Objective: Marijuana has been approved for a number of psychiatric conditions in many states in the US including posttraumatic stress disorder (PTSD), agitation in Alzheimer’s disease, and Tourette’s disorder. In this systematic review, we examine the strength of evidence for the efficacy of marijuana and other cannabinoids for these psychiatric indications.
Data Sources: The literature (MEDLINE) was searched for studies published between January 1980 and March 2015 using search terms related to marijuana and other cannabinoids and the specific diagnosis.
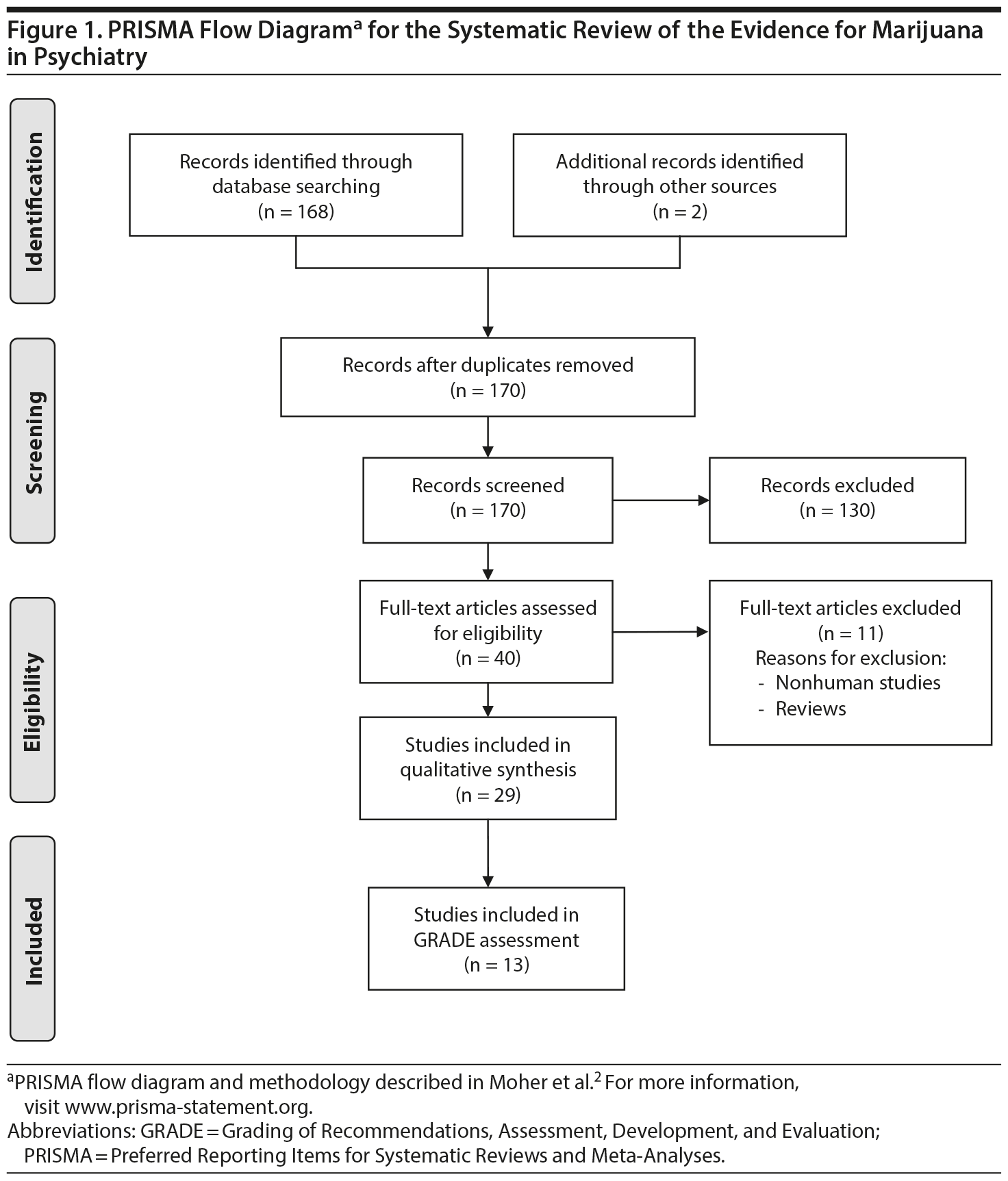
Study Selection: The best quality of evidence, namely placebo-controlled, randomized clinical trials (RCTs) and meta-analyses, was sought per PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. In the absence of RCTs, the next best available evidence (eg, observational studies, case reports) was reviewed. Of 170 publications that were screened, 40 were related to the topic, 29 were included in the qualitative synthesis, and 13 studies examined the efficacy of cannabinoids in humans.
Data Extraction: The evidence was rated using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) method.
Results: No RCTs have thus far examined the efficacy of marijuana for Tourette’s disorder, PTSD, or Alzheimer’s disease. Lower-quality studies examined the efficacy of marijuana, Δ9-tetrahydrocannabinol, and nabilone; the strength of evidence for the use of cannabinoids for these conditions is very low at the present time. The consequences of chronic cannabinoid exposure includes tolerance, dependence, and withdrawal. Early and persistent marijuana use has been associated with the emergence of psychosis. Marijuana impairs attention, memory, IQ, and driving ability.
Conclusions: Given its rapidly changing legal status, there is an urgent need to conduct double-blind, randomized, placebo- or active-controlled studies on the efficacy and safety of marijuana or its constituent cannabinoids for psychiatric conditions. Physicians and policy-makers should take into account the limited existing evidence and balance that with side effects before approving medical marijuana for psychiatric indications.
‘ ‹

A Systematic Review of the Evidence for Medical Marijuana in Psychiatric Indications
ABSTRACT
Objective: Marijuana has been approved for a number of psychiatric conditions in many states in the US including posttraumatic stress disorder (PTSD), agitation in Alzheimer’s disease, and Tourette’s disorder. In this systematic review, we examine the strength of evidence for the efficacy of marijuana and other cannabinoids for these psychiatric indications.
Data Sources: The literature (MEDLINE) was searched for studies published between January 1980 and March 2015 using search terms related to marijuana and other cannabinoids and the specific diagnosis.
Study Selection: The best quality of evidence, namely placebo-controlled, randomized clinical trials (RCTs) and meta-analyses, was sought per PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. In the absence of RCTs, the next best available evidence (eg, observational studies, case reports) was reviewed. Of 170 publications that were screened, 40 were related to the topic, 29 were included in the qualitative synthesis, and 13 studies examined the efficacy of cannabinoids in humans.
Data Extraction: The evidence was rated using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) method.
Results: No RCTs have thus far examined the efficacy of marijuana for Tourette’s disorder, PTSD, or Alzheimer’s disease. Lower-quality studies examined the efficacy of marijuana, Δ9-tetrahydrocannabinol, and nabilone; the strength of evidence for the use of cannabinoids for these conditions is very low at the present time. The consequences of chronic cannabinoid exposure includes tolerance, dependence, and withdrawal. Early and persistent marijuana use has been associated with the emergence of psychosis. Marijuana impairs attention, memory, IQ, and driving ability.
Conclusions: Given its rapidly changing legal status, there is an urgent need to conduct double-blind, randomized, placebo- or active-controlled studies on the efficacy and safety of marijuana or its constituent cannabinoids for psychiatric conditions. Physicians and policy-makers should take into account the limited existing evidence and balance that with side effects before approving medical marijuana for psychiatric indications.
J Clin Psychiatry 2016;77(8):1050-1064
dx.doi.org/10.4088/JCP.15r10036
© Copyright 2016 Physicians Postgraduate Press, Inc.
‘ ¡Shared first authorship.
aAbraham Ribicoff Research Facilities, Connecticut Mental Health Center, New Haven
bDepartment of Psychiatry, Yale University School of Medicine, New Haven, Connecticut
cPsychiatry Service, VA Connecticut Healthcare System, West Haven
*Corresponding author: Deepak Cyril D’ Souza, MD, Psychiatry Service, 116A, VA Connecticut Healthcare System, 950 Campbell Ave, West Haven, CT 06516 ([email protected]).
There is a growing movement across the United States legalizing the medical and recreational use of marijuana. As of June 2016, the District of Columbia and 25 states in the United States have passed legislation removing state-level penalties for the use of marijuana by patients who have obtained certification from a physician that their medical condition would very likely benefit from the use of marijuana. Most of these states now have approved dispensaries operating where individuals with physician certification can obtain marijuana. Marijuana, however, continues to be a Schedule I substance under US federal law.
