Article Abstract
Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
Mr A is an 82-year-old married white man who presented with his family to the memory clinic at Banner Alzheimer’s Institute for evaluation and treatment of cognitive impairment. His family initially noted some problems with memory after he was rear-ended in a motor vehicle accident in 2001. It was unclear whether any loss of consciousness occurred at that time or whether a brain scan was performed in the emergency room.

CASE CONFERENCE
The Banner Alzheimer’s Institute Case Conference is a weekly event in which physicians and staff discus challenging and/or teaching cases of patients seen at the Institute’s Memory Disorders Clinic. These conferences are attended by a multidisciplinary group that includes Banner Alzheimer’s Institute dementia specialists, community physicians (internal medicine, family medicine, and radiology), physician assistants, social workers, nurses, medical students, residents, and fellows.
BANNER ALZHEIMER’ S INSTITUTE
The Banner Alzheimer’s Institute located in Phoenix, Arizona, has an unusually ambitious mission: to end Alzheimer’s disease without losing a generation, set a new standard of care for patients and families, and forge a model of collaboration in biomedical research. The Institute provides high-level care and treatment for patients affected by Alzheimer’s disease, dementia, and related disorders. In addition, the Institute offers extensive support services for families and many unique and rewarding research opportunities.
Prim Care Companion CNS Disord 2016;18(1):doi:10.4088/PCC.15alz01922
© Copyright 2016 Physicians Postgraduate Press, Inc.
Submitted: December 1, 2015; accepted December 17, 2015.
Published online: February 25, 2016.
AUTHORS
Anna D. Burke, MD, is a geriatric psychiatrist and dementia specialist at the Stead Family Memory Clinic of Banner Alzheimer’s Institute and a clinical assistant professor of psychiatry at the University of Arizona College of Medicine, Phoenix.
Garrett H. Riggs, MD, is a behavioral neurologist and dementia specialist at the Stead Family Memory Clinic of Banner Alzheimer’s Institute.
David A. Weidman, MD, is a neurologist and dementia specialist at the Stead Family Memory Clinic of Banner Alzheimer’s Institute.
Helle Brand, PA, is a physician assistant at the Stead Family Memory Clinic of Banner Alzheimer’s Institute.
William J. Burke, MD, is a geriatric psychiatrist and the director of the Stead Family Memory Clinic of Banner Alzheimer’s Institute and a research professor of psychiatry at the University of Arizona College of Medicine, Phoenix.
Corresponding author: Anna D. Burke, MD, Banner Alzheimer’s Institute, 901 E. Willetta St, Phoenix, AZ 85006 ([email protected]).
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. This special series of case reports about dementia was deemed valuable for educational purposes by the Publisher, Editor in Chief, and CME Institute Staff. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation.
CME Objective
After studying this article, you should be able to:
- Evaluate an elderly patient with cognitive impairment for dementia
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1.0 hour of Category I credit for completing this program.
Date of Original Release/Review
This educational activity is eligible for AMA PRA Category 1 Creditâ„¢ through February 28, 2018. The latest review of this material was February 2016.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Larry Culpepper, MD, MPH, Editor in Chief, has been a consultant for Forest, Lundbeck, Merck, Shire, Takeda, and Elsevier Press; has been a stock shareholder of M3 My Mood Monitor; and has received royalties from UpToDate and Oxford University Press. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
HISTORY OF PRESENTING ILLNESS
Mr A is an 82-year-old married white man who presented with his family to the memory clinic at Banner Alzheimer’s Institute for evaluation and treatment of cognitive impairment. His family initially noted some problems with memory after he was rear-ended in a motor vehicle accident in 2001. It was unclear whether any loss of consciousness occurred at that time or whether a brain scan was performed in the emergency room. His family reported that the doctors seemed most concerned about a potential cervical-spine injury. However, his wife reported that after the accident she began to notice her husband was “not as sharp” as he had been before. He forgot conversations and events and became lost while driving. He had occasional periods of confusion during conversations and difficulty organizing and completing more complex tasks. As a result of these symptoms, he underwent biofeedback sessions, which he felt were helpful in improving his cognitive symptoms. His wife reported that around that time, Mr A began to have more sleep disturbances including dream enactment, which he referred to as “restless legs syndrome.” His family confirmed that rather than feeling an urge to move his lower extremities, Mr A seemed to thrash and act out his dreams while sleeping.
