Schizophrenia is a chronic disorder that can be effectively controlled but will likely require lifelong treatment. Unfortunately, most patients experience numerous relapses, ongoing symptoms, and impairment, often because of treatment interruptions. Continuous treatment is imperative to prevent relapse and the potentially devastating consequences that can follow a return of psychotic symptoms. Clinicians must better monitor a common cause of relapse, nonadherence, and offer strategies to improve adherence. Strategies can target the patient, the environment, or treatment. One treatment-related strategy is the use of long-acting injectable (LAI) antipsychotics, which have the potential to reduce relapse and rehospitalization for many patients with schizophrenia.

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation.
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the planning teleconference series “Managing Transitions in Care and Adherence to Improve Outcomes in Schizophrenia,” which was held in July and August 2018. This report was prepared and independently developed by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by an educational grant from Alkermes, Inc.
CME Objective
After studying this article, you should be able to:
- Ensure that patients receive continuous, evidence-based treatment for schizophrenia as well as medical care
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Release, Expiration, and Review Dates
This educational activity was published in February 2019 and is eligible for AMA PRA Category 1 Creditâ„¢ through February 28, 2021. The latest review of this material was January 2019.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
J Clin Psychiatry 2019;80(1):al18010ah2c
To cite: Lauriello J, Perkins DO. Enhancing the treatment of patients with schizophrenia through continuous care. J Clin Psychiatry. 2019;80(1):al18010ah2c
To share: https://doi.org/10.4088/JCP.al18010ah2c
© Copyright 2019 Physicians Postgraduate Press, Inc.
Notice of correction 3/26/19: On page 25, in the “Pharmacologic Advantages of LAIs” section, formulation distinctions are now made between aripiprazole monohydrate and aripiprazole lauroxil, and on page 26, the fourth and fifth bullet points now describe supplementation distinctions between aripiprazole monohydrate and aripiprazole lauroxil. In Table 2, corrections were made to the time to peak for aripiprazole monohydrate and to the injection frequency for aripiprazole lauroxil.
Schizophrenia is a lifelong disorder, and the possibility of recovery is largely dependent on the successful prevention of relapses. Relapses can have serious consequences, such as damaging relationships, interrupting education or employment, and even harming oneself or others.1 Currently, the best strategy for preventing relapse is ensuring continuous maintenance treatment. In this Academic Highlights, Drs John Lauriello and Diana O. Perkins review evidence on the dangers of treatment interruptions, explain causes of treatment interruptions such as nonadherence, and offer strategies for enhancing treatment adherence to improve outcomes.
THE CASE FOR CONTINUOUS TREATMENT
Antipsychotic medications are effective for ongoing control of psychotic symptoms,2 but these medications are only successful if taken regularly. Even very short gaps in treatment increase the likelihood of relapse.3 For example, Subotnik and colleagues3 conducted a longitudinal study assessing the effect of treatment interruptions on the return of positive symptoms in 49 patients with recent-onset schizophrenia. Adherence to treatment was assessed through pill counts, plasma levels, patient reports, and clinician assessment. The investigators found that even brief periods of partial adherence, defined as missing 50%-75% of the prescribed dosage for 2 weeks, increased a patient’s risk of psychotic relapse nearly 6-fold (P < .04).3 Thus, ensuring continuous antipsychotic treatment is crucial to achieving and maintaining recovery.
Causes of Treatment Interruptions
Several factors can lead to an interruption in a patient’s treatment. These can include difficulties accessing treatment due to financial, geographic, or even transportation-related barriers.4 Interruptions can also occur due to transfers from inpatient to outpatient care, release from incarceration, or a change in providers. Nonadherence, however, is perhaps the leading cause of treatment interruptions.
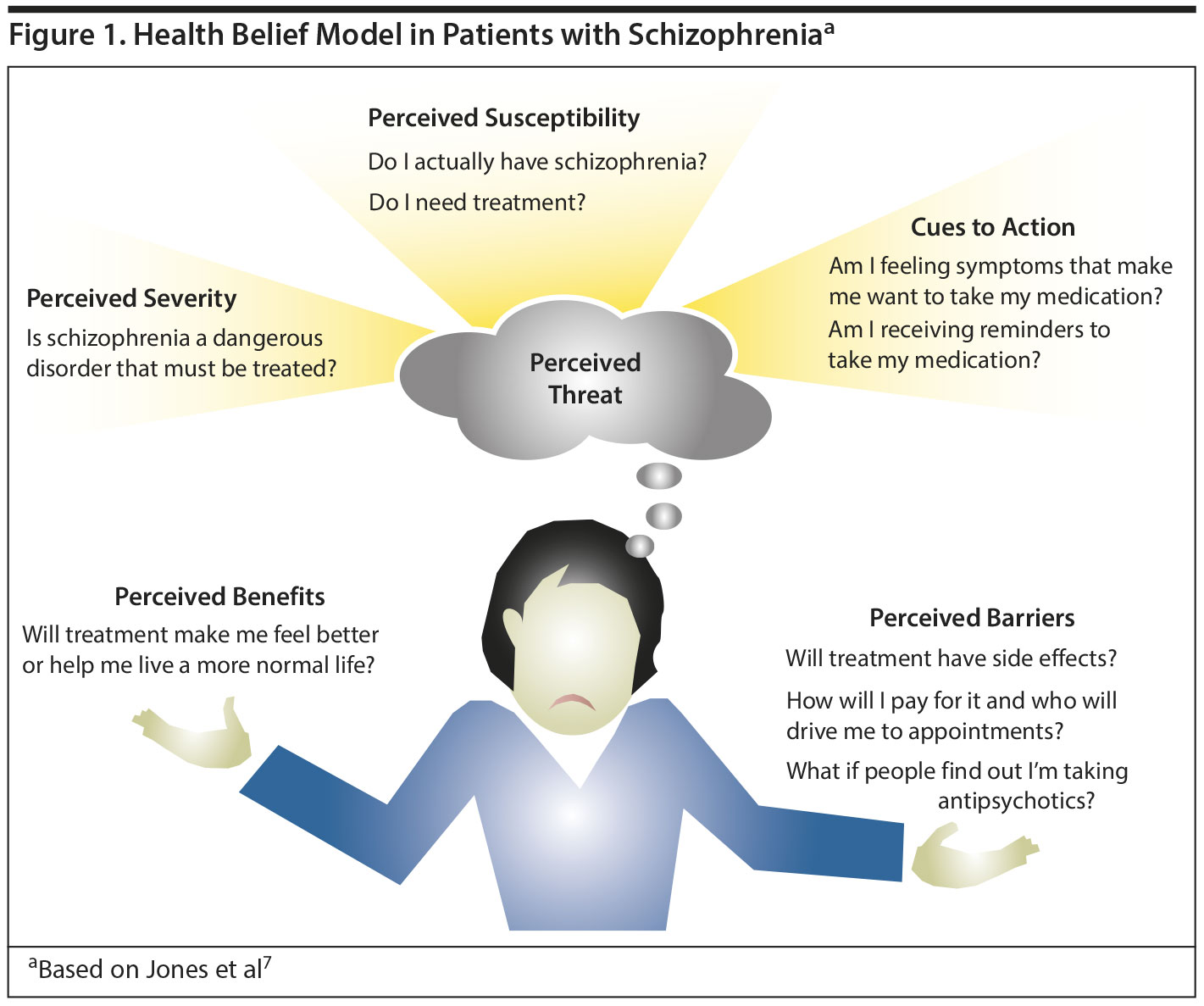