While the list of conditions varies from state to state (Table 1), the most commonly listed conditions include cancer, glaucoma, HIV/AIDS, cachexia, nausea, pain, muscle spasticity, and epilepsy. Several states have included psychiatric conditions such as posttraumatic stress disorder (PTSD), agitation in Alzheimer’s disease, and Tourette’s disorder. The conditions approved for medical marijuana have been selected on the basis of anecdotal reports, inputs from public interest groups, advocates of medical marijuana, and presumably, a review of the existing science. Typically, individuals with a medical condition on the approved list have to be certified by a physician in order to be eligible for the program. In some states, physicians have the option to certify patients for “other” conditions not specifically listed if, in the physician’s opinion, the benefits of medical marijuana outweigh the potential risks.
In addition to crude marijuana, a number of products containing individual cannabinoids have been manufactured, tested, and approved by regulatory agencies in the United States, Europe, and Canada. Dronabinol consists of purified oral Δ9-tetrahydrocannabinol (THC) and is approved by the US Food and Drug Administration (FDA) for the treatment of cachexia associated with HIV/AIDS. Dronabinol and nabilone (an oral THC analog) are approved in the United States for the treatment of nausea and vomiting associated with chemotherapy. Nabiximols is a combination (approximately 1:1 ratio) of THC and cannabidiol in oromucosal spray formulation and is approved in Canada and many European countries to treat spasticity in multiple sclerosis. To date, there is no approval by the FDA (or equivalent agencies) for any cannabinoid for the treatment of a psychiatric disorder.
Thus far, there are few meta-analyses or systematic reviews of medical marijuana for psychiatric indications to guide policy-makers and mental health clinicians. In this article, we systematically review the evidence base for cannabis in psychiatric conditions with a secondary goal to review the side effects and adverse outcomes observed in studies.
METHODS
The literature was systematically searched (MEDLINE) for studies published between January 1980 and March 2015 using search terms [marijuana OR cannabis OR cannabinoid OR Dronabinol OR Nabilone OR THC] AND [posttraumatic stress disorder OR Tourette OR dementia OR Alzheimer’s ]. (See PRISMA [Preferred Reporting Items for Systematic Reviews and Meta-Analyses] flowchart1,2). Studies or conference proceedings were also identified using cross-references and data were sought by contacting authors when it was unavailable from published manuscripts. The best quality of evidence, namely placebo-controlled, randomized clinical trials (RCTs) and meta-analyses was sought. In the absence of RCTs, the next best available evidence (observational studies, case reports, etc) was reviewed. When good evidence for marijuana was lacking, supportive evidence from the use of synthetic cannabinoids was sought. Of 170 manuscripts that were screened, 40 were related to the topic, 29 were included in the qualitative synthesis, and 13 studies examined the efficacy of cannabinoids in humans (Figure 1). Studies were rated independently by 2 raters (R.R., S.T.W.) on quality, consistency, generalizability, and effect size using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) method.3 Discrepancies were reconciled by mutual discussion. The study was considered exempt from Institutional Review Board approval.

- Marijuana has been “approved” as medication for various indications in 25 states and the District of Columbia, yet the evidence of efficacy and safety is generally less than that required by the US Food and Drug Administration for approval of a new medication.
- Psychiatric conditions for which marijuana has been approved in various states include posttraumatic stress disorder, Tourette’s disorder, and agitation in Alzheimer’s disease.
- The quality of the evidence for marijuana in the treatment of these condition is generally very low and mostly consists of retrospective surveys and chart reviews, case reports, and anecdotal evidence—as opposed to randomized controlled trials.
- Potential harmful effects of marijuana include addiction and dependence, psychosis, cognitive deficits, and the potential for pulmonary complications.
PHARMACOLOGY OF MARIJUANA
Marijuana is not a single drug, but rather a complex and highly variable4 blend of approximately 400 chemical compounds that include phytocannabinoids, terpenoids, and flavonoids that produce individual, interactive, and “entourage” effects.5 Different strains of marijuana contain different proportions of cannabinoids,4 making it difficult to compare uncontrolled studies. Unlike FDA-approved medications, the proportion, content, and potency of marijuana’s active constituents may vary considerably. Thus, attributing marijuana’s positive or negative effects to any of its many constituents remains challenging.
Δ9-tetrahydrocannabinol, the principal psychoactive constituent of cannabis, is a partial agonist at cannabinoid-1 receptor (CB1R). However, there are more than 70 other phytocannabinoids present in marijuana including cannabidiol, cannabigerol, cannabichromene, etc, some of which may possess individual pharmacologic effects6,7 and may modify the effects of THC.5 For example, cannabidiol may have anxiolytic and antipsychotic-like effects that offset some of the effects of THC.8 Other cannabinoids have been shown to potentiate the analgesic effects of THC,9 have anti-inflammatory and antioxidant properties,10 have anticonvulsant properties,5 and even appetite-stimulating effects.11 Furthermore, the cannabinoids and terpenoids present in marijuana may also have entourage effects.5
Cannabinoids have multiphasic, dose-dependent effects in preclinical studiesSR1-SR3* with anxiolytic effects at lower doses and anxiogenic effects at higher doses.12 THC has been shown to have biphasic effects on blood pressure, fear-coping strategies, and intracranial self-stimulation.SR3,SR4 This multiphasic profile of effects has important implications for the therapeutic application of cannabinoids.
Overview of the Endocannabinoid System
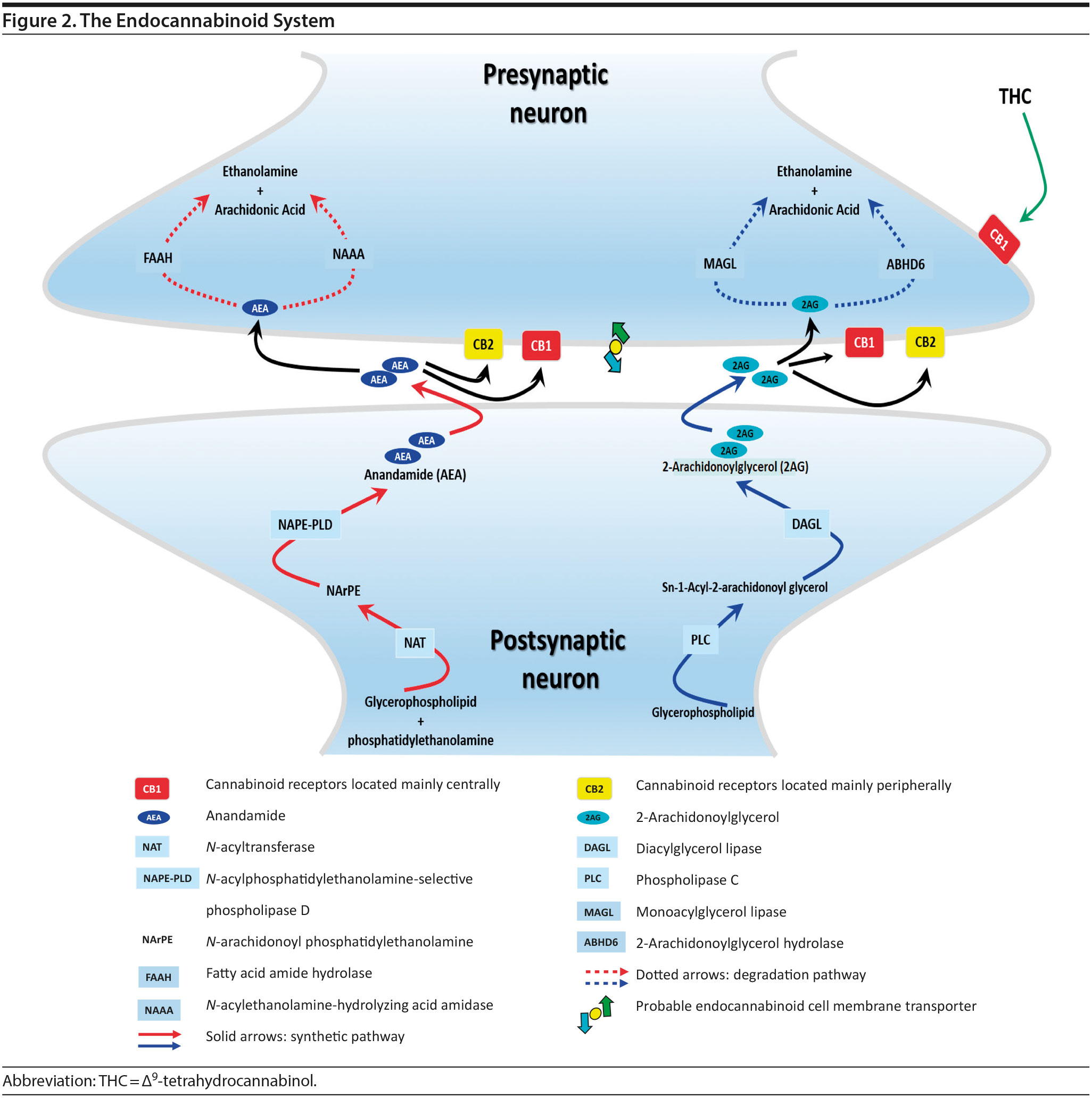
The endocannabinoid (eCB) system is a neuromodulatory system consisting of two G-protein-coupled receptors, cannabinoid-1 receptor (CB1R) and cannabinoid-2 receptor (CB2R); lipid ligands including anandamide (AEA) and 2-arachidonoylglycerol (2-AG); and enzymes involved in eCB biosynthesis(N-acylphosphatidylethanolamine-selective phospholipase D [NAPE-PLD] and diacylglycerol lipase [DAG-L]) and degradation (fatty acid amide hydrolase [FAAH], monoacylglycerol lipase [MAG-L], and alpha/beta-hydrolase domain containing 6 [ABHD6], aka, monoacylglycerol lipase ABHD6 or 2-arachidonoylglycerol hydrolase) (for reviews see references 7, 13, and 14) (Figure 2). CB1Rs are critical in mediating the psychoactive effects of cannabinoids and are expressed mainly in the brain, whereas CB2Rs are mostly expressed peripherally. eCBs are synthesized and released on demand, after which they travel back to activate the presynaptic CB1R, resulting in braking the further release of neurotransmitters.SR5-SR9 eCBs are rapidly removed by a transport system that is yet to be fully characterized. Anandamide is hydrolyzed by FAAH while 2-AG is hydrolyzed by both FAAH and MAG-L. Inhibition of FAAH and MAG-L prolongs the activity of anandamide and 2-AG, respectively, and may offer an alternative to treatment with phytocannabinoids (components of cannabis).