The family reported that several years after the accident, Mr A began to display a steady slow decline in his cognitive abilities. His short-term memory worsened and more functional impairments in instrumental activities of daily living began to emerge. His family had discussed concerns regarding his memory problems with their primary care physician, who administered the Mini-Mental State Examination (MMSE; Folstein et al, 1975) and told Mr A that he was scoring below what would be expected. However, no formal diagnosis was made at that time. The primary care physician suggested that a medication such as donepezil might be helpful but did not start the therapy.
In addition to short-term memory problems, changes in stress tolerance had begun to gradually emerge. The family reported increasing irritability and occasional verbal agitation. There was no physical aggression. He also had developed paranoid ideations regarding financial matters and fear of others stealing. No auditory or visual hallucinations were present. Mr A displayed increasing anxiety and sporadic tearfulness.
Mr A also had developed dizziness and orthostasis. Because of his ulcerative colitis, it was difficult to assess whether constipation had emerged. He reported changes in his taste and sense of smell. Decreased processing speed and physical agility were also reported. Mild tremors of both hands were present as was mild hypophonia. His writing had changed, becoming more tremulous and micrographic. Fluctuations in cognition were present with good days and bad days and appeared to have worsened over the past several years.
Mr A remained independent in personal hygiene and grooming. He reported no significant problems with zippers or buttons or other fine motor skills. He did have difficulty with use of certain household appliances such as the TV remote, telephone, microwave, and computer. He did not cook nor did he handle any household chores. His wife had managed these tasks for many years. Mr A’s wife also had taken over his medications, filling his pillbox regularly and reminding him to take the medications. He reported that without his wife’s help, he would frequently miss doses of his medications. He limited his driving to rural roads and low-traffic areas and times of day. He reported getting lost more often and was more easily distracted or confused on the road.
PAST MEDICAL HISTORY
Mr A had a history of ulcerative colitis, hypothyroidism, orthostasis, hypercholesterolemia, diverticulitis, and prostate cancer treated with radiation and hormone therapy. His surgical history was notable for cholecystectomy. He was taking metoprolol for an unclear cardiac condition described by the family as “a fast heart rate.” There was a questionable history of previous strokes or “mini strokes” reported by the family.
ALLERGIES
Mr A reported an allergy to penicillin. He also reported abnormal reactions to anesthesia and any “motion sickness or antinausea pills.” He had experienced akathisia while taking sleep aids.
MEDICATIONS
Mr A’s current medications included mesalamine 800 mg 3 times/d, atorvastatin 10 mg/d, paroxetine 15 mg/d, metoprolol 12 mg twice/d, levothyroxine 88 μg/d, memantine 10 mg/d, lecithin 1,200 mg/d, vitamin C, coenzyme Q10, fish oil, and melatonin. He was previously treated with caprylidene but was unable to tolerate the nutraceutical due to gastrointestinal distress.
SOCIAL HISTORY
Mr A had a high school education. He worked as a farmer for 60 years. He was retired and lived independently with his wife.
SUBSTANCE ABUSE HISTORY
Mr A denied any history of alcohol, tobacco, or illicit drug abuse.
Family History
Mr A’s mother and father had dementia of uncertain type. Mr A believed that both his parents suffered from strokes. There was no family history of Parkinson’s disease or mental illness.
On the basis of the information so far, do you think a major neurocognitive disorder is present?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Yes | 92% |
| B. No | 0% |
| C. Not enough information | 8% |
The majority of the participants felt that the functional and cognitive deficits present in the clinical history were sufficient to warrant a diagnosis of a major neurocognitive disorder. Several participants felt that a diagnosis could not be established without additional information from a physical and neurologic examination.
