Background: Although lamotrigine may be useful for treating patients with treatment-resistant bipolar disorder, some lamotrigine-associated adverse effects, including mild to moderate skin rash, may prevent the continuation of treatment.
Methods: We investigated lamotrigine rechallenge for the treatment of bipolar disorder. The present study was based on retrospective chart review of outpatients with bipolar disorder (DSM-5 criteria) who visited the hospital’s psychiatric department between July 2011 and August 2017. The review revealed 12 patients with bipolar disorder who underwent lamotrigine rechallenge following lamotrigine discontinuation due to various adverse reactions, including skin rash. None of the patients showed Stevens-Johnson syndrome. All patients suffered from treatment-resistant bipolar disorder that was refractory to treatments other than lamotrigine. For each patient, the severity of the adverse reaction to lamotrigine was weighed against the potential for therapeutic benefit.
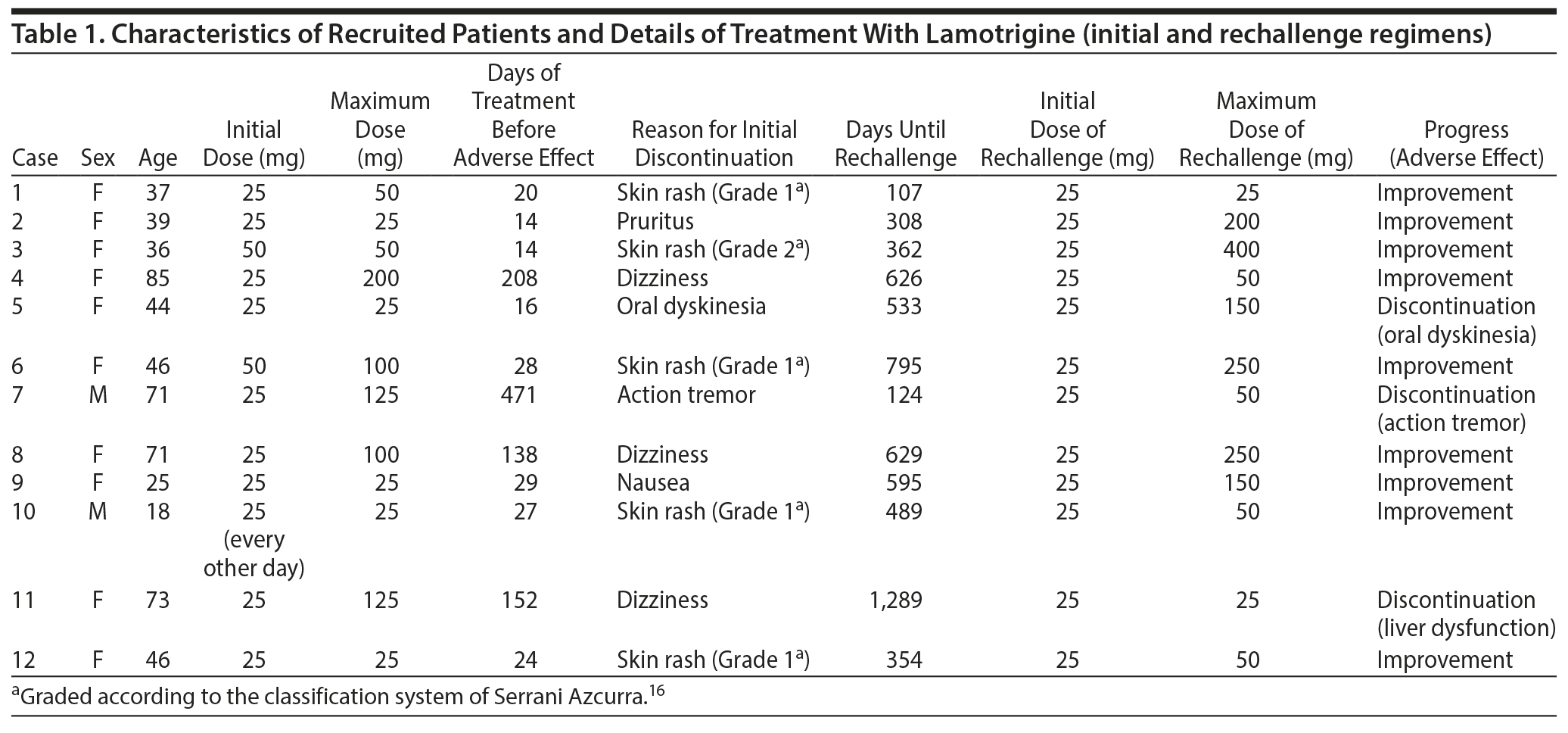
Results: In 9 of 12 cases, a positive outcome of lamotrigine rechallenge was observed. In all cases with initial skin rash with very slow titration of lamotrigine, rechallenge was successful with no recurrence of the rash. In the 3 cases for which lamotrigine was unsuccessful, lamotrigine was discontinued owing to movement disorders, ie, oral dyskinesia and action tremor, and liver dysfunction, respectively.