In contrast to eCBs, THC is metabolized over several hours before being excreted. Therefore, the duration of effects of THC and eCBs is very different, with eCBs having brief effects whereas THC has prolonged effects.
Notably, the eCB system may have an important role in neurodevelopment that may explain why adolescence is a critical period wherein individuals are particularly susceptible to the negative effects of cannabis (discussed in the section on tolerance, dependence, and withdrawal).
Review of Evidence About Marijuana for Psychiatric Indications
Tourette’s Disorder
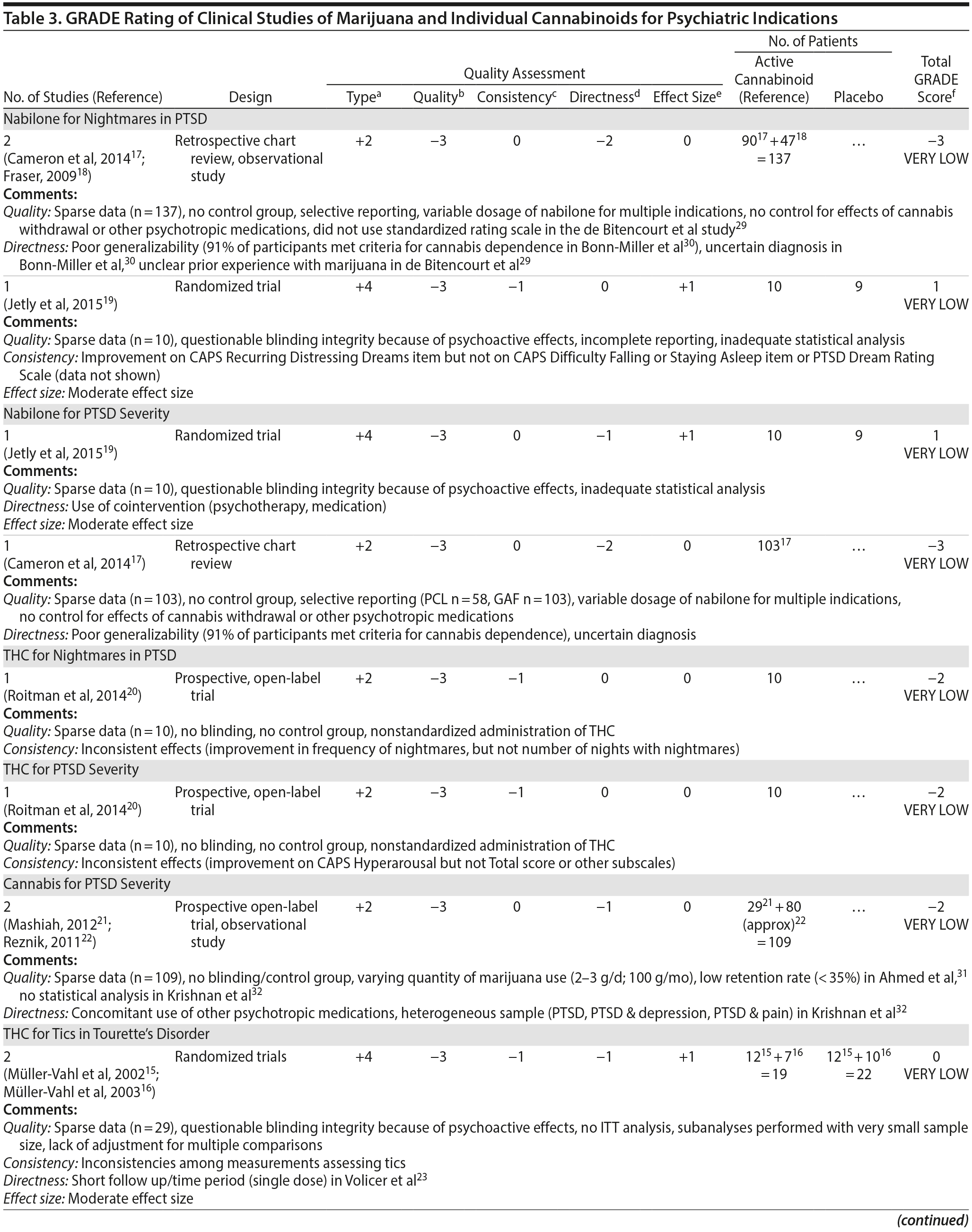
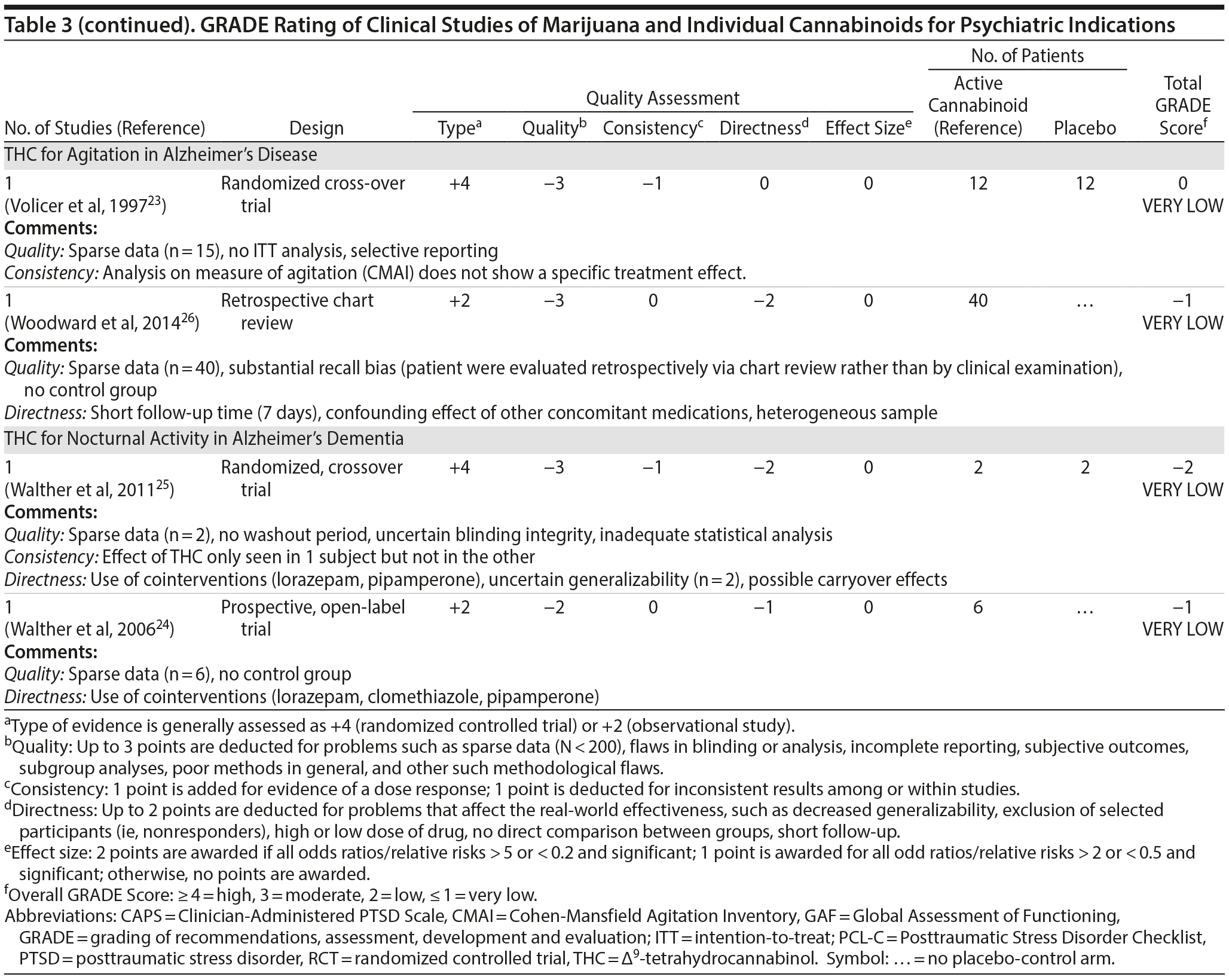
There are no published RCTs of marijuana for Tourette’s disorder. Müller-Vahl et al15 conducted a double-blind, randomized, placebo-controlled crossover trial of dronabinol (THC) in patients with Tourette’s disorder (n = 12) who received a single dose of THC (5-10 mg) or placebo, followed by a 4-week washout period before crossing over to the other treatment condition. Tic severity 3-4 hours after dosing was reduced significantly relative to before drug on a self-administered tic severity scale but not on the clinician-administered scales (Table 2).