A. Evidence of significant cognitive decline from a previous level of performance in 1 or more areas of cognitive domains (complex attention, executive function, learning and memory, language, perceptual-motor, or social cognition) based on:The DSM-5 (American Psychiatric Association, 2013) defines a major neurocognitive disorder as follows:
- Concern of the individual, a knowledgeable informant, or the clinician that there has been a significant decline in cognitive function and
- Substantial impairment in cognitive performance, preferably documented by standardized neuropsychological testing or, in its absence, another quantified clinical assessment.
B. The cognitive deficits interfere with independence in everyday activities.
C. The cognitive deficits do not occur exclusively in the context of a delirium.
D. The cognitive deficits are not better explained by another mental disorder.
On the basis of the information so far, what would you expect to see on the neurologic examination?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Normal | 8% |
| B. Objective nonfocal neurologic findings (including frontal release signs) | 90% |
| C. Focal neurologic findings | 2% |
The majority of the participants present expected objective nonfocal neurologic findings such as frontal release signs to be present. Also, 8% believed the examination would be normal and 2% felt that due to the patient’s cardiac history and questionable history of mini stroke, objective focal neurologic findings might be present.
Different dementias may be associated with various physical examination findings. However, most often the physical examination is normal in the early stages. Some subtle general findings can include frontal release signs such as a positive snout, glabellar, or palmomental reflex (Links et al, 2010).
NEUROLOGIC EXAMINATION
The cranial nerve examination was notable for broken smooth pursuits and bilateral decreased hearing to finger rub. Hypomimia and hypophonia were present. Strength was symmetric. A rest tremor was present bilaterally in the hands, more on the right. Tone was increased in a paratonic fashion, but no lead pipe rigidity or cogwheeling was noted. Sensory screening demonstrated no localizing abnormalities. There was no ataxia. Deep tendon reflexes were symmetrically hypoactive throughout and absent in the ankles. Gait was shuffling, slow, and festinating with en bloc turning. Arm swing was decreased bilaterally.
PHYSICAL EXAMINATION
The physical examination was significant for mild kyphosis and psychomotor retardation evidenced by a slowing of gait and general movement as well as delayed response time when answering questions or formulating thoughts. Otherwise, the physical examination was unremarkable. Mr A’s blood pressure was 99/61 mm Hg, pulse was 58 bpm, and weight was 209.8 lb.
MENTAL STATUS EXAMINATION
Mr A was a well-groomed man displaying mild psychomotor slowing. He described his mood as “good.” His affect was constricted. His thought process was coherent, logical, and goal directed. He denied any suicidal or paranoid ideations or auditory or visual hallucinations. His insight was limited, and his fund of knowledge was decreased.
LABORATORY AND RADIOLOGY RESULTS
Laboratory studies including complete blood count with differential, a comprehensive metabolic panel, vitamin B12 level, thyroid-stimulating hormone level, and urinalysis and urine culture available at the time of the visit revealed no clinically significant abnormalities. A magnetic resonance image available at the time of the visit revealed moderate white matter disease primarily in the posterior and medial left occipital lobe and the right occipital lobe. Small to moderate foci of T2 signal hyperintensity were also noted in the posterior and high left frontal lobes. Hippocampal volumes were normal to subjective evaluation.