Conclusions: The present results suggest that lamotrigine rechallenge may be a viable option for treatment-resistant bipolar disorder.
Lamotrigine Rechallenge in Treatment-Resistant Bipolar Disorder

ABSTRACT
Background: Although lamotrigine may be useful for treating patients with treatment-resistant bipolar disorder, some lamotrigine-associated adverse effects, including mild to moderate skin rash, may prevent the continuation of treatment.
Methods: We investigated lamotrigine rechallenge for the treatment of bipolar disorder. The present study was based on retrospective chart review of outpatients with bipolar disorder (DSM-5 criteria) who visited the hospital’s psychiatric department between July 2011 and August 2017. The review revealed 12 patients with bipolar disorder who underwent lamotrigine rechallenge following lamotrigine discontinuation due to various adverse reactions, including skin rash. None of the patients showed Stevens-Johnson syndrome. All patients suffered from treatment-resistant bipolar disorder that was refractory to treatments other than lamotrigine. For each patient, the severity of the adverse reaction to lamotrigine was weighed against the potential for therapeutic benefit.
Results: In 9 of 12 cases, a positive outcome of lamotrigine rechallenge was observed. In all cases with initial skin rash with very slow titration of lamotrigine, rechallenge was successful with no recurrence of the rash. In the 3 cases for which lamotrigine was unsuccessful, lamotrigine was discontinued owing to movement disorders, ie, oral dyskinesia and action tremor, and liver dysfunction, respectively.
Conclusions: The present results suggest that lamotrigine rechallenge may be a viable option for treatment-resistant bipolar disorder.
Prim Care Companion CNS Disord 2018;20(2):17m02231
To cite: Inaba T, Sogawa R, Mizoguchi Y, et al. Lamotrigine rechallenge in treatment-resistant bipolar disorder. Prim Care Companion CNS Disord. 2018;20(2):17m02231.
To share: https://doi.org/10.4088/PCC.17m02231
© Copyright 2018 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Faculty of Medicine, Saga University, Saga, Japan
bDepartment of Pharmacy, Saga University Hospital, Saga, Japan
cDepartment of Neuropsychiatry, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
*Corresponding author: Akira Monji, MD, Saga University, Department of Psychiatry, Nabeshima 5-1-1, Saga-city, Saga 849-8501, Japan ([email protected]).
Lamotrigine is a structurally novel anticonvulsant that was approved by the US Food and Drug Administration for the treatment of epilepsy in 1994. Lamotrigine is generally well tolerated by children and adults.1 The mechanism of action of lamotrigine involves sodium channel blocking and may entail the activation of N-methyl-d-aspartate receptors.2 Lamotrigine is also beneficial for the treatment of psychiatric disorders, especially mood disorders. Evidence supports lamotrigine as an effective maintenance treatment for bipolar disorder, particularly in the prevention of depressive episodes.1 In acute bipolar depression, there is modest support for its efficacy from meta-analysis,1 especially in more severely depressed subjects. A double-blind randomized controlled trial3 in unipolar depression reported benefits on subsets of symptoms and improved response in the more severely depressed subjects. Although the preventive effect of lamotrigine on recurrence or relapse of mood episodes in bipolar I disorder has been established in clinical studies, it has recently been reported that lamotrigine may be more suitable for maintenance treatment in bipolar II disorder than in bipolar I disorder.2 Another recent report5 suggested that lamotrigine alone or in combination with quetiapine may be effective in the prevention of postpartum depression in women with bipolar II disorder.