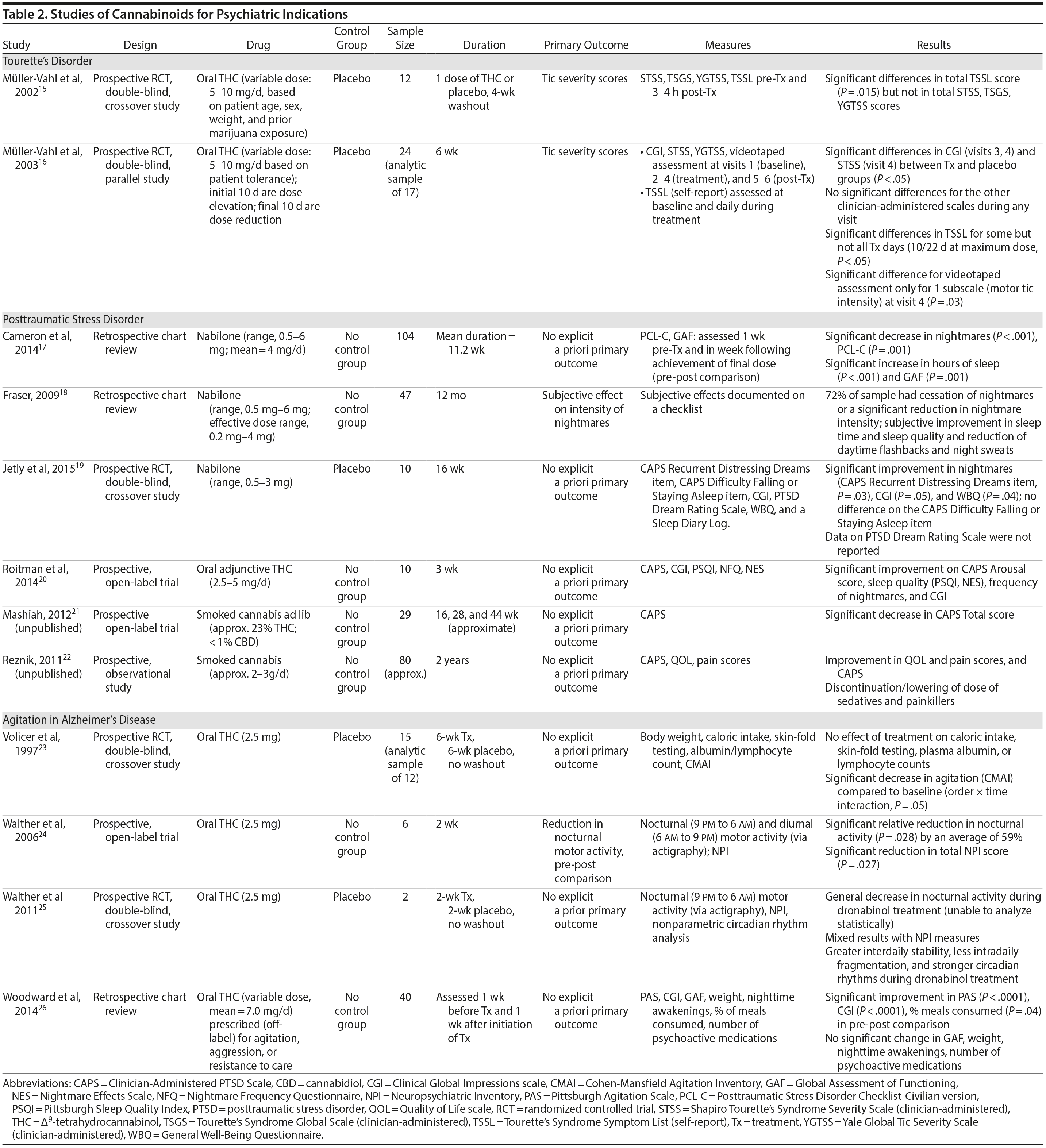
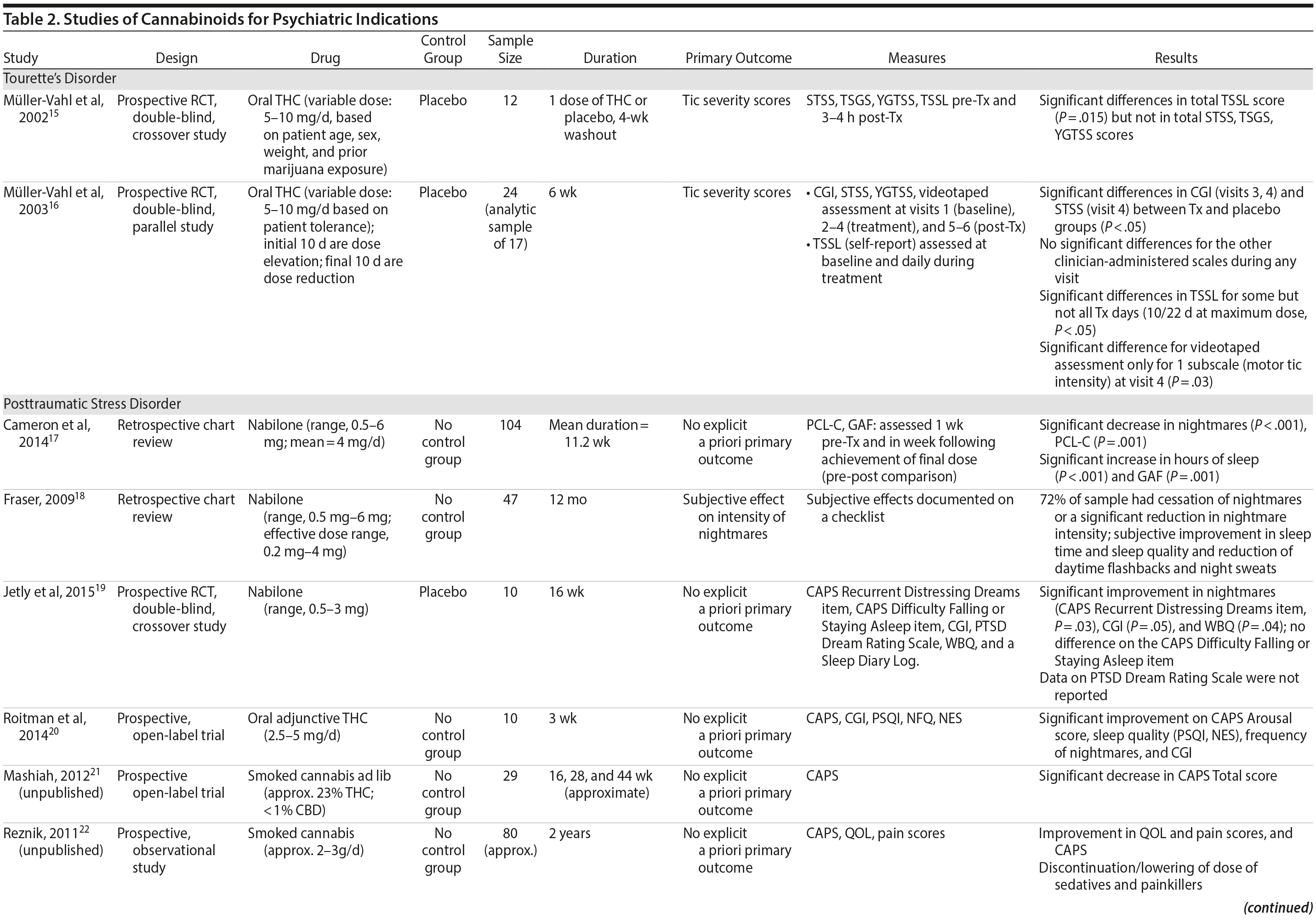
In a second double-blind, placebo-controlled trial,16 patients with Tourette’s disorder (n = 24) were randomized to 6 weeks of dronabinol or placebo, with the first and last 10 days to titrate on and off the medication, respectively. There were significant improvements in some (but not all) visits between treatment groups in some (but not all) of the clinical scales assessing tic severity (Table 2). There were statistically significant improvements in videotape-based ratings of motor tic intensity for 1 of the 2 visits during full-dose treatment, but not motor tic frequency, phonic tics, or number of areas of body involved.15-26
A Cochrane systematic review27 concluded that there is insufficient evidence to support the use of cannabinoids for the treatment of tics in Tourette’s disorder.