On the basis of the information so far, what would you expect the MMSE score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 26-30 | 0% |
| B. 21-25 | 85% |
| C. 16-20 | 15% |
| D. 11-15 | 0% |
| E. < 11 | 0% |
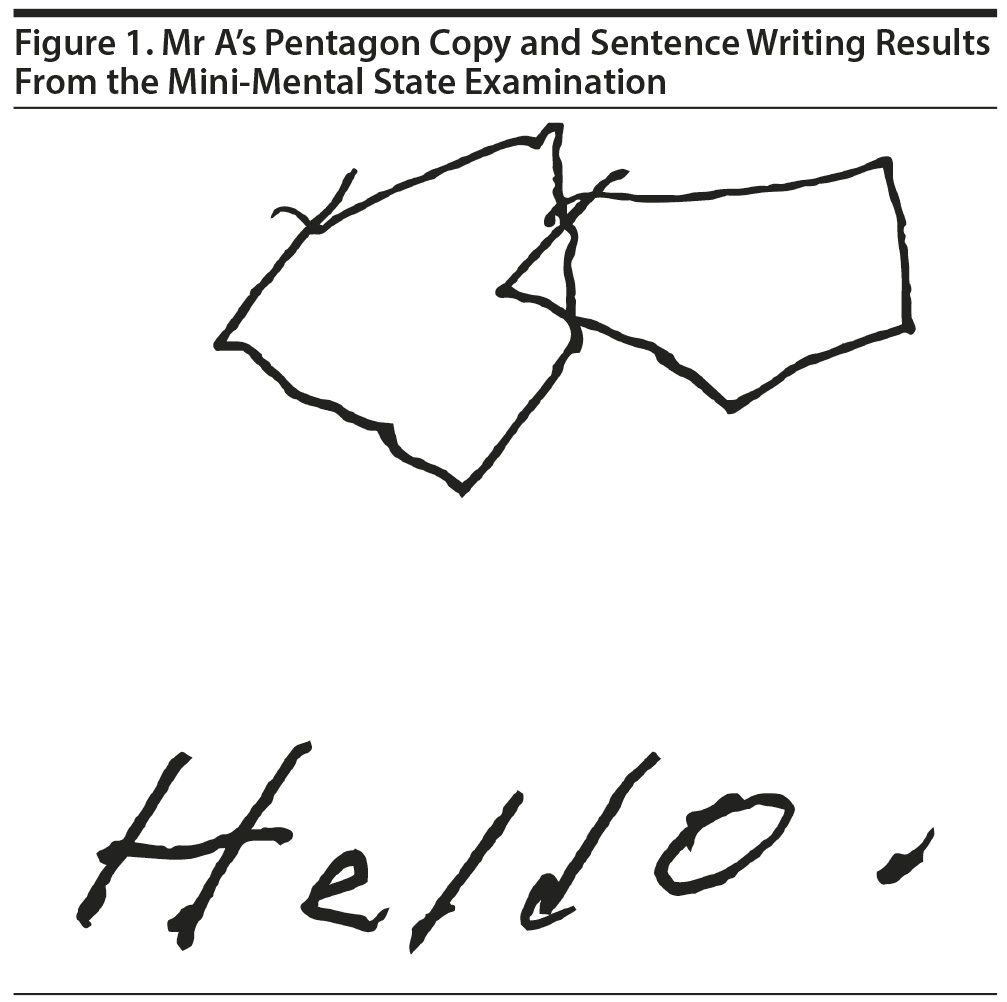
The majority of the participants believed that Mr A would fall within the range of 21 to 25 points on the MMSE. Mr A scored 23/30 on the MMSE, with impairments in orientation, delayed recall, pentagon copy, and sentence writing (Figure 1).
