure by the end of the study was 135/89 supine and 129/84 standing. The only reported adverse events in the trial were dizziness and mild orthostatic hypotension (2 patients). During the washout period, 5 patients experienced a return to distressing nightmares after the discontinuation of prazosin.
Raskind and colleagues26 evaluated 34 US military veterans who were randomly assigned to receive prazosin or placebo for 8 weeks in a parallel-group study. The majority of patients were undergoing psychotherapy before the trial, and half had been prescribed 1 or more psychotropic medications for the relief of PTSD-induced symptoms. Patients receiving psychotherapy and/or pharmacologic therapy remained on these therapies throughout the trial. Prazosin was started at 1 mg at bedtime for 3 days, and then the dose was increased to 2 mg until day 7. The dose of prazosin could be titrated thereafter to a maximum of 15 mg if patients had no relief and adverse effects were tolerable. Primary outcomes included the CAPS recurrent distressing dreams item, Pittsburgh Sleep Quality Index, and CGI-C. Secondary outcomes included the total CAPS score, the Nightmare Frequency Questionnaire-Revised, and the Hamilton Depression Rating Scale (HDRS). The mean daily dose of prazosin was 13 ± 3 mg. By the end of the 8-week period, 6 patients had discontinued therapy (4 due to unspecified adverse events and 2 for unknown reasons). The CAPS distressing dreams item was not significantly improved from baseline to week 4 (P = .09), but at week 8 significant improvement was demonstrated (P = .02). The effect size was found to be large at 0.94. The same pattern was seen for the Pittsburgh Sleep Quality Index at week 4 and week 8 (P = .5 and P = .008, respectively). CGI-C scores at week 4 and week 8 were both significant (P = .02 and P = .002, respectively). The Nightmare Frequency Questionnaire-Revised “nights with military trauma-related nightmares” and “total number of military trauma-related nightmares” items were both significantly improved (P = .02 and P = .01, respectively). The PTSD Dream Rating Scale was also found to be significantly improved from baseline (P < .001). No significant improvement was demonstrated on the HDRS (P = .08). Mean blood pressures were 129 ± 10.7/82 ± 6.8 mm Hg supine and 126 ± 12/83 ± 9.2 mm Hg standing at baseline and 128 ± 17/81 ± 10.1 mm Hg supine and 125 ± 18.3/83 ± 11.8 mm Hg standing at study conclusion. Dizziness and nasal congestion were among the reported adverse effects experienced with prazosin, and orthostatic hypotension was similar between the groups at the end of the trial.
Taylor and colleagues27 conducted a randomized, crossover trial of 13 patients, with chronic civilian trauma PTSD, for a period of 7 weeks. Patients received prazosin or placebo for 3 weeks, followed by a 1-week washout period, before receiving the other treatment for 3 weeks. As with the other trials, prazosin was initiated at 1 mg at bedtime. The dose was increased in 1-mg increments every few days for the first 10 days in order to achieve the lowest effective dose with the least adverse effects (mean daily dose = 9.6 mg). The CAPS and an NNDA scale were used to assess the efficacy of the treatment. The PTSD Checklist-Civilian and Clinical Global Impressions-Improvement scale were also utilized. In addition, during the last 3 nights of each treatment, patients wore a monitor (REMView) to objectively measure sleep duration and REM sleep time. The “recurrent distressing dreams” item of the CAPS score and of the NNDA scale were both significantly improved (P = .04 and P = .05, respectively). The effect size of prazosin on the CAPS nightmare score was large at 0.96. Clinical Global Impressions-Improvement scale scores, PTSD Dream Rating Scale scores, and PTSD Checklist-Civilian scores were significantly improved from baseline (P = .002, P = .006, and P = .025, respectively). Total sleep time and REM sleep time were both statistically and clinically greater in the prazosin group (374 ± 86 min vs 280 ± 105 min, P < .01; and 138 ± 63 min vs 97 ± 70 min, P < .01, respectively). Although overall changes in blood pressure were not discussed in this study, the mean blood pressure in the prazosin group at study conclusion was 140 ± 23/78 ± 6 mm Hg. Dizziness and orthostatic hypotension were similar between groups.