Treatment adherence is poor in a number of conditions,5 particularly chronic medical and psychiatric diseases including schizophrenia. Dr Perkins explained that the Health Belief Model (HBM) as described by Rosenstock6 provides a useful conceptual framework for understanding patients’ decision-making regarding whether or not to adhere to treatment for schizophrenia (Figure 1). According to the HBM,6,7 patients accept treatment when they believe they have a disorder (1) that is sufficiently severe to warrant treatment (perceived threat), (2) that the treatment addresses the disorder (perceived benefit), and (3) that the benefits of treatment outweigh the risks (perceived barriers).
Nonadherence can stem from a breakdown at any point in the HBM. For example, during acute episodes, patients with active psychosis often have difficulty perceiving the threat of their illness because they may have poor insight and either do not perceive themselves as ill or they minimize the severity. Patients may also have difficulty perceiving the threat of their illness once their acute episode has resolved and they have begun maintenance treatment because during acute episodes, the distressing symptoms are a reminder about the need for medication, and, thus, an intrinsic cue to action. In contrast, once the patient has transitioned to maintenance antipsychotics, symptoms may be reduced or remitted and, therefore, no longer serve as a reminder to take medication. The patient will need to develop other reasons for continuing to take the medication, such as relapse prevention, but a patient, particularly one who has just recovered from a first episode, may have difficulty perceiving relapse as a threat and understanding that medication is effective in reducing relapse risk. This misperception is because patients with schizophrenia may lack insight into their disorder or knowledge of schizophrenia (causes, symptoms, diagnosis, course, treatments, and self-management), which has been found to contribute to poor adherence and disengagement from treatment.8
According to Dr Perkins, in some instances, patients in an acute psychotic episode may be experiencing active symptoms such as psychosis, anxiety, or sleep difficulty, and may want treatment but have difficulty believing the treatment will benefit them. In other cases, patients fully acknowledge the benefits of and need for treatment, but they may still be concerned that the risks outweigh the benefits. Two of the most frequently cited reasons for discontinuing antipsychotic medications are inadequate response and the experience of side effects.4 Clearly, when patients feel that they are not responding to treatment, or treatment is causing more harm than good, they will be less inclined to continue. In addition, patients may encounter practical barriers to medication adherence, such as medication costs or lack of transportation to the pharmacy or to the clinic.4
Following is the personal account of a patient who struggled with adhering to treatment due to lack of efficacy and unpleasant side effects (oral communication, August 2018).
 Patient Perspectives
Patient Perspectives
“Sarah”: “[From] 2003 through 2007, I stopped taking medication and I didn’ t see my psychiatrist either. . . . And I mean I was psychotic, you know, but I was able to work like part-time, so I just kept on trying and fighting it because I didn’ t want to go on disability. Because nothing I took seemed to work, so you know, back in 1998, 1999, the doctor put me on [olanzapine] and I gained like 20, 30 pounds in 3 weeks, and then . . . he put me on [quetiapine] and [sertraline]. He thought I had bipolar and OCD . . . and it just seemed like no matter what dose—and I didn’ t want to take too much because I wanted to be able to still work—but it seemed like whatever dosage I took . . . it didn’ t alleviate the symptoms . . . and, God, I mean, I hate the weight gain!”
 Case Practice Question
Case Practice Question
Case 1. Martin is a 21-year-old college student who was hospitalized after disrobing on campus in response to command hallucinations that the safety of the world depended on his becoming “one with nature.” He began an oral antipsychotic, his psychosis remitted, and he returned to college the following year. At a recent visit, you ask Martin about missing any doses of medication, and he admits to missing about 25%-50% of his doses most days, and sometimes not taking medication at all on weekends. Does Martin’s partial adherence influence his risk of psychosis relapse?
- Yes, missing 50%-75% of medication over a period of 2 or more weeks increases relapse risk 6-fold compared to adherent patients.