On the basis of the information so far, what would you expect the Montreal Cognitive Assessment (MoCA) score to be?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. 26-30 | 0% |
| B. 21-25 | 0% |
| C. 16-20 | 90% |
| D. 11-15 | 10% |
| E. < 11 | 0% |
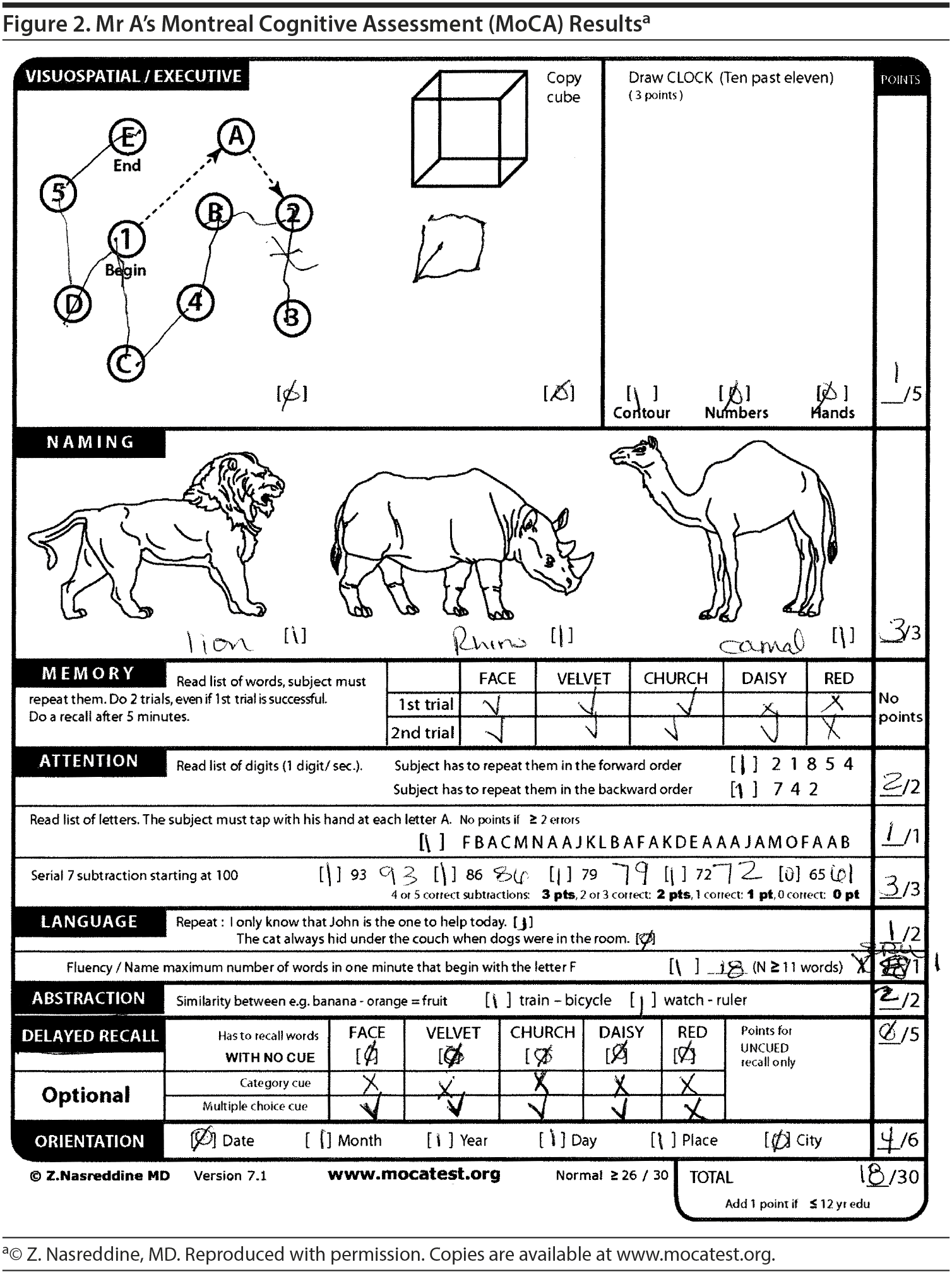
The majority of the participants believed that Mr A would fall within the range of 16 to 20 points on the MoCA. His MoCA test revealed a score of 18/30, with impairments not only in short-term memory abilities but also in executive function and visuospatial abilities. The pattern of recall (0/5 spontaneous recall, but 4/5 recognition recall) suggested a retrieval-based deficit rather than an amnestic (storage) deficit (Figure 2).
The Montreal Cognitive Assessment (MoCA) has been shown to have a better sensitivity and specificity in detecting more subtle cognitive impairments, such as mild cognitive impairment (MCI), when compared to the MMSE. Nasreddine et al (2005) found that the MMSE had a sensitivity of 18% to detect MCI, whereas the MoCA detected 90% of MCI subjects. In the mild Alzheimer’s disease group, the MMSE had a sensitivity of 78%, whereas the MoCA detected 100%. Specificity was excellent for both the MMSE and MoCA (100% and 87%, respectively).