Observational studies and case reports. Among patients with Tourette’s disorder who had used marijuana (n = 17/64), most (82%) reported a reduction or remission of symptoms with marijuana use.28 There are a number of case reports SR10-SR15 in which oral THC or marijuana has been reported to reduce tic severity in patients with Tourette’s .
Summary. The overall GRADE of evidence for studies of cannabinoids in Tourette’s disorder is very low (Table 3) based on small sample sizes, lack of intention-to-treat analysis, possible selection bias, lack of adjustment for multiple comparisons, and inconsistent results across different scales that were used to measure the same symptom (ie, tics).
Posttraumatic Stress Disorder
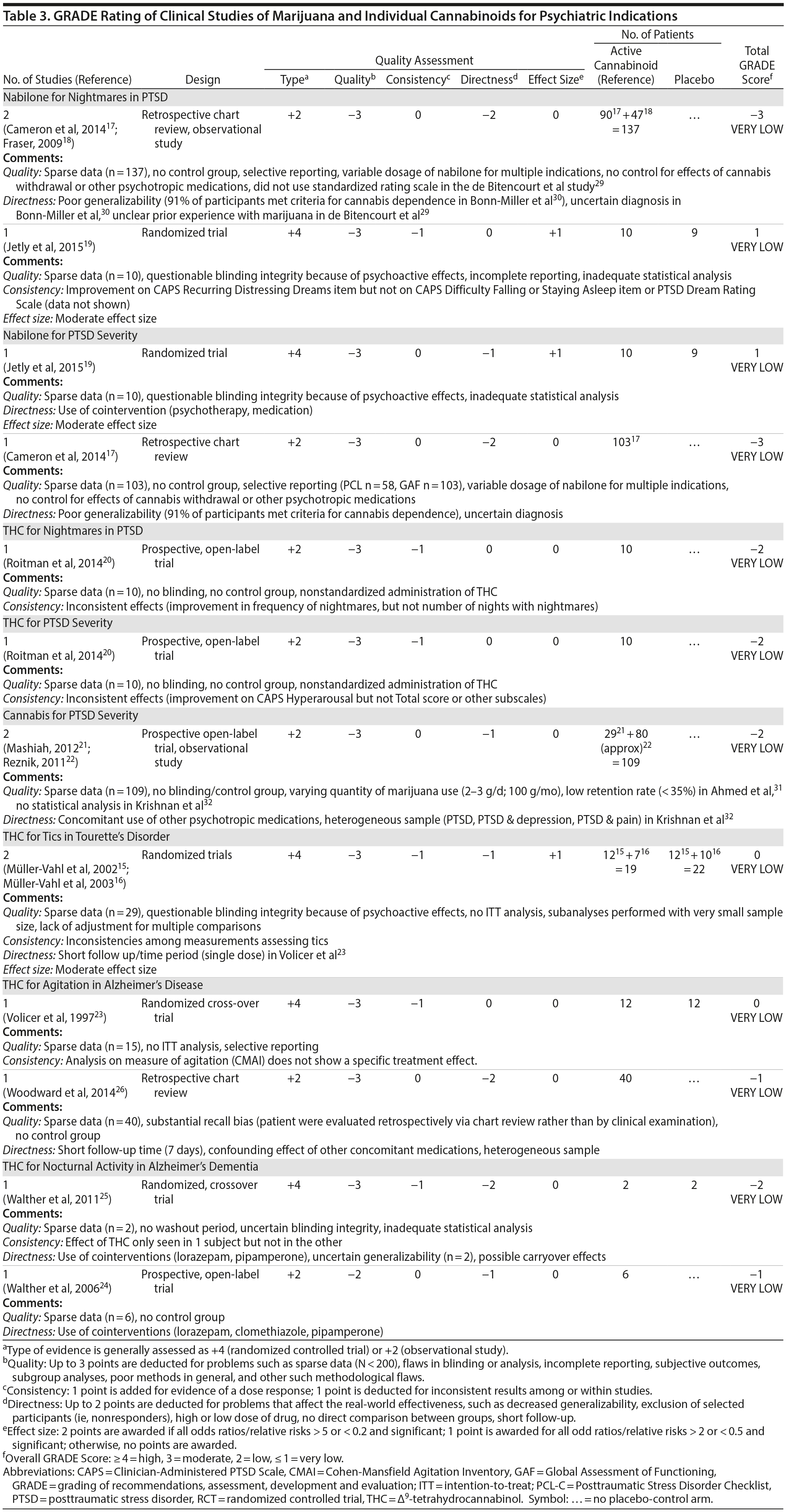
There are no RCTs examining the efficacy or safety of marijuana in PTSD. Four published studies17-20 examined the efficacy of cannabinoids while 2 unpublished studies21,22 reported on the efficacy of marijuana in PTSD (Table 2).
Cameron et al17 conducted a retrospective chart review of patients (n = 104) at a mental health and correctional facility that treated adult male offenders with seriously mental illness. While approximately 90% of these patients were diagnosed with PTSD, the review included patients with varying comorbid diagnoses (including mood, psychotic, and substance use disorders). The patients were prescribed nabilone (a synthetic THC-analog) at varying doses (range, 0.5-6 mg) for a variety of indications, including insomnia, nightmares, chronic pain, nausea/vomiting, and anorexia. Of note, 91% met criteria for marijuana dependence prior to admission. Nabilone was found to be associated with a significant improvement in duration of sleep and functioning, significant reduction in nightmares, and improvement in PTSD symptom severity.
In a retrospective chart review18 of PTSD patients (n = 47) with nightmares despite pharmacotherapy who were treated with adjunctive nabilone (0.5-6 mg), 72% of patients reported experiencing either cessation of nightmares or a significant reduction in nightmare intensity, improvement in sleep time, sleep quality, daytime flashbacks, and night sweats based on self-report. Nabilone was discontinued in 28% of patients because of side-effects.
In a double-blind, crossover-design RCT,19 10 male soldiers diagnosed with PTSD, with trauma-related nightmares despite standard pharmacotherapy, received nabilone (0.5 mg, titrated to effect or maximum of 3 mg) or placebo for 7 weeks followed by a 2-week washout period prior to crossing over. Nabilone resulted in significant reduction in nightmares as measured by mean reduction in the CAPS Recurrent Distressing Dreams item. However, there were no differences on the Clinician-Administered PTSD Scale (CAPS) Difficulty Falling or Staying Asleep item. The study also used the PTSD Dream Rating Scale, but data on this measure were not reported.
In a 3-week, open-label study, Roitman et al20 administered oral THC (2.5-5 mg twice daily) as an add-on to current pharmacologic treatment in patients (n = 10) with chronic PTSD (symptoms > 1 year using DSM-IV criteria). The study reported significant improvement on CAPS Arousal subscore, but not on Avoidance or Intrusion subscores or CAPS Total score; significant improvement in sleep quality and frequency of nightmares, but not number of nights with nightmares; and significant improvement on the Clinician Global Impressions scale.
In an unpublished open-label study, Mashiah21 examined the efficacy of adjunctive marijuana in 29 Israeli male combat veterans diagnosed with PTSD. Patients smoked marijuana ad-lib until they felt relaxed, up to a maximum of 100 g per month. The authors reported a significant decrease in CAPS Total score from baseline to first follow-up (n = 26) at approximately 4.3 months, second follow-up (n = 25) at approximately 7.6 months, and last follow-up (n = 10) at approximately 11.3 months. As noted, patient retention was poor. In a conference abstract, Reznik22 reported an observational study of 160 Israeli military personnel with PTSD who applied for a medical marijuana license. Participants who received licenses (about 50%) were followed up for 2 years. The authors report that with daily cannabis use of 2-3 g/d, “in most cases” there was an improvement in quality of life, pain, CAPS scores, and a discontinuation or lowering of dosage of pain medication and sedatives. The abstract, however, lacked details of statistical analysis.