- Yes, missing even 1 dose of antipsychotic doubles the risk of relapse compared to adherent patients.
- No, relapse risk increases when patients cease using antipsychotics for 2 or more weeks.
- No, antipsychotics have little benefit in preventing psychotic relapse.
 Discussion of Case Practice Question
Discussion of Case Practice Question
Preferred response is (a)
Yes, missing 50%-75% of medication over a period of 2 or more weeks increases relapse risk 6-fold compared to adherent patients.
Studies such as the one conducted by Subotnik and colleagues3 have discovered that even relatively brief periods of partial nonadherence will significantly increase a patient’s risk of relapse. In other words, if patients who are prescribed a twice-daily antipsychotic regularly forget either the morning or evening dose for a period of just 2 weeks, they are 6 times more likely to experience a psychotic relapse.
STRATEGIES FOR ENSURING CONTINUOUS TREATMENT
Because nonadherence is a multifaceted problem, this issue will need to be addressed in multiple contexts. Clinicians can take steps engaging directly with the patient or indirectly with their family and their physical environment to improve treatment adherence and continuity.
Strategies for Accurately Assessing Adherence
Before deciding the strategies that are most likely to ensure continuous treatment for individual patients, clinicians must determine each patient’s actual adherence pattern. Debate has long existed over the best methods for assessing adherence. Self-report, family member reports, prescription refills, and pill counts, each have their potential flaws and pitfalls.4,9 A practical and reasonably effective option for evaluating adherence is for the clinician to systematically ask patients a few simple, straightforward questions, such as how much medication they are supposed to take each day, how many days over the past month they did not take any medication, and how many days over the past month did they take less than their prescribed amount of medication. One study10 found that clinician estimates of adherence based on answers to questions similar to these were comparable to those obtained through electronic monitoring.
Patient-Centered Strategies
A critical component of patient-centered strategies for improving adherence is adopting a collaborative treatment model. Dr Perkins pointed out that medical management models fall along a continuum with a medical-, clinician-driven model at one end and a collaborative model, in which the clinician and patient share decision-making, at the other. The medical model is appropriate in straightforward conditions, such as bacterial pneumonia or a gunshot wound, but in conditions such as schizophrenia, in which the optimal intervention is not as clear and the relative importance of potential risks and benefits varies between people, the collaborative model is more appropriate. This model allows clinicians and patients to discuss problems specific to each patient and work together to determine the best strategies for the patient to obtain mastery over his or her illness.
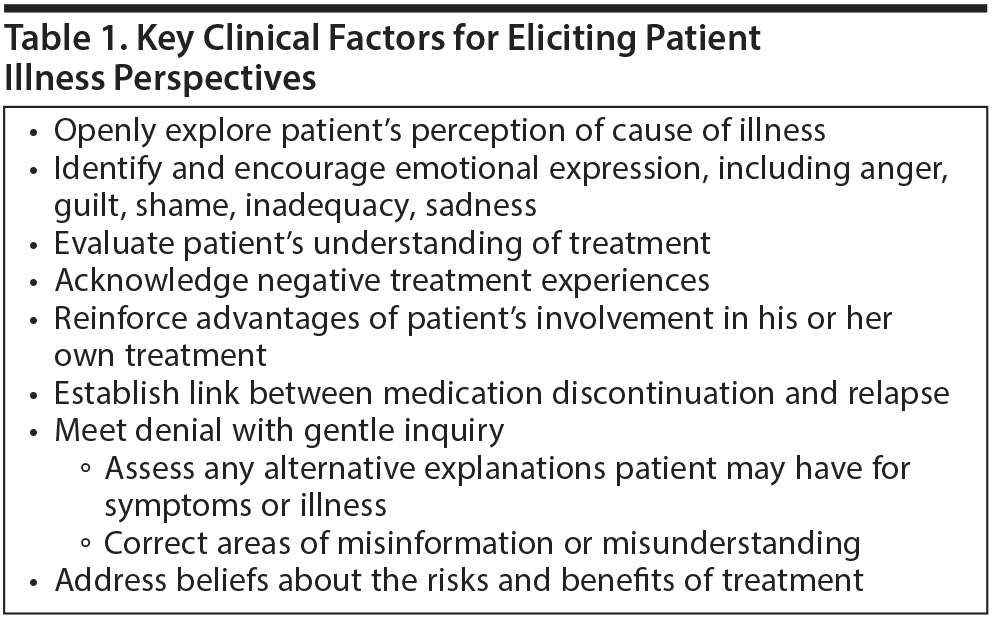
Dr Perkins discussed ways in which clinicians can use behavioral and educational strategies to address patients’ attitudes and beliefs about the use of antipsychotic medications and the role these strategies play in engaging patients in the recovery process. For example, various forms of cognitive-behavioral therapy have been found to help patients with psychosis adapt to their illness and improve their insight, attitudes toward treatment, and adherence.11 Motivational interviewing can be used to emphasize patients’ personal choice and responsibility for their illness, thereby enhancing adherence.4 The likelihood of adherence improves when clinicians take a nonjudgmental and empathetic stance and seek to elicit patients’ concerns and beliefs about medication. Dr Perkins emphasized a number of additional key factors that may improve patient engagement (Table 1), especially understanding the patients’ perspective on and prior experience with their illness and treatment, particularly negative experiences.
Psychoeducation is also a critical component of schizophrenia treatment. Through psychoeducation, patients gain a better understanding of their illness and learn about available treatments and their side effects.4 The families and caregivers of patients with schizophrenia can also greatly benefit from psychoeducation, which can improve adherence by helping patients accept the necessity of long-term treatment. One study12 followed a group of 41 patients with schizophrenia for 7 years. All participants were encouraged to continue maintenance antipsychotic treatment, and some of the patients were randomly assigned to also receive psychoeducation. After 7 years, compliance in both groups was rated as “good” or “very good,” but the group that received psychoeducation had experienced a better course of illness and significantly lower hospitalization rates (74.7 days vs 243.4 days; P < .03).12
Following is the account of an actual patient with schizophrenia discussing the ways in which her physician’s collaborative approach encouraged her to adhere to treatment (oral communication, August 2018).