On the basis of the information so far, what underlying etiologic subtype of major neurocognitive disorder is present?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Alzheimer’s disease | 0% |
| B. Frontotemporal lobar degeneration | 0% |
| C. Lewy body disease | 90% |
| D. Vascular disease | 0% |
| E. Traumatic brain injury | 0% |
| F. Parkinson’s disease | 5% |
| G. Due to another medical condition | 0% |
| H. Due to multiple etiologies | 5% |
The majority of participants felt that Mr A’s clinical presentation, including insidious onset of symptoms, fluctuating course, rapid eye movement sleep-behavior disturbances, parkinsonian symptoms, and affective lability, were most consistent with Lewy body disease. However, several participants questioned whether his symptoms may be related to an independent Parkinson’s disease pathology or to multiple causes including vascular pathology. Most participants felt it would be important to personally inspect neuroimaging data to maximize diagnostic information.
THE TREATING PHYSICIAN’ S IMPRESSION
On the basis of the history and clinical presentation as well as the results of the cognitive and physical examinations, the treating physician felt that Mr A had a major neurocognitive disorder of mild severity (per DSM-5 criteria). The physical examination revealed parkinsonian symptoms consistent with Lewy body pathology. Mr A also endorsed other clinical symptoms consistent with Lewy body disease including fluctuations in cognitive status, dream reenactment behaviors, sensitivities to multiple medications, hypophonia, autonomic dysfunction, and prominent visuospatial deficits on cognitive testing. However, Mr A’s neuroimaging revealed significant cerebrovascular intracranial pathology that cannot fully be excluded as a contributing factor but was not felt to be the sole cause of his symptoms. In the differential diagnosis, the physician included major neurocognitive disorder due to Lewy body disease versus a mixed Lewy body and vascular disease diagnosis. Further workup was ordered to help clarify the diagnosis.
Which of the following evaluations would you schedule next?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Neuropsychological testing | 100% |
| B. FDG-PET | 0% |
| C. Amyloid PET | 0% |
| D. DaT SPECT | 0% |
All of the participants felt that neuropsychological testing would yield the most benefit in establishing an etiology for Mr A’s symptoms as well as a pattern of cognitive strengths and weaknesses that could be helpful in nonpharmacologic management. The testing also could establish a baseline for future objective monitoring of the progression of illness.
THE TREATING PHYSICIAN’ S PLAN
The treating physician ordered neuropsychological testing to assess for the presence of a pattern of cognitive strengths and weaknesses that could help clarify the exact etiology of symptoms. This testing also would establish a baseline for future monitoring of progression of illness as well as help with nonpharmacologic treatment planning, utilizing the patient’s strengths and weaknesses.
What treatment would you start?
Your colleagues who attended the Banner Alzheimer’s Institute Case Conference answered as follows:
| A. Cholinesterase inhibitor | 70% |
| B. NMDA antagonist | 0% |
| C. Carbidopa/levodopa | 30% |
| D. SSRI | 0% |
The majority of the participants stated that they would initiate a cholinesterase inhibitor as first-line therapy. They felt that since Mr A most likely had Lewy body disease, his positive response to the medication may be more pronounced. Also, 30% of the participants would opt to start with carbidopa/levodopa since they felt that this could alleviate Mr A’s motor symptoms relatively quickly and perhaps improve apathy, mood, and motivation. The treating physician started a rivastigmine patch 4.6 mg/d. Although some physicians may choose to start both a cholinesterase inhibitor and carbidopa/levodopa at the same visit, we typically recommend the addition of one agent at a time and thorough assessment of tolerability and response. Individuals with dementia with Lewy bodies are prone to medication sensitivities. If several medications are started at once and side effects occur, it may be difficult to identify the offending agent.