Adverse Effects
In all studies evaluated, adverse effects were reportedly tolerable, and, in some cases, PTSD symptoms returned if the patient discontinued the treatment regimen. The most common reported adverse effects of prazosin were dizziness and orthostatic hypotension. Most cases of orthostatic hypotension were considered mild, but one study27 indicated that diastolic blood pressure readings for patients receiving prazosin were significantly lower than for those receiving placebo. Headache, nausea, and dizziness led to discontinuation of therapy by 15 patients in the treatment group of one study.21 Nocturnal urinary incontinence, dry mouth, and morning sedation were noted as well. Case reports18,19,28 have further documented extreme sedation, alterations in normal behavior, and a feeling described by one patient as “blurred awareness of the line between dreaming and reality.” 28(p774)
DISCUSSION
The evidence presented in this review is promising regarding the use of prazosin to provide relief of nightmares associated with PTSD. Every study reviewed has demonstrated that prazosin is effective at providing nightmare relief in these patients. Of the placebo-controlled studies evaluating prazosin, all had an effect size greater than 0.8. This implies that not only did prazosin statistically improve symptoms compared to placebo, but it also had a clinically significant impact. This collection of studies, however, is not without weaknesses.
The greatest flaw of the studies published is that, in total, only 252 patients received prazosin therapy, for an average duration of 8 weeks. In fact, considering only the high-quality, randomized, placebo-controlled studies, a mere 40 patients received prazosin therapy. Given the large population of patients who suffer from PTSD, drawing firm conclusions and making recommendations for the role of prazosin from a total of only 252 patients who received therapy for approximately 8 weeks is difficult.
An additional weakness of the studies reviewed was that 10 of the 11 studies were published by Dr Murray Raskind and his group of colleagues. Interestingly, 8 of the studies were from VA Northwest Network Mental Illness Research, Education and Clinical Center (Raskind is the director), which is in Veterans Integrated Service Network 20 (Dr Elaine Peskind is a member of the Executive Committee).17-19,21,22,25-27 Six of these 8 studies were also from the Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle.17,18,20,22,26,27 None of the studies published were multiregional, nor did they utilize a large, diverse sample. Although all 11 studies support the efficacy of prazosin in reducing nightmares associated with PTSD, the lack of diversity in investigators and regional locations reduces the external validity of this data. In reality, the lack of variety and diversity can be explained by the fact that these authors are experts in the field, and few other clinicians are investigating prazosin in this manner. Interestingly, a geographic surveillance study evaluated the spread of knowledge regarding the use of prazosin to treat PTSD.29 This study demonstrated that while over one-third of veterans with PTSD were prescribed prazosin in the Veterans Affairs Puget Sound Health Care System, the number dropped significantly the further away from Washington they looked. In fact, only 6.7%, 4.0%, and 1.9% of patients with PTSD were prescribed prazosin when they were 500-999 miles, 1,000-2,499 miles, and greater than 2,500 miles away, respectively. This finding highlights the need for future studies to be conducted by outside contributors utilizing larger and more diverse samples from multiple locations to confirm the results of these preliminary studies.
Three recent or ongoing studies have been initiated to address these concerns. One is a large (200 estimated participants), single-location (University of Pittsburgh), randomized, double-blind, placebo-controlled study with an estimated completion date of June 2011 (as listed on ClinicalTrials.gov).30 Another study,31 overseen by Raskind, was a dual-location (Walter Reed Army Medical Center, Washington, DC, and Madigan Army Medical Center, Washington State), double-blind, placebo-controlled efficacy study comparing prazosin to paroxetine to placebo. It began in 2004 but was terminated due to poor recruitment of subjects (only 89 of an anticipated 201 were enrolled). The most promising of the three is a large (326 estimated participants), multicenter, randomized, double-blind, placebo-controlled study32 that is being overseen by Raskind and has an estimated completion date of 2012. This research is being conducted within the VA health system and includes hospitals in 12 states. While these studies are still primarily overseen by Raskind and are focused on veterans, they may address our concerns about location and size of previous samples.
A final limitation of the studies published is that dosing of prazosin was not standardized. In the studies reviewed, prazosin dosing was typically initiated at 1 mg prior to bedtime and then titrated by 1 mg weekly to an effective dose with minimal side effects. The mean doses deemed to be effective in all studies ranged from 2.3 ± 0.7 mg in elderly patients to 13.3 ± 3 mg in combat veterans. While van Liempt and colleagues9 identified 1 to 4 mg as the optimal dosing range, this may be conservative dosing, considering the large amount of patients who required greater dosages.