 Patient Perspectives
Patient Perspectives
“Gina”: “I think one thing that really impacted my treatment adherence is the doctor’s bedside manner. Like, if I feel they’ re on my team, then it makes me . . . trust them more and I open up to them more and, like, it’s easier to have a conversation about my health versus if they’ re really, like, bossy. . . . [S]o many, so my favorite [doctors] are the ones where I feel like there’s a good rapport and that they care about my whole experience, not just prescribing medicines for symptoms. They try to kind of get to the root of the cause of today: I forgot my medicine pill—they suggest that I do something to improve that, or if my cholesterol is a little bit high, they don’ t just say, okay, well take this pill for that. They say, well, you can make lifestyle changes. So they kind of see the big picture.”
Environment-Centered Strategies
Cognitive impairments are a core feature of schizophrenia that are often evident before the onset of illness and persist even when psychosis remits.13 Among patients with schizophrenia, those with more severe cognitive impairments are more likely to be nonadherent to treatment.14 Environment-centered treatment strategies are particularly effective for combating nonadherence that stems from cognitive impairments. Environmental support such as signs, checklists, pill boxes, or special arrangements of belongings can be used to help patients with schizophrenia and cognitive difficulties remember to take medications, refill prescriptions, or go to appointments.15 Environmental supports such as these have been found to improve not only treatment adherence but also functioning in general.15
Treatment-Centered Strategies: Focus on LAIs
Clinicians may be able to ensure continuous treatment by modifying a patient’s medication regimen. Simple steps such as simplifying the dosing schedule, adjusting the dose, treating side effects, or switching to a more effective agent can make patients more likely to continue taking their medications.4 Perhaps the most reliable way to ensure a patient is receiving continuous treatment is to switch the patient to a long-acting injectable (LAI) antipsychotic formulation.
Interpersonal advantages of LAIs. Monitoring adherence to oral medication is extremely difficult, and definitions of what constitutes nonadherence vary, as do ideas about the best methods for monitoring adherence.4 The use of LAIs eliminates this uncertainty because clinicians know immediately if patients are adherent based on whether they come for their follow-up injections. This increased certainty is also beneficial for families and caregivers because it can reduce conflicts over taking medications or discussions over compliance issues. As a result, family relations can become more stable and less confrontational.16
Pharmacologic advantages of LAIs. All antipsychotic drugs have short plasma half-lives, which means that oral formulations require frequent dosing to maintain therapeutic effect.17 Injectable formulations eliminate the need for daily dosing while also maintaining a more steady plasma level than can be achieved through oral medications.18 Before considering the pharmacokinetics of LAIs, Dr Lauriello stated that the formulations of these treatments differ, particularly in regard to the carrier in which the drug is suspended, and is an important consideration when prescribing. Both first- and second-generation antipsychotics are available in LAI formulations.17 To create depot formulations of first-generation antipsychotics (FGAs), agents such as haloperidol and fluphenazine were able to be converted into esters that were highly soluble in oil. Thus, FGA injections consisted of active drug esters dissolved in a vegetable oil that were then injected intramuscularly. The oil formed a depot that slowly released the drug into the bloodstream. However, Dr Lauriello pointed out that these injections could be quite painful because the oil from the injection would take up space within the patient’s muscle and be treated as a foreign object by the body, resulting in soreness and swelling.19
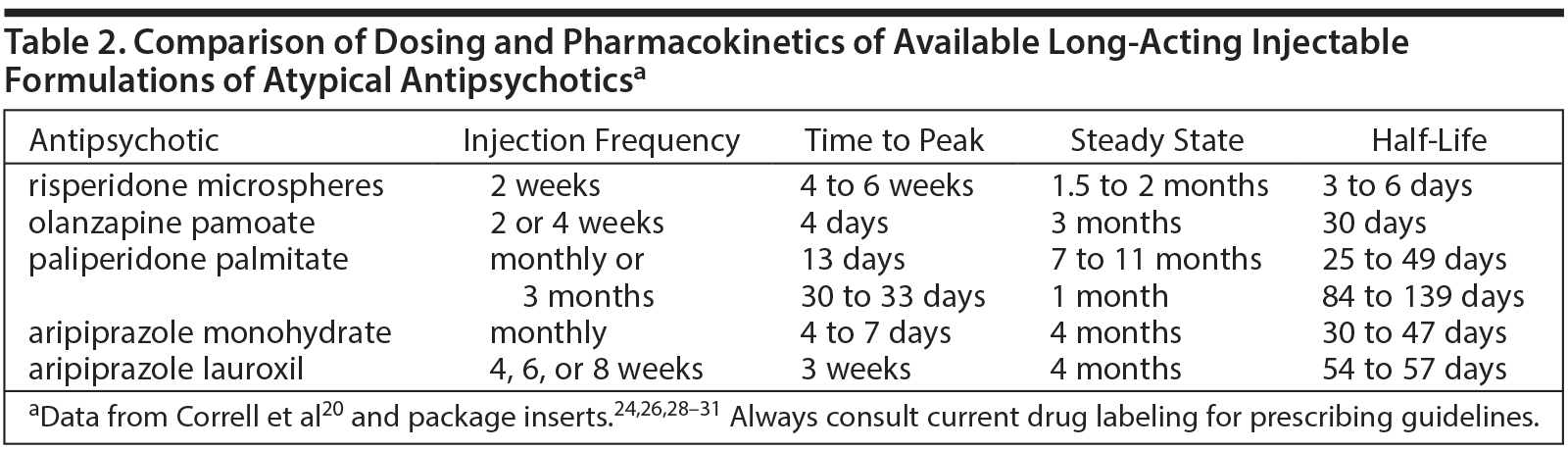
Second-generation antipsychotics (SGAs) cannot be suspended in oil; therefore, these agents had to be modified through different means to create long-acting formulations. All injectable formulations of SGAs use water-based formulations and are less painful than their oil-based counterparts.20 For risperidone LAI (risperidone microspheres), the active drug is contained in microspheres made of a copolymer that, after being injected intramuscularly, slowly breaks down and releases the medication over several weeks.21 Olanzapine LAI (olanzapine pamoate) consists of a combination of olanzapine and the salt of pamoic acid. After intramuscular injection, the olanzapine slowly dissociates from the salt and is gradually released into the bloodstream over several weeks.22 Paliperidone LAI (paliperidone palmitate) is contained in particle-sized esters that slowly dissolve and are hydrolyzed, releasing the active drug into circulation.23 Aripiprazole is provided in 2 different LAI drugs, aripiprazole monohydrate and aripiprazole lauroxil, which have different doses and dosing schedules. Aripiprazole monohydrate is a lyophilized powder; absorption into systemic circulation is prolonged due to the low solubility of the aripiprazole particles.24 Aripiprazole lauroxil contains a nonester prodrug of aripiprazole that is attached to a fatty acid tail, which serves to lower solubility and extend systemic release.25 The SGA LAIs are listed in Table 2 and described below.