Some lamotrigine-associated adverse effects, such as skin rash, may prevent continuation of lamotrigine treatment, particularly in the first 2 months of treatment during ongoing or completion of lamotrigine titration.6 Tak et al7 compared the adverse effects of lamotrigine in adolescent epilepsy patients and adolescent psychiatric patients. They reported that within 7 weeks of initiation of lamotrigine, the likelihood of developing a rash was not significantly different between psychiatric and nonpsychiatric patients.7 A recent report8 has demonstrated that psychiatrists, neurosurgeons, and pediatricians should strictly comply with the guidelines for the proper use of lamotrigine and that allergologists should endeavor to ensure the early detection of severe lamotrigine-induced skin eruptions.
Several reports9-16 have described the outcome of lamotrigine rechallenge following a skin rash in the treatment of either epilepsy or psychiatric disorders. There may be racial differences in susceptibility to adverse effects of lamotrigine, and probably also to rechallenge of lamotrigine. Here, we report on lamotrigine rechallenge in the treatment of bipolar disorder after discontinuation of lamotrigine due to various adverse effects, including skin rash.
METHODS
Between July 2011 and August 2017, we retrospectively reviewed charts from the psychiatric department of our hospital for outpatients with bipolar disorder. Among this group, we were looking for patients who had experienced an adverse reaction to lamotrigine on initial exposure to the drug (Table 1), even though lamotrigine had been titrated according to the manufacturer suggested protocol, and who had discontinued the drug because of the reaction. We found all of the patients had been diagnosed by the same psychiatrist (T.I.) according to the DSM-5 classification, and all of the patients were Japanese and thus have the same ethnic traits. Lamotrigine had been used in combination with other drugs, such as antipsychotics, antidepressants, other anticonvulsants, and benzodiazepines, and patients’ prescription regimens had been maintained during the rechallenge.

- Lamotrigine is an effective maintenance treatment for bipolar disorder, particularly in the prevention of depressive episodes.
- Some lamotrigine-associated adverse effects, such as skin rash, may prevent continuation of lamotrigine treatment, particularly in the first 2 months of treatment during ongoing or completion of lamotrigine titration.
- Lamotrigine rechallenge with very slow titration may be a viable option for bipolar disorder that is treatment resistant against drugs other than lamotrigine.
For those who had skin rash as their reason for lamotrigine discontinuation, grade of the rash was based on the intensity and location of the outbreak according to the classification system of Serrani Azcurra.16 For example, Grade 1 is "Macular or papular outbreak or erythema without related symptoms"; Grade 2 is "Macular or papular outbreak or erythema with itching or other related symptoms. Localized peeling or other lesions that cover < 50% of body surface area"; and Grade 5 is "Severe life-threatening Stevens-Johnson syndrome."16
Because our patients suffered from treatment-resistant bipolar disorder that was refractory to treatments other than lamotrigine, they were offered lamotrigine rechallenge by their responsible physician (A.M.). For each patient, the severity of the adverse reaction to lamotrigine was weighed against the potential for therapeutic benefit with the drug. Informed consent had been obtained at that time for all those considered appropriate for rechallenge.