Observational studies and case reports. PTSD diagnosis has been found to be associated with lifetime history of marijuana use as well as past year daily marijuana use after controlling for anxiety, mood disorder, and type and frequency of trauma.33 Bonn-Miller et al34 reported that lower degree of change in PTSD severity during the course of residential treatment was associated with significantly greater frequency of marijuana use at 4-month follow-up. This association was not seen with alcohol or other drugs. Greer et al35 found that participants (n = 80) seeking enrollment into New Mexico’s Medical Marijuana Program reported improvement in PTSD symptoms when they were using marijuana compared to when they were not, based on retrospective recall. Boden et al36 reported that PTSD symptom severity was positively associated with marijuana use as a coping mechanism, greater degree of problems associated with marijuana use, greater severity of marijuana withdrawal, and greater degree of craving related to compulsivity and emotionality. Patients with high PTSD scores reported using marijuana primarily as a coping mechanism and to help with sleep.30,36 The association between PTSD symptom severity and degree of marijuana use may be moderated by degree of avoidance, in that marijuana use enables avoidance among patients with severe PTSD.37 Degree of marijuana use is also related to prior experience with marijuana and expectancy of its effects on PTSD symptoms.38
Summary. The overall GRADE of evidence for studies of cannabinoids in PTSD is very low (Table 3). Emerging data about the effects of the eCB system on extinction learning29 may provide a stronger basis for future hypothesis-driven clinical trials. Especially given recent evidence suggesting poorer outcomes in PTSD patients who use marijuana,39 there is an urgent need for adequately powered, double-blind RCTs with adequate control for effects of prior expectancy and symptoms of marijuana withdrawal that overlap with symptoms of PTSD (namely, sleep disturbance, nightmares, and anxiety). Future studies should strive to measure specific effects on PTSD symptoms that are distinct from the effects of marijuana as an avoidance strategy or coping mechanism.
Agitation in Alzheimer’s Disease and Other Dementias
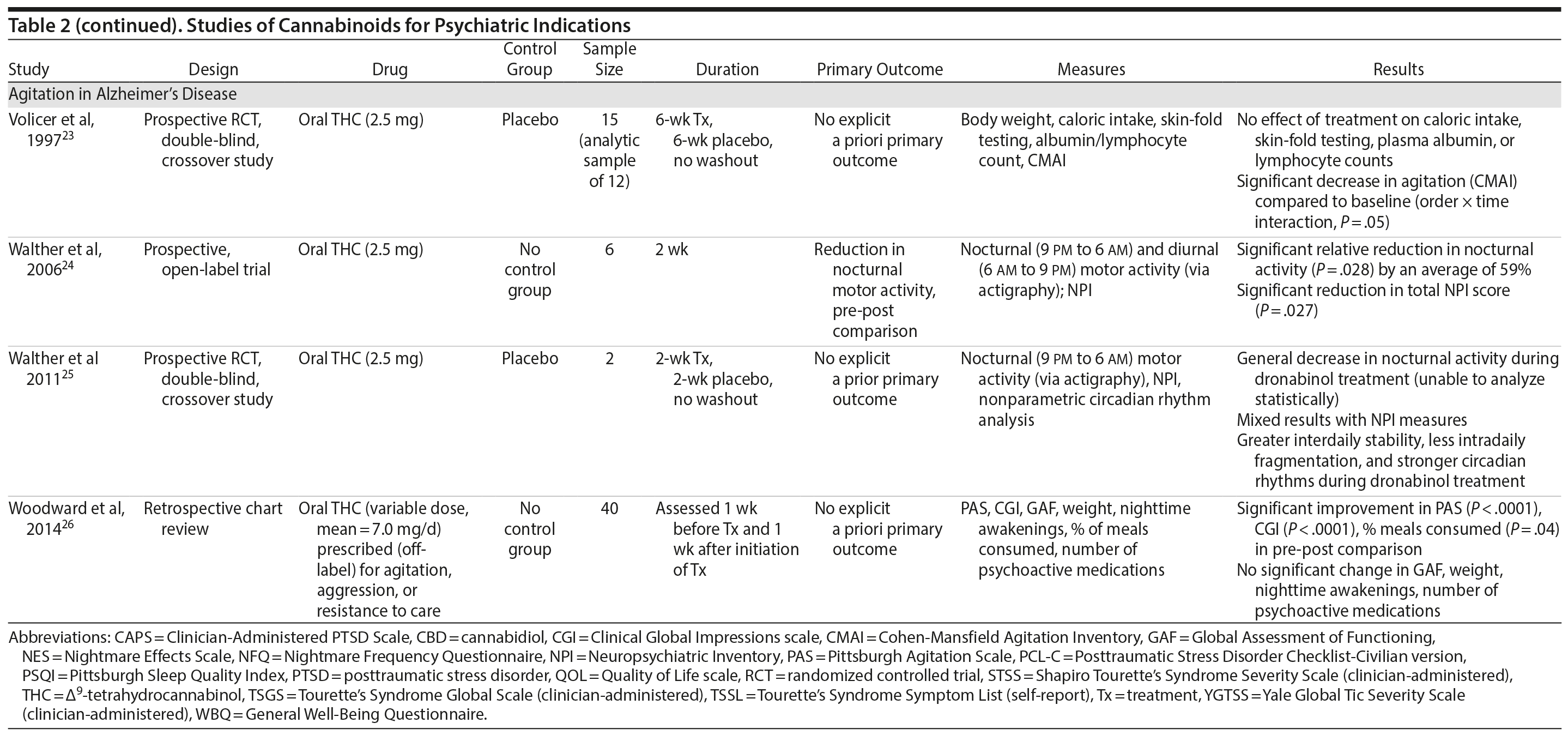
There are no published RCTS with marijuana for agitation in Alzheimer’s disease or other dementias. There are 4 prospective studies, 1 retrospective study, and a Cochrane systematic review assessing the efficacy of oral THC for the treatment of various symptoms associated with dementia (Table 2). In a retrospective study,26 inpatients (n = 40) with dementia showed statistically significant improvements in measures of agitation, aggressiveness, resisting care, aberrant vocalization, and percent of meals consumed after 7 days of treatment dronabinol compared to pretreatment. No changes were found in weight, number of psychoactive medications, number of nighttime awakenings, GAF, or observed sleep time. Frequent side effects were sedation and delirium.
Volicer et al23 evaluated the efficacy of dronabinol (2.5 mg twice daily) in 12 inpatients with dementia who exhibited food-refusing behaviors in a randomized, 6-week, double-blind, placebo-controlled, crossover design. Body weight increased significantly during the course regardless of treatment order (P = .006); the effect of treatment on weight gain was greater for those receiving dronabinol first (P < .017). Skin-fold thickness increased significantly (P = .016), but there was no effect of treatment or order. Caloric intake, plasma albumin, and lymphocyte counts did not change significantly. The only reported statistically significant behavioral change was an interaction of treatment order ×— time (P = .05) wherein subjects who received dronabinol first showed greater improvement in measures assessing agitation. Notably, the rate of tiredness/somnolence was approximately twice as high during active treatment phase compared to placebo phase.
Walther et al24 evaluated the efficacy of dronabinol for the treatment of nighttime agitation in an open-label trial of 6 patients with dementia, using nocturnal motor activity (measured by actigraphy) as a proxy. In a pre-post comparison, nocturnal motor activity was significantly reduced after 14 days of treatment. Composite score on the Neuropsychiatric Inventory (NPI), a measure of dementia outcomes, was also significantly lower after treatment compared to baseline, with significant changes notable in the subscores measuring aberrant motor activity, nighttime behaviors, agitation, appetite disturbances, and irritability. In a follow-up RCT,25 2 patients were randomized to receive treatment with oral THC (2.5 mg) or placebo for two weeks before crossing over (no wash out). Both patients reportedly showed more stability, less fragmentation, and stronger circadian rhythm patterns in circadian rhythm analyses; results of dementia outcomes (measured by NPI) were mixed.
Ahmed et al31 studied the safety and efficacy of oral THC (0.75-1.5 mg) or placebo in a RCT of 10 dementia patients. Six THC-related adverse events were reported including dizziness, fatigue (n = 2), and agitation (n = 3), all of which were judged to be transient and minor in severity; efficacy outcomes have not yet been published (ClinicalTrials.gov identifier: NCT01302340).
A Cochrane systematic review of the literature32 concluded that there is insufficient evidence that cannabinoids are effective for the treatment of behavioral disturbances associated with dementia. Another systematic review40 noted that there is a lack of adequately powered trials showing efficacy and safety of cannabinoids in older adults generally, though small studies have suggested an acceptable safety profile in both healthy41 and demented31 geriatric patients.