Future trials should be conducted to address some of the concerns and weakness addressed in this review. Studies of a larger and more diverse patient population should be conducted to ensure prazosin is efficacious in patients (other than combat veterans) with PTSD-associated nightmares. Additionally, long-term studies, greater than 8 weeks in duration, should be conducted to ensure prazosin retains its efficacy while not leading to any intolerable adverse effects.
CONCLUSION
The data analyzed in this review indicate that prazosin is effective in reducing nightmares associated with PTSD. However, there are weaknesses that must be addressed in future studies before prazosin can be used in the general population for this indication. We recommend that future studies should be conducted over a longer period of time in a larger and more diverse patient population.
Drug names: mirtazapine (Remeron and others), paroxetine (Paxil, Pexeva, and others), phenelzine (Nardil), prazosin (Minipress and others), propranolol (Inderal, InnoPran, and others), quetiapine (Seroquel), risperidone (Risperdal and others), sertraline (Zoloft and others), trazodone (Oleptro and others).
Author affiliations: Harrison School of Pharmacy, Auburn University (all authors), Auburn, Alabama, and College of Medicine, University of South Alabama, Mobile (Dr Lorenz).
Potential conflicts of interest: None reported.
Funding/support: None reported.
REFERENCES
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000:429-484.
2. Kirkwood CK, Makela EH, Wells BG. CH 74: Anxiety disorders II: posttraumatic stress disorder and obsessive-compulsive disorder. DiPiro J, Talbert R, Yee G, et al, eds. Pharmacotherapy, A Pathophysiological Approach, 7th Edition. New York, NY: McGraw-Hill Companies, Inc. 2008: 1179-1184.
3. Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048-1060. PubMed
4. Pitman RK, Delahanty DL. Conceptually driven pharmacologic approaches to acute trauma. CNS Spectr. 2005;10(2):99-106. PubMed
5. Yehuda R. Risk and resilience in posttraumatic stress disorder. J Clin Psychiatry. 2004;65(suppl 1):29-36. PubMed
6. Kulka R, Schlenger W, Fairbanks J. Trauma and the Vietnam War Generation: Report of Findings From the National Vietnam Veterans Readjustment Study. New York, NY: Brunnel/Mazel; 1990.
7. Hoge CW, Auchterlonie JL, Milliken CS. Mental health problems, use of mental health services, and attrition from military service after returning from deployment to Iraq and Afghanistan. JAMA. 2006;295(9):1023-1032. PubMed
8. McGhee LL, Maani CV, Garza TH, et al. The effect of propranolol on posttraumatic stress disorder in burned service members. J Burn Care Res. 2009;30(1):92-97. PubMed doi:10.1097/BCR.0b013e3181921f51
9. van Liempt S, Vermetten E, Geuze E, et al. Pharmacotherapy for disordered sleep in post-traumatic stress disorder: a systematic review. Int Clin Psychopharmacol. 2006;21(4):193-202. PubMed doi:10.1097/00004850-200607000-00001
10. Miller LJ. Prazosin for the treatment of posttraumatic stress disorder sleep disturbances. Pharmacotherapy. 2008;28(5):656-666. PubMed doi:10.1592/phco.28.5.656
11. Amitriptyline, Citalopram, Escitalopram, Fluoxetine, Fluvoxamine, Imipramine, Mirtazapine, Paroxetine, Phenelzine, Prazosin, Sertraline, and Venlafaxine. In: Lexi-Comp Online [AUHSOP Intranet]. Hudson, OH: Lexi-Comp, Inc. Updated September 22, 2010. Accessed September 27, 2010. http://online.lexi.com/crlsql/servlet/crlonline.
12. Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract. 2007;13(2):72-78. PubMed doi:10.1097/01.pra.0000265763.79753.c1
13. Taylor HR, Freeman MK, Cates ME. Prazosin for treatment of nightmares related to posttraumatic stress disorder. Am J Health Syst Pharm. 2008;65(8):716-722. PubMed doi:10.2146/ajhp070124
14. McIntyre CK, Power AE, Roozendaal B, et al. Role of the basolateral amygdala in memory consolidation. Ann N Y Acad Sci. 2003;985(1):273-293. PubMed doi:10.1111/j.1749-6632.2003.tb07088.x
15. Weathers FW, Keane TM, Davidson JRT. Clinician-Administered PTSD Scale: a review of the first ten years of research. Depress Anxiety. 2001;13(3):132-156. PubMed doi:10.1002/da.1029
16. Penava SJ, Otto MW, Pollack MH, et al. Current status of pharmacotherapy for PTSD: an effect size analysis of controlled studies. Depress Anxiety. 1996-1997;4(5):240-242. PubMed doi:10.1002/(SICI)1520-6394(1996)4:5<240::AID-DA6>3.0.CO;2-H
17. Raskind MA, Dobie DJ, Kanter ED, et al. The alpha1-adrenergic antagonist prazosin ameliorates combat trauma nightmares in veterans with posttraumatic stress disorder: a report of 4 cases. J Clin Psychiatry. 2000;61(2):129-134 PubMed doi:10.4088/JCP.v61n0208
18. Taylor FB, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clin Psychopharmacol. 2002;22(1):82-85. PubMed doi:10.1097/00004714-200202000-00013
19. Peskind ER, Bonner LT, Hoff DJ, et al. Prazosin reduces trauma-related nightmares in older men with chronic posttraumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16(3):165-171. PubMed doi:10.1177/0891988703256050
20. Thompson CE, Taylor FB, McFall ME, et al. Nonnightmare distressed awakenings in veterans with posttraumatic stress disorder: response to prazosin. J Trauma Stress. 2008;21(4):417-420. PubMed doi:10.1002/jts.20351
21. Raskind MA, Thompson CE, Petrie EC, et al. Prazosin reduces nightmares in combat veterans with posttraumatic stress disorder. J Clin Psychiatry. 2002;63(7):565-568. PubMed doi:10.4088/JCP.v63n0705
22. Daly CM, Doyle ME, Radkind MA, et al. Clinical case series: the use of prazosin for combat-related recurrent nightmares among Operation Iraqi Freedom combat veterans. Mil Med. 2005;170(6):513-515. PubMed
23. Boynton L, Bentley J, Strachan E, et al. Preliminary findings concerning the use of prazosin for the treatment of posttraumatic nightmares in a refugee population. J Psychiatr Pract. 2009;15(6):454-459. PubMed doi:10.1097/01.pra.0000364287.63210.92
24. Byers MG, Allison KM, Wendel CS, et al. Prazosin versus quetiapine for nighttime posttraumatic stress disorder symptoms in veterans: an assessment of long-term comparative effectiveness and safety. J Clin Psychopharmacol. 2010;30(3):225-229. PubMed doi:10.1097/JCP.0b013e3181dac52f
25. Raskind MA, Peskind ER, Kanter ED, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160(2):371-373. PubMed doi:10.1176/appi.ajp.160.2.371
26. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61(8):928-934. PubMed doi:10.1016/j.biopsych.2006.06.032
27. Taylor FB, Martin P, Thompson CE, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry. 2008;63(6):629-632. PubMed doi:10.1016/j.biopsych.2007.07.001
28. Reardon CL, Factor RM. Bizarre behavior in a patient treated with prazosin for PTSD [letter]. Am J Psychiatry. 2008;165(6):774-775. PubMed doi:10.1176/appi.ajp.2008.07111710
29. Harpaz-Rotem I, Rosenheck RA. Tracing the flow of knowledge: geographic variability in the diffusion of prazosin use for the treatment of posttraumatic stress disorder nationally in the Department of Veterans Affairs. Arch Gen Psychiatry. 2009;66(4):417-421. PubMed doi:10.1001/archgenpsychiatry.2008.536
30. Efficacy of Sleep Interventions for Posttraumatic Stress Disorder (PTSD) (EASI-P). ClinicalTrials.gov Web site. http://www.clinicaltrials.gov/ct2/show/NCT00393874?term=00393874&rank=1. Updated February 18, 2011. Accessed January 23, 2012.
31. Prazosin vs Paroxetine in Combat Stress-Related Post-Traumatic Stress Disorder (PTSD) Nightmares & Sleep Disturbance. ClinicalTrials.gov Web site. http://www.clinicaltrials.gov/ct2/show/NCT00202449?term=00202449&rank=1. Updated April 25, 2011. Accessed January 23, 2012.
32. CSP #563—Prazosin and Combat Trauma PTSD (PACT). ClinicalTrials.gov Web site. http://clinicaltrials.gov/ct2/show/NCT00532493?term=NCT00532493&rank=1. Updated October 7, 2010. Accessed October 8, 2010.