RESULTS
Twelve patients fit our criteria; their clinical data are shown in Table 1. In all cases, adverse effects showed improvement after withdrawal of lamotrigine. In 5 of 12 cases, the reason for initial lamotrigine discontinuation was skin rash. Four patients showed a Grade 1 skin rash and 1 patient showed a Grade 2 skin rash. None of the patients showed Stevens-Johnson syndrome. In 9 of 12 cases, a positive outcome following lamotrigine rechallenge was observed in psychiatric symptoms. In all cases with an initial skin rash, lamotrigine rechallenge was successful with no recurrence of the rash. In 3 of 12 cases, lamotrigine rechallenge was discontinued because of oral dyskinesia, action tremor, and liver dysfunction, respectively.
DISCUSSION
To our knowledge, few reports have described lamotrigine rechallenge for the treatment of bipolar disorder following skin rash. In case studies of single patients, reports are mixed. Buzan and Dubovsky10 reported an unsuccessful case of lamotrigine rechallenge, while Manfredi et al12 reported a successful case of lamotrigine rechallenge. In a larger study of a group of 27 patients with bipolar disorder, Aiken and Orr15 investigated the efficacy of lamotrigine rechallenge and reported a positive outcome in 21 cases. Another study16 of 10 patients with bipolar disorder undergoing rechallenge reported that 8 patients had a positive outcome. These 2 reports15,16 that included groups of patients support the use of lamotrigine rechallenge as a viable option for treatment-resistant bipolar disorder following a benign lamotrigine-induced rash, and they demonstrated that lamotrigine rechallenge should be avoided within 4 weeks of the initial rash.
In the present study, we investigated lamotrigine rechallenge in several patients with bipolar disorder that was resistant to treatments other than lamotrigine. These patients had discontinued their treatment owing to various adverse effects induced by lamotrigine, including skin rash. In all of the cases with initial skin rash, lamotrigine rechallenge with very slow titration of lamotrigine occurring for several weeks was successful and there were no recurrences of the rash.
In some cases (case 1 and case 4 in Table 1), maximum doses of lamotrigine in rechallenge were lower than those of lamotrigine in the first challenge; however, psychiatric symptoms were ameliorated in these cases. The reason for these results should be clarified in future studies. The present results suggest that lamotrigine rechallenge is a viable option for treatment-resistant bipolar disorder. However, the clinicians should consider the ethnicity of the patients because there might be different reactions of lamotrigine in other populations.
In 3 cases, lamotrigine rechallenge was unsuccessful. In 2 of them, patients experienced movement disorders such as oral dyskinesia and action tremor twice during the treatment of lamotrigine. This side effect is surprising as lamotrigine has rarely been linked to the development of movement disorders17; although, a few studies18 reporting on adverse effects following long-term use of lamotrigine report movement disorders among the symptoms experienced. Mackay et al18 reported that early in lamotrigine treatment, adverse effects experienced by epileptic patients included skin rash and Stevens-Johnson syndrome. Lamotrigine rechallenge may have unacceptable risk in patients who had previously had severe (Stevens-Johnson syndrome) rash with lamotrigine. However, after more than 6 months of treatment, adverse effects experienced included mood disorder, ataxia, visual blurring, and diplopia.18 Other reports19-21 demonstrated late neurologic disturbances induced by lamotrigine in epileptic patients, including ataxia, headache, oculogyric crises, involuntary eye blinking, and tic disorder. Therefore, clinicians should bear in mind the potential for movement disorders induced by lamotrigine, as seen in the present study.
This study was limited by its small number of subjects and use of a retrospective design. Future prospective studies with more subjects are necessary to draw more definitive conclusions.
Submitted: October 13, 2017; accepted January 18, 2018.
Published online: March 29, 2018.
Potential conflicts of interest: The authors declare no conflicts of interest.
Funding/support: No external funding was received in support of the present study.