Case report. Additionally, 1 case report documents significant and sustained improvement after nabilone treatment in a 72-year-old demented man with severe behavioral problems refractory to antipsychotics, gabapentin, citalopram, lorazepam, and trazodone.42
Summary. The overall GRADE score for the use of cannabinoids in the treatment of dementia-related symptoms is very low (Table 3). Of note, there is some evidence for the involvement of the eCB system in Alzheimer’s disease.43 The limitations of published trials include small sample size, lack of control groups, retrospective assessments, lack of randomization and blinding, use of pre-post comparison, short durations, lack of intention-to-treat analysis, possible carry-over effects in the crossover study, and inconsistent results using different measures designed to assess the same outcomes. One positive aspect of this emerging literature was the use of objective measures (eg, actigraphy) to assess nighttime agitation. Notably, several other objective measures showed no differences by group (treatment vs placebo) or in pre-post comparisons.23,24,26 It remains possible that the purported “calming” effect of THC in agitated dementia patients is explained by nonspecific sedation. Indeed, sedation was one of the most frequent side effects of dronabinol in these studies.23,26 Given the well-known effects of cannabinoids on cognitive domains that are already impaired in dementia, future studies must also weigh the purported benefits against the cognitive impairing effects of cannabinoids.
Other Indications
Medical marijuana has also been approved for a number of medical (eg, Crohn’s disease), neurologic (eg, epilepsy and Parkinson’s disease), and general conditions (eg, pain) that are associated with psychiatric and psychological comorbidity.SR16-SR23 Thus, mental health clinicians might be involved in caring for clients who are taking medical marijuana for other conditions. The quality of the evidence for other such indications is reviewed elsewhere (see Supplementary References SR24-SR26).
Discussion
The overall GRADE of evidence for studies of cannabinoids in Tourette’s disorder, PTSD, and agitation in Alzheimer’s disease and other dementias is very low. Clinicians should exercise caution in certifying medical marijuana for these psychiatric disorders.
The data for this systematic review were derived from published manuscripts of the studies and published conference proceedings or obtained through personal communication with the authors. While it is hoped that this systematic review accurately captures the efficacy measures in individual studies, additional data on specific measures such as sleep or pain that may have been collected by authors but not included in their publications were not available and is a potential limitation.
To enable clinicians and patients to make an informed decision in light of the absence of evidence, and since clinical decisions are often based on a discussion about risk versus benefit, we review the risks of medical marijuana in the following section.
Risks of Medical Marijuana
Side Effects
Since few RCTs have specifically assessed the safety of crude marijuana, most of the data on safety has to be extrapolated from clinical trials with cannabinoids (eg, dronabinol and nabilone) or from data on marijuana abuse. The most commonly reported side effects in trials of cannabinoids include dizziness, tiredness, sedation, lightheadedness, headache, anxiety, disorientation, dry mouth, falls, fatigue, weakness, nausea, feeling of intoxication or “stoned,” euphoria, and oromucosal discomfort.15,16,31,SR27-SR36 In trials of smoked marijuana, cough, throat irritation, and mouth pain or a sensation of burning have been reported.SR34-SR36 Other side effects included seizure, emotional lability, anxiety/nervousness, hallucinations, paranoid reactions, and euphoria. Generally, these are rated as mild in severity. Patients with preexisting psychosis have been reported to experience a worsening of psychosis; other side effects may be greater in those who are marijuana-naׯve, with adverse events occurring in 44% of marijuana-naׯve patients compared to 28% of marijuana dependent patients in one study.17 In most studies, side effects were based on self-report and not systematic assessments.
Tolerance, Dependence, and Withdrawal
Tolerance. Tolerance to the analgesic, hypothermic, and hypomotor effects of CB1R agonists has been shown in animal species and develops within days; tolerance to memory and endocrine effects takes longer to develop.SR37-SR40 In humans, tolerance develops to effects on mood, memory and cognition, heart rate, blood pressure, and hormonesSR41-SR44; the magnitude of tolerance is proportional to the dose and duration of exposure. The rate and time-course of the development of tolerance to the various effects of CB1R agonists varies.
Dependence. Approximately 9% of people who use marijuana are estimated to develop dependence.44 The risks of marijuana dependence decrease over a 10-year period. However, people who use marijuana at least 5 times a year are likely to continue the same level of use for at least 10 years.45 Furthermore, lifetime rates of addiction in those who begin use in adolescence have been reported close to 17%.46 Although the lifetime prevalence of dependence in marijuana users (9%) is lower than rates of dependence for tobacco (32%), heroin (23%), cocaine (17%), and alcohol (15%),44 this portion represents a significant number given the overall high prevalence of marijuana use.
Withdrawal syndrome. The administration of CB1R antagonists has been shown to precipitate a withdrawal syndrome in animals chronically exposed to CB1R agonists.47,48 In humans, a marijuana withdrawal syndrome has been described in retrospective self-report studies, prospective studies, and human laboratory studies involving the administration and discontinuation of cannabinoids.49,SR45-SR50 Now recognized in DSM-5 as a distinct entity, cannabis withdrawal syndrome is characterized by anger, aggression, appetite change, weight loss, irritability, anxiety, restlessness, altered sleep, strange dreams, marijuana craving, and physical discomfort.49,50,SR44-SR54 Less common symptoms include chills, depressed mood, stomach pain, and sweating. Most symptoms appear within 1 day of abstinence, peak within 2-3 days, and resolve within 1-3 weeks. Two studies49,50 evaluated symptoms for at least 4 weeks and observed prominent withdrawal symptoms during the initial 2 to 3 weeks of abstinence, some of which persisted through the entire study period. The findings of these studies suggest that withdrawal symptoms may persist longer than 4 weeks.49,50 Characteristic of a true withdrawal syndrome, abstinence symptoms occur with blind discontinuation and resolve with CB1R agonist re-administration.SR44,SR45,SR49,SR52,SR55
Adaptation of the CB1R system associated with tolerance. Exposure to CB1R agonists is accompanied by receptor down-regulation, desensitization of receptor-mediated G-protein activation, and alterations in CB1R mRNA levels.SR56-SR65 More recently, CB1R down-regulation associated with chronic marijuana use in humans has been demonstrated both postmortem and in vivo.51 These changes are related to the duration and magnitude of exposure to cannabinoids and have a distinct regional and temporal profile, with CB1R down-regulation occurring first in the cerebral cortex and hippocampus followed by the basal ganglia and cerebellum.SR40,SR61,SR66-SR69
Reversal of adaptation with abstinence. With prolonged abstinence, there is recovery in the number and function of CB1Rs over 2 weeks.52 In vivo human imaging studies suggest that CB1R down-regulation associated with marijuana dependence recovers within 2-4 weeks of abstinence.51
Psychosis and Other Psychiatric Disorders
Transient53 as well as persistent psychosis54 has been associated with marijuana use. Transient, marijuana-induced psychosis can outlast the period of acute intoxication and can persist for as long as 30 days.55 The risk of psychosis associated with marijuana is increased in early and chronic use.56 The relationship between marijuana and persistent psychosis fulfills many but not all of the standard criteria for causality.55 Marijuana use has also been shown to exacerbate the course of illness in individuals with established psychotic disorders and may be a component cause in the etiology of schizophrenia.55 Observational studies of patients with psychotic disorders indicate that those with a history of marijuana use (compared to those with no such history) have an earlier age at onset of illness by 2.7 years.56 Given that medical marijuana is mostly prescribed for chronic conditions and that chronicity heightens the risk of psychosis in marijuana use, psychosis represents a real risk. Emerging evidence suggests marijuana use has also been shown to be associated with worsening manic symptoms in patients with bipolar disorder.SR70-SR72 Heavy marijuana use has also been associated with increased risk for depressive disorders.57
Cognitive Deficits
Acute effects. Marijuana and other cannabinoids can acutely impair several domains of cognitive function, including reaction time, attention, divided attention, signal detection, allocation of attention, information processing speed, spatial working memory and maze accuracy, verbal learning and recall, procedural memory, associative learning, tracking accuracy, time estimation, distance estimation, set shifting, motor coordination, and danger perception.53,SR73-SR105
Long-term effects. Adverse effects associated with long-term marijuana use also include cognitive decline in the form of deficits in attention, executive functioning, memory, and IQ.58-61 It is thought that these impairments persist beyond the period of intoxication and are related to dose, duration, frequency, and age at first marijuana use.62 In a longitudinal cohort following over 1,000 subjects from birth to age 38, Meier et al61 showed that those with persistent marijuana use experience a significant decline in IQ. Moreover, this decline was greatest in those who began use in adolescence versus adulthood (8-point vs 6-point decline in IQ). This trend did not reverse after cessation of marijuana. Alternate hypothesis that this association was confounded by low socioeconomic status of cannabis users or conscientiousness, one of the traits of Five-Factor Model of personality, were not supported in this cohort.SR106-SR108 The association between cannabis use and poor academic performance persisted despite controlling for socioeconomic status in a separate cohort as well.63 Other studies show that deficits in neuropsychological functioning can reverse after cessation of marijuana use, but recovery times can vary from a week to 3 months to 2 years of abstinence.SR109-SR112
Effects on Driving
Marijuana is the most common illicit drug implicated in motor vehicle fatalities.64 Epidemiologic data suggest that recent marijuana use increases risk of a motor vehicle accident by approximately 2-fold.65 Marijuana and cannabinoids are well known to impair a number of driving-related conditions including attention, reaction time, working memory, processing speed, working memory, procedural memory, tracking accuracy, time estimation and distance estimation.66 Marijuana and THC have been shown to significantly impair on-road driving to levels equivalent to driving with blood alcohol levels of 0.05%-0.1%.SR113-SR115 Occasional users of marijuana are more sensitive to the driving impairing effects of marijuana and THC than heavy users.