- Risperidone microspheres. After an initial injection of risperidone, the main release of the drug begins after a 3-week delay, thus requiring oral supplementation during the first 3 weeks of treatment. Subsequent injections should be given every 2 weeks, and steady-state plasma levels are reached after 4 injections and continue for 4 to 6 weeks after the final injection.26
- Olanzapine pamoate. Olanzapine LAI reaches peak plasma levels 2 to 4 days after injection, and, therefore, no oral supplementation is required.20 Additional injections are required every 2 to 4 weeks with steady-state plasma levels reached after 3 months. Clinically relevant levels of olanzapine may remain up to 3 months after the last injection.27
- Paliperidone palmitate. Paliperidone palmitate is available in 2 formulations, one of which is given monthly and the other is given every 3 months. Both formulations begin to release the active drug within a day of injection, eliminating the need for oral supplementation. The once-monthly injection reaches peak plasma concentrations after approximately 2 weeks and therapeutic levels will remain in circulation for 3 to 5 months after the final injection,28 whereas the every-3-month formulation takes about a month to reach peak plasma levels and therapeutic levels may remain in the body for 11 to 12 months following deltoid injection and 15 to 18 months following gluteal injection.29
- Aripiprazole monohydrate. Supplementation with oral aripiprazole is required for 2 weeks after the first injection of aripiprazole monohydrate.24 Aripiprazole monohydrate requires monthly injections. Plasma concentrations peak 4 to 7 days after injection, steady-state levels are achieved after approximately 4 months, and the drug remains in the body for 4 to 6 months after the final injection.24
- Aripiprazole lauroxil. Aripiprazole lauroxil comes in different strengths that can be administered every 4, 6, or 8 weeks.30 When initiating aripiprazole lauroxil, clinicians have 2 options for supplementation. If oral aripiprazole supplementation is used, it is required for 3 weeks after the first injection of aripiprazole lauroxil.30 The other option is supplementation with a faster-acting formulation of aripiprazole lauroxil that is now available, and this initial injection is to be used along with a single 30-mg oral dose of aripiprazole and the first injection of the maintenance lauroxil formulation.31 The first injection of the maintenance formulation of aripiprazole lauroxil can be administered either at the same time as the initial, fast-acting injection and the single oral dose or up to 10 days later.31
The LAIs offer a much simpler dosing regimen than oral antipsychotics, with injections every 2 weeks to every 3 months, depending on the agent, which is a much easier medication schedule for patients with schizophrenia to maintain. Furthermore, the steady plasma levels provided by these long-acting formulations may provide a more reliable therapeutic effect. Thus, 2 of the main reasons that patients with schizophrenia become nonadherent to medication—difficulty remembering and lack of efficacy—may both be remedied with LAI formulations.
Advantages of LAIs in patients with comorbid substance abuse and/or a history of incarceration. Substance abuse is common in patients with schizophrenia and is associated with a number of poor outcomes including nonadherence, relapse, and involvement with the criminal justice system.32 Because patients with these types of complex problems are frequently excluded from drug trials, the Paliperidone Palmitate Research in Demonstrating Effectiveness (PRIDE) study32 was designed to compare LAI paliperidone with oral antipsychotics in real-world treatment settings. The 444 participants were recruited from across the United States and all had to have had a relatively recent history of incarceration. Patients were randomly assigned to receive either a once-monthly injection of paliperidone palmitate or 1 of 7 oral antipsychotics.
Over the 15 months of the study period, 39.8% of the paliperidone palmitate group and 53.7% of the oral antipsychotic group experienced a treatment failure, which was most commonly either an arrest/incarceration or psychiatric hospitalization. Paliperidone palmitate was significantly superior to oral antipsychotics in delaying the time to first hospitalization or arrest/incarceration (P = .019).32 The median time until first treatment failure among the patients receiving treatment with paliperidone palmitate was 190 days longer than that of the patients receiving oral antipsychotics. This finding is particularly remarkable because about 60% of study participants had comorbid substance abuse.33 Additionally, studies34,35 of LAI risperidone in patients with schizophrenia and comorbid substance abuse have found that use of this agent is associated with reductions in substance use and improvement in schizophrenia symptoms.35 LAI formulations may, therefore, be a helpful treatment option in this difficult-to-treat population.