Evidence from open-label studies (Van Der Putt et al, 2006) has indicated that patients with dementia with Lewy bodies and Parkinson’s disease dementia do better than those with other diagnoses, including Alzheimer’s disease. In addition, those with dementia of moderate severity do better than those with mild severity. Although this may seem counterintuitive, it was the finding of Van Der Putt et al (2006) that patients who were in the moderate stage of dementia with Lewy bodies had a more robust response to acetylcholinesterase inhibitors than those who were in the early and mild stages.
DISCUSSION
Dementia with Lewy bodies is the second most common form of major neurocognitive impairment seen by physicians. Dementia with Lewy bodies presents as a progressive dementia with pronounced deficits in attention and executive function. Unlike Alzheimer’s dementia, prominent memory impairment may not be evident in the early stages.
Other core features of the disease include fluctuating cognition with pronounced variations in attention and alertness; recurrent complex visual hallucinations, typically well-formed and detailed; and spontaneous features of parkinsonism. Suggestive features include rapid eye movement sleep-behavior disorder, which can appear years before the onset of dementia and parkinsonism; severe sensitivity to neuroleptics, which occurs in up to 50% of Lewy body dementia patients who take them (Baskys A, 2004); and low dopamine transporter uptake in the brain’s basal ganglia as seen on SPECT and PET imaging scans. Supportive features include repeated falls and syncope; transient, unexplained loss of consciousness; autonomic dysfunction; hallucinations of other senses such as touch or hearing; visuospatial abnormalities; and other psychiatric disturbances.
DRUG NAMES
Atorvastatin (Lipitor and others), donepezil (Aricept and others), levothyroxine (Synthroid, Tirosint, and others), memantine (Namenda), metoprolol (Lopressor and others), paroxetine (Paxil, Pexeva, and others), rivastigmine (Exelon and others).
Disclosure of off-label usage
The authors have determined that, to the best of their knowledge, rivastigmine and memantine are not approved by the US Food and Drug Administration for the treatment of dementia with Lewy bodies.
FUNDING/SUPPORT
None reported.
FINANCIAL DISCLOSURE
Drs Burke, Riggs, Weidman, and Burke and Ms Brand have no personal affiliations or financial relationships with any commercial interest to disclose relevant to the activity.
CASE CONFERENCE
The Banner Alzheimer’s Institute Case Conference is a weekly event in which physicians and staff discuss challenging and/or teaching cases of patients seen at the Institute’s Stead Family Memory Clinic. These conferences are attended by a multidisciplinary group that includes Banner Alzheimer’s Institute dementia specialists, community physicians (internal medicine, family medicine, and radiology), physician assistants, social workers, nurses, medical students, residents, and fellows.
DISCLAIMER
The opinions expressed are those of the authors, not of Banner Health or Physicians Postgraduate Press.
Clinical Points
- Currently, no therapies are approved by the US Food and Drug Administration for dementia with Lewy bodies, but studies indicate that the cognitive symptoms associated with this disorder may respond well to cholinesterase inhibitor therapy.
- Dementia with Lewy bodies is the second most common form of major neurocognitive impairment seen by physicians. Dementia with Lewy bodies presents as a progressive dementia with pronounced deficits in attention, executive function, visuospatial abilities, and memory that coincide with onset of extrapyramidal symptoms and autonomic dysfunction.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
References
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
Baskys A. Lewy body dementia: the litmus test for neuroleptic sensitivity and extrapyramidal symptoms. J Clin Psychiatry. 2004;65(suppl 11):16-22. PubMed
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed doi:10.1016/0022-3956(75)90026-6
Links KA, Merims D, Binns MA, et al. Prevalence of primitive reflexes and parkinsonian signs in dementia. Can J Neurol Sci. 2010;37(5):601-607. PubMed doi:10.1017/S0317167100010763
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. PubMed doi:10.1111/j.1532-5415.2005.53221.x
Van Der Putt R, Dineen C, Janes D, et al. Effectiveness of acetylcholinesterase inhibitors: diagnosis and severity as predictors of response in routine practice. Int J Geriatr Psychiatry. 2006;21(8):755-760. PubMed doi:10.1002/gps.1557
Enjoy this premium PDF as part of your membership benefits!