REFERENCES
1. Reid JG, Gitlin MJ, Altshuler LL. Lamotrigine in psychiatric disorders. J Clin Psychiatry. 2013;74(7):675-684. PubMed CrossRef
2. Lamictal (lamotrigine) [prescribing information]. Brentford, UK: GlaxoSmithKline plc; 2016.
3. Barbee JG, Thompson TR, Jamhour NJ, et al. A double-blind placebo-controlled trial of lamotrigine as an antidepressant augmentation agent in treatment-refractory unipolar depression. J Clin Psychiatry. 2011;72(10):1405-1412. PubMed CrossRef
4. Terao T, Ishida A, Kimura T, et al. Preventive effects of lamotrigine in bipolar II versus bipolar I disorder. J Clin Psychiatry. 2017;78(8):e1000-e1005. PubMed CrossRef
5. Sharma V, Sommerdyk C. Lamotrigine in the prevention of bipolar II postpartum depression. Prim Care Companion CNS Disord. 2016;18(6):doi:10.4088/PCC.16l01964. PubMed CrossRef
6. Abe Y, Yasugawa S, Miyamoto K, et al. Valproate as a risk factor for lamotrigine discontinuation. J Affect Disord. 2013;150(3):1197-1199. PubMed CrossRef
7. Tak H-J, Ahn J-H, Kim K-W, et al. Rash in psychiatric and nonpsychiatric adolescent patients receiving lamotrigine in Korea: a retrospective cohort study. Psychiatry Investig. 2012;9(2):174-179. PubMed CrossRef
8. Saeki H, Yamada K, Morikawa N, et al. Severe drug eruptions due to lamotrigine in Japan based on data from the relief system of the Pharmaceuticals and Medical Devices Agency. Allergol Int. 2017;66(1):156-158. PubMed CrossRef
9. Tavernor SJ, Wong ICK, Newton R, et al. Rechallenge with lamotrigine after initial rash. Seizure. 1995;4(1):67-71. PubMed CrossRef
10. Buzan RD, Dubovsky SL. Recurrence of lamotrigine-associated rash with rechallenge. J Clin Psychiatry. 1998;59(2):87. PubMed CrossRef
11. Besag FMC, Ng GYT, Pool F. Successful re-introduction of lamotrigine after initial rash. Seizure. 2000;9(4):282-286. PubMed CrossRef
12. Manfredi G, Pacchiarotti I, Kotzalidis GD, et al. Successful rechallenge with slowly titrated lamotrigine after rash. Bipolar Disord. 2004;6(4):338-339. PubMed CrossRef
13. P-Codrea (Tigaran) S, Sidenius P, Dam M. Lamotrigine-induced rash—worth a rechallenge. Acta Neurol Scand. 2005;111(3):191-194. PubMed CrossRef
14. Lorberg B, Youssef NA, Bhagwagar Z. Lamotrigine-associated rash: to rechallenge or not to rechallenge? Int J Neuropsychopharmacol. 2009;12(2):257-265. PubMed CrossRef
15. Aiken CB, Orr C. Rechallenge with lamotrigine after a rash: a prospective case series and review of the literature. Psychiatry (Edgmont). 2010;7(5):27-32. PubMed
16. Serrani Azcurra DJL. Lamotrigine rechallenge after a skin rash: a combined study of open cases and a meta-analysis. Rev Psiquiatr Salud Ment (Barc.). 2013;6(4):144-149. PubMed CrossRef
17. Yang JH, Chung SW, Kim JS. Action tremor associated with lamotrigine monotherapy. J Mov Disord. 2010;3(1):18-19. PubMed CrossRef
18. Mackay FJ, Wilton LV, Pearce GL, et al. Safety of long-term lamotrigine in epilepsy. Epilepsia. 1997;38(8):881-886. PubMed CrossRef
19. Sotero de Menezes MA, Rho JM, Murphy P, et al. Lamotrigine-induced tic disorder: report of five pediatric cases. Epilepsia. 2000;41(7):862-867. PubMed CrossRef
20. Kim DG, Oh SH, Kim OJ. A case of lamotrigine-induced excessive involuntary eye blinking. J Clin Neurol. 2007;3(2):93-95. PubMed CrossRef
21. Thome-Souza S, Moreira B, Valente KD. Late adverse effects of the co-administration of valproate and lamotrigine. Pediatr Neurol. 2012;47(1):47-50. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!