 Case Practice Question
Case Practice Question
Case 2. Carl is a 56-year-old man who is about to be released from prison after having served 12 years for robbery and assault charges. Carl was diagnosed with schizophrenia at age 19 and, since his diagnosis, his illness has never been well controlled, resulting in numerous hospitalizations and arrests. His parents, who are now quite elderly, are reluctant to allow Carl back into their home due to his past difficulties adhering to treatment and controlling his symptoms and behavior. The past 12 years, while he has been in prison, have been the longest period during which Carl has remained adherent to treatment and, as a result, he feels better than he has since he was diagnosed. He wants to remain well but is concerned that he will not be able to keep up with his oral medications after he is released. His doctor recommends that he begin long-acting injections of his atypical antipsychotic, but Carl is resistant because during one of his earliest hospitalizations he received long-acting injections of fluphenazine and he still remembers how painful they were. He says that the pain lasted for hours and sometimes days. Carl is also reluctant because his current treatment is working and he says, “If it isn’ t broken, don’ t fix it.”
- Newer atypical LAIs require less frequent injections than older LAIs such as fluphenazine.
- Atypical LAIs are formulated differently than older injectable antipsychotics, which causes the injections to be less painful.
- The pharmacokinetics of LAI treatments mirror those of the oral medications Carl has had success with; therefore, he should notice no difference in treatment effect between the oral and the injectable formulations of his antipsychotic.
- Adherence to LAI atypical antipsychotic treatment is easy to maintain and monitor, which reduces interpersonal conflict and may make his parents more willing to let him move home.
 Discussion of Case Practice Question
Discussion of Case Practice Question
Preferred response is (c)
The pharmacokinetics of LAI treatments mirror those of the oral medications Carl has had success with; therefore, he should notice no difference in treatment effect between the oral and the injectable formulations of his antipsychotic.
Many valid arguments exist in support of Carl’s use of LAI antipsychotics upon release from prison, including responses a, b, and d. The frequency of atypical antipsychotic injections varies from every 2 weeks up to every 3 months, unlike the older injections that sometimes had to be administered weekly. Furthermore, the water-based formulations of atypical LAIs are far less painful than first-generation LAIs, which were oil-based and caused pain due to the space they occupied in the muscle. Finally, because monitoring adherence to injectable treatments is simple and straightforward, the struggle to take medication that frequently occurs between patients and caregivers is dramatically reduced. Option c is not a valid argument because the pharmacokinetics of LAI antipsychotics are quite different from oral formulations, but this difference is usually positive because it results in a more steady plasma level and a more level and consistent therapeutic effect.
Disadvantages of LAIs. Although the use of LAIs confers numerous benefits, several potential disadvantages of these agents must also be considered. Some providers, families, and patients may have difficulty accepting this form of treatment.36 According to Dr Lauriello, families are likely to be the most accepting of it because they see the utility of guaranteed delivery. Patients have been found to have the most negative attitudes toward LAI treatment compared with physicians and their family members because they fear the pain of the injections and the loss of their autonomy.36 Indeed, LAI antipsychotics are unfortunately associated with a great deal of stigma, and many clinicians and patients lack knowledge of these treatment options.20 Dr Perkins pointed out that clinicians may be reluctant to prescribe LAIs due to practical reasons such as a lack of resources in their practice to administer the injections, or because differences in the way that insurance plans cover oral vs injectable formulations.
 Clinical Points
Clinical Points
- Routinely inquire about adherence as even a few missed doses over a period of a couple of weeks greatly increase a patient’s risk of relapse.
- Provide psychotherapeutic approaches such as motivational interviewing and psychoeducation to improve treatment engagement and adherence.
- Use environmental modifications such as pill boxes and alarms as reminders to improve adherence with maintenance antipsychotic treatment.
- Use a collaborative treatment model to involve patients in decision making and encourage self-monitoring strategies.
- Consider pharmacology of a particular drug, such as oral vs oil-based LAI vs water-based LAI, when selecting the best agent for a particular patient.
- Keep medication regimens as simple as possible.
- Include discussion of the option for a LAI for any patient on maintenance antipsychotic treatment.
- Keep in mind that injectable antipsychotics may have added benefit in certain patient populations, including those with a history of substance abuse or incarceration.
- Stay informed about emerging delivery systems because many agents currently being developed hold great promise for improving treatment outcomes.
In regard to most side effects, the LAI versions of antipsychotics are associated with side effects similar to oral formulations,20 but because of their delivery route, physical injection site complications are possible with LAIs. A risk exists for postinjection delirium/sedation syndrome (PDSS) with olanzapine pamoate if the agent is inadvertently injected intravascularly, which leads to the drug’s being released too quickly, mimicking an overdose.22 For this reason, all patients receiving an injection of olanzapine pamoate must be observed for 3 hours. As the risk of PDSS illustrates, another disadvantage of LAIs is that the dose cannot be easily changed. Dr Lauriello pointed out that dose increases can be achieved by adding an oral agent, but dose reductions cannot be achieved quickly.
Addressing barriers to the use of LAIs. For many patients, the benefits of LAI treatment will outweigh the disadvantages, but these barriers must still be addressed. Negative perceptions of LAIs that may be held by patients, families, or clinicians should be addressed through education because perceptions of LAIs have been found to improve as knowledge of these agents increases.20 Clinicians facing practical barriers to prescribing LAIs should consider using specialty pharmacies to administer injections. Most states allow pharmacies to administer LAIs, which allows clinicians to prescribe these agents while keeping costs down by avoiding both the need to hire personnel to administer injections and the costs that can be incurred through buy-and-bill reimbursement programs.37
Patients who are resistant to LAI treatment due to fears of injection pain can be offered a topical anesthetic such as lidocaine, which, when applied an hour prior to receiving an antipsychotic injection, has been found to greatly reduce injection-site pain.38
 Case Practice Question
Case Practice Question
Case 3. Jim is 18 years old and you have been treating him for schizophrenia for the past 3 years. His psychosis has been in remission for the past year, and he has been accepted to a university about 2 hours away where he wants to live in the dorm. Jim’s parents are supportive of this decision. Jim states he wants to continue taking medication to avoid relapse. He is interested in a LAI formulation. He wants to continue treatment with you, but your office does not have mechanisms in place to perform medication injections. What is the best option to ensure Jim receives continuous, effective treatment that he will find acceptable?
- You could advise Jim that although you cannot provide LAI treatment, the known benefits of continuing your therapeutic relationship will outweigh the potential benefits of switching to a new provider to obtain access to LAIs.
- You could work with Jim and his family to determine whether any local pharmacies or primary care providers would administer LAI formulations on your prescription.
- Jim could self-administer the long-acting injectable formulation.
- One of Jim’s parents could administer the injection.
 Discussion of Case Practice Question
Discussion of Case Practice Question
Preferred response is (b)
You could work with Jim and his family to determine whether any local pharmacies or primary care providers would administer LAI formulations on your prescription.
Laws concerning who may administer long-acting antipsychotic injections vary by state, and you could assist Jim and his family in determining the laws that apply to the state where his university is located. Most states will allow pharmacists to administer the injection; the physician must simply write the prescription and monitor the response just as with oral medications. If this is not an option, Jim may be able to receive his injection from a primary care clinic or the student health center at his university using a prescription that you have written. Unfortunately, because LAIs have to be injected intramuscularly, they cannot be self-administered.
CONCLUSION
Schizophrenia is a chronic disorder that will most likely require lifelong treatment. To ensure optimal outcomes, patients must understand the nature of this disorder and accept the reality that they may need medication for the rest of their lives. Clinicians must also understand that continuous treatment is imperative to prevent relapse and the potentially devastating consequences that can follow a return of psychotic symptoms. Currently, no ideal treatments are available for schizophrenia and different treatments will be better suited for different patients, but LAIs hold great promise for improving adherence and maintaining recovery.
Many new formulations of available atypical antipsychotics are currently in development that will use new delivery technologies. According to Dr Lauriello, these new agents, which may become available in the near future, may have advantages over available antipsychotics. For example, risperidone has recently been approved as a subcutaneous rather than intramuscular injection.39 Subcutaneous injections may have advantages because they can be given with smaller needles and potentially be self-administered. In addition, risperidone is being developed as a subcutaneous implant that would last for 6 months, and, as an additional benefit over an injectable formulation, the implant could be removed if the treatment needed to be discontinued.40 Clinicians should watch for approvals of these and other new agents that may be beneficial in their practice. By selecting the best pharmacologic agent, providing psychoeducation and behavioral interventions to promote adherence, and developing a collaborative therapeutic relationship with patients, clinicians will provide the most successful treatment.
Published online: February 19, 2019.
Disclosure of off-label usage: Dr Lauriello has determined that, to the best of his knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this activity.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
The teleconference was chaired by John Lauriello, MD, from the University of Missouri School of Medicine, Columbia. The faculty was Diana O. Perkins, MD, from the University of North Carolina School of Medicine, Carrboro.
Financial disclosure
Dr Lauriello has received grant/research support from Sunovion, Otsuka, Janssen, and Alkermes; is on the advisory panel for Alkermes, Janssen, Osmotica, Otsuka, Reckitt Benckiser, Sunovion, and Teva; has received speaker’s honoraria from Roche; and has received travel expenses from Alkermes. Dr Perkins is a consultant for Sunovion and Alkermes and has received other financial or material support from American Psychiatric Association Publishing. The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the commercial supporter.
Review Process
The faculty member(s) agreed to provide a balanced and evidence-based presentation and discussed the topic(s) and CME objective(s) during the planning sessions. The faculty’s submitted content was validated by CME Institute staff, and the activity was evaluated for accuracy, use of evidence, and fair balance by the Chair and a peer reviewer who is without conflict of interest.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Emsley R. Antipsychotic maintenance treatment in schizophrenia and the importance of preventing relapse. World Psychiatry. 2018;17(2):168-169. PubMed CrossRef
2. Correll CU, Rubio JM, Kane JM. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry. 2018;17(2):149-160. PubMed CrossRef
3. Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286-292. PubMed CrossRef
4. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216-226. PubMed CrossRef
5. DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42(3):200-209. PubMed CrossRef
6. Rosenstock I. Historical origins of the health belief model. Health Educ Behav. 1974;2(4):328-335.
7. Jones CJ, Smith H, Llewellyn C. Evaluating the effectiveness of health belief model interventions in improving adherence: a systematic review. Health Psychol Rev. 2014;8(3):253-269. PubMed CrossRef
8. Myers N, Bhatty S, Broussard B, et al. Clinical correlates of initial treatment disengagement in first-episode psychosis. Clin Schizophr Relat Psychoses. 2017;11(2):95-102. PubMed CrossRef
9. Byerly M, Fisher R, Whatley K, et al. A comparison of electronic monitoring vs clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res. 2005;133(2-3):129-133. PubMed CrossRef
10. Byerly MJ, Nakonezny PA, Rush AJ. The Brief Adherence Rating Scale (BARS) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophr Res. 2008;100(1-3):60-69. PubMed CrossRef
11. Penn DL, Waldheter EJ, Perkins DO, et al. Psychosocial treatment for first-episode psychosis: a research update. Am J Psychiatry. 2005;162(12):2220-2232. PubMed CrossRef
12. Bפuml J, Pitschel-Walz G, Volz A, et al. Psychoeducation improves compliance and outcome in schizophrenia without an increase of adverse side effects: a 7-year follow-up of the Munich PIP-Study. Schizophr Bull. 2016;42(suppl 1):S62-S70. PubMed CrossRef
13. Nuechterlein KH, Ventura J, Subotnik KL, et al. The early longitudinal course of cognitive deficits in schizophrenia. J Clin Psychiatry. 2014;75(suppl 2):25-29. PubMed CrossRef
14. El-Missiry A, Elbatrawy A, El Missiry M, et al. Comparing cognitive functions in medication adherent and non-adherent patients with schizophrenia. J Psychiatr Res. 2015;70:106-112. PubMed CrossRef
15. Velligan DI, Diamond P, Mueller J, et al. The short-term impact of generic versus individualized environmental supports on functional outcomes and target behaviors in schizophrenia. Psychiatry Res. 2009;168(2):94-101. PubMed CrossRef
16. Kane JM, Garcia-Ribera C. Clinical guideline recommendations for antipsychotic long-acting injections. Br J Psychiatry suppl. 2009;195(S52):S63-S67. PubMed CrossRef
17. Taylor D. Psychopharmacology and adverse effects of antipsychotic long-acting injections: a review. Br J Psychiatry suppl. 2009;195(S52):S13-S19. PubMed CrossRef
18. Ereshefsky L, Mascarenas CA. Comparison of the effects of different routes of antipsychotic administration on pharmacokinetics and pharmacodynamics. J Clin Psychiatry. 2003;64(suppl 16):18-23. PubMed
19. Paquette SM, Dawit H, Hickey MB, et al. Long-acting atypical antipsychotics: characterization of the local tissue response. Pharm Res. 2014;31(8):2065-2077. PubMed CrossRef
20. Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24. PubMed CrossRef
21. Kane JM, Eerdekens M, Lindenmayer J-P, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry. 2003;160(6):1125-1132. PubMed CrossRef
22. Lindenmayer JP. Long-acting injectable antipsychotics: focus on olanzapine pamoate. Neuropsychiatr Dis Treat. 2010;6:261-267. PubMed CrossRef
23. Samtani MN, Vermeulen A, Stuyckens K. Population pharmacokinetics of intramuscular paliperidone palmitate in patients with schizophrenia: a novel once-monthly, long-acting formulation of an atypical antipsychotic. Clin Pharmacokinet. 2009;48(9):585-600. PubMed CrossRef
24. Abilify Maintena [package insert]. Rockville, MD: Otsuka America Pharmaceutical, Inc.; 2017.
25. Hard ML, Mills RJ, Sadler BM, et al. Aripiprazole lauroxil: pharmacokinetic profile of this long-acting injectable antipsychotic in persons with schizophrenia. J Clin Psychopharmacol. 2017;37(3):289-295. PubMed CrossRef
26. Risperdal Consta [package insert]. Titusville, NJ: Janssen CNS. 2007.
27. Heres S, Kraemer S, Bergstrom RF, et al. Pharmacokinetics of olanzapine long-acting injection: the clinical perspective. Int Clin Psychopharmacol. 2014;29(6):299-312. PubMed CrossRef
28. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2015.
29. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2015.
30. Aristada [package insert]. Waltham, MA; Alkermes, 2018.
31. Aristada Initio [package insert]. Waltham, MA: Alkermes, 2018.
32. Alphs L, Benson C, Cheshire-Kinney K, et al. Real-world outcomes of paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: a randomized, open-label, review board-blinded 15-month study. J Clin Psychiatry. 2015;76(5):554-561. PubMed CrossRef
33. Alphs L, Bossie C, Mao L, et al. Treatment effect with paliperidone palmitate compared with oral antipsychotics in patients with recent-onset versus more chronic schizophrenia and a history of criminal justice system involvement. Early Interv Psychiatry. 2018;12(1):55-65. PubMed CrossRef
34. Akerman SC, Brunette MF, Noordsy DL, et al. Pharmacotherapy of co-occurring schizophrenia and substance use disorders. Curr Addict Rep. 2014;1(4):251-260. PubMed CrossRef
35. Rubio G, Mart×nez I, Ponce G, et al. Long-acting injectable risperidone compared with zuclopenthixol in the treatment of schizophrenia with substance abuse comorbidity. Can J Psychiatry. 2006;51(8):531-539. PubMed CrossRef
36. Jaeger M, Rossler W. Attitudes towards long-acting depot antipsychotics: a survey of patients, relatives and psychiatrists. Psychiatry Res. 2010;175(1-2):58-62. PubMed CrossRef
37. Baldini CG, Culley EJ. Estimated cost savings associated with the transfer of office-administered specialty pharmaceuticals to a specialty pharmacy provider in a Medical Injectable Drug program. J Manag Care Pharm. 2011;17(1):51-59. PubMed
38. Bloch Y, Levkovitz Y, Atshuler A, et al. Use of topical application of lidocaine-prilocaine cream to reduce injection-site pain of depot antipsychotics. Psychiatr Serv. 2004;55(8):940-941. PubMed CrossRef
39. Multiple Ascending Dose Study of the Safety, Tolerability, Pharmacokinetic/Efficacy. Full Text View. ClinicalTrials.gov.
40. Navitha A, Jogala S, Krishnamohan C, et al. Development of novel risperidone implants using blends of polycaprolactones and in vitro in vivo correlation studies. J Adv Pharm Technol Res. 2014;5(2):84-89. PubMed CrossRef
This PDF is free for all visitors!