In driving simulation studies, marijuana and THC produce dose-related impairments in a number of driving outcomes including speed variance, lane deviation, steering instability, and braking distance.67,68 Interestingly, while people underestimate their driving impairments under the influence of alcohol, under the influence of marijuana or THC, people seem to be more aware of impairment and therefore drive more slowly.67,SR116-SR118 Finally, emerging data suggest that under the influence of both alcohol and marijuana, driving impairments may be more than additive.68,SR119-SR123
Pulmonary Effects
Although still controversial, the effects of long-term marijuana smoking on pulmonary function , as well as the risk of lung cancer,SR124-SR126 are a persistent concern. Heavy and chronic marijuana smoking may lead to symptoms of bronchitis, increased airway resistance, and airway inflammation.69 However, lower levels of marijuana use do not appear to be associated with these symptoms.70 (The effect of cannabis use on other aspects of physical health is reviewed elsewhere.SR127)
Interactions With Other Drugs
Both in vitro and in vivo studies suggest that many cannabinoids can significantly inhibit a wide range of cytochrome P450 (CYP) enzymes including CYP2C9, CYP2D6, CYP2C19, and CYP3A4,SR128-SR131 though the clinical implications of potential drug interactions requires further study. Marijuana may increase the anticoagulant effect of warfarin by inhibiting its metabolism and its displacement from protein-binding sites.71 Marijuana also decreases the peak concentration of antiretroviral therapies, though the clinical significance of this is unclear.72 The potential drug-drug interactions induced by cannabinoids will need further attention.
Special Considerations in Children and Adolescents
Considering that adolescence may represent a period of increased vulnerability for the emergence of psychosis and for greater cognitive effects such as significant decline in IQ associated with cannabis use, there is increasing concern regarding the use of medical marijuana for conditions such as Tourette’s disorder in children and adolescents.73 Although medical marijuana is currently approved only for adults > 18 years and older, studies suggest that it invariably makes its way into the hands of children and adolescents as reflected by the increasing number of emergency department admissions for unintentional marijuana ingestion.74
Clinical Implications
Clinicians who certify, prescribe, or care for clients receiving marijuana need to be aware that because of tolerance, over time, patients may require more marijuana to achieve a desired effect, that abrupt discontinuation may precipitate a withdrawal syndrome, and that in patients who have been abstinent for weeks, if medical marijuana is resumed, lower doses would be advisable. Similarly, clinicians need to be aware that marijuana impairs cognition, and when combined with other prescribed drugs (eg, benzodiazepines) or when used in disorders of cognition (eg, dementia), impairment may be heightened. Clinicians should also be aware that marijuana impairs driving, and when combined with alcohol or prescribed drugs (eg, opioids or benzodiazepines), driving may be further impaired. Finally, potential interactions between marijuana and other drugs and the clinical significance of these interactions are not well studied.
CONCLUSIONS
There are few RCTs with medical marijuana or cannabinoids for psychiatric indications. The strength of evidence for the use of medical marijuana for psychiatric indications of PTSD, Tourette’s disorder, and agitation in Alzheimer’s disease is very low at the present time. While states have approved the use of the whole plant, most of the existing evidence is about individual constituents of marijuana. For drugs to be approved by the US FDA, the gold standard of evidence, ie, RCT data are required. To that extent, little gold standard evidence exists for psychiatric indications. Of note, cannabinoid medications that have been approved by the FDA already exist, namely dronabinol and nabilone, but public interest in them is limited. Furthermore, the varied conditions for which medical marijuana is approved have no common etiology, pathophysiology, or phenomenology, raising questions about a mechanism that could explain why it could be beneficial for all these conditions.
The demonstration of efficacy in studies using medical marijuana is made challenging by multiple constraints related to blinding, expectancy, and multiphasic dose-dependent effects of constituent cannabinoids. Unlike FDA-approved medications, the proportion, content, and potency of marijuana’s active constituents may vary considerably across strains, making it difficult to compare uncontrolled studies. Taken collectively, attributing marijuana’s positive or negative effects to any of its many constituents remains challenging.
Finally, certifying physicians and those treating patients who use medical marijuana need to be aware that chronic and persistent use of marijuana is associated with adverse effects including potential deleterious effects on cognition and driving ability and risks of emergence or worsening of psychosis.
Future Directions
There is an urgent need for adequately powered, double-blind, randomized, placebo/active-controlled studies in carefully characterized patient populations with a specific psychiatric diagnosis or target symptom. Study designs should strive to minimize confounders such as expectancy and the possibility of unblinding due to the psychoactive effects of marijuana by using an active control,75 low doses of marijuana, or innovative study-designs such as balanced-placebo design.76 The standardization of the dose of medical marijuana, the proportion of its constituent cannabinoids, and the route of administration remains a challenge. The effects of tolerance and withdrawal should be minimized. The use of well-validated outcome measures that are not exclusively based on self-report but also include objective measures would help distinguish disease-specific effects from nonspecific anxiolytic and euphoric effects. Additionally, there is a need to rigorously assess short- and long-term neuropsychiatric side effects. Future research on the safety and efficacy of individual constituents (eg, cannabinoids) of marijuana and efforts to accelerate the advance of compounds with therapeutic promise would eliminate many current difficulties associated with establishing the efficacy of marijuana in RCTs.
Finally, states should consider establishing programs to screen for specific psychiatric disorders, eg, schizophrenia, prior to issuing medical marijuana prescriptions, prospectively monitoring negative outcomes (eg, new cases of psychosis), providing risk and safety information to all patients, and including medical marijuana in prescription monitoring databases as has been done for opioids and benzodiazepines.
Submitted: April 8, 2015; accepted December 29, 2015.
Drug names: citalopram (Celexa and others), dronabinol (Marinol and others), gabapentin (Neurontin, Gralise, and others), lorazepam (Ativan and others), nabilone (Cesamet), warfarin (Coumadin, Jantoven, and others).
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, citalopram, dronabinol, gabapentin, lorazepam, nabilone, and trazodone are not approved by the US Food and Drug Administration for the treatment of dementia; dronabinol is not approved for the treatment of Tourette’s disorder; nabilone is not approved for the treatment of posttraumatic stress disorder; and nabiximols is not approved for any indication.
Financial disclosure: Dr D’ Souza has in the past 3 years or currently receives research grant support administered through Yale University School of Medicine from AbbVie and Pfizer Inc and is a consultant for Bristol-Meyers Squibb and Johnson & Johnson. He also serves on the Connecticut Medical Marijuana Program Board of Physicians. Drs Radhakrishnan and Wilkinson have received funding from APIRE/Janssen Resident Psychiatric Research Scholar Fellowship and are supported by NIMH R25 IMPORT training grant (R25MH071584). Dr Wilkinson has also received funding from the Thomas P. Detre Research Fellowship (nonprofit).
Funding/support: The authors wish to acknowledge support from the (1) Department of Veterans Affairs; (2) National Institute of Mental Health, National Institute on Drug Abuse; (3) National Institute on Alcohol Abuse and Alcoholism (NIAAA); and (4) the Yale Center for Clinical Investigation (YCCI).
Role of the sponsor: None of the funding agencies had a role in the conduct or publication of this manuscript.
Disclaimer: The views expressed herein are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs.
Supplementary material: Supplementary References are available at PSYCHIATRIST.COM.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. PubMed doi:10.1371/journal.pmed.1000100
2. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed doi:10.1371/journal.pmed.1000097
3. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines, 1: introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383-394. PubMed doi:10.1016/j.jclinepi.2010.04.026
4. ElSohly MA, Ross SA, Mehmedic Z, et al. Potency trends of Δ9-THC and other cannabinoids in confiscated marijuana from 1980-1997. J Forensic Sci. 2000;45(1):24-30. PubMed doi:10.1520/JFS14636J
5. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br J Pharmacol. 2011;163(7):1344-1364. PubMed doi:10.1111/j.1476-5381.2011.01238.x
6. Galal AM, Slade D, Gul W, et al. Naturally occurring and related synthetic cannabinoids and their potential therapeutic applications. Recent Patents CNS Drug Discov. 2009;4(2):112-136. PubMed doi:10.2174/157488909788453031
7. Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64(1):21-47. PubMed doi:10.1146/annurev-psych-113011-143739
8. Schubart CD, Sommer IE, Fusar-Poli P, et al. Cannabidiol as a potential treatment for psychosis. Eur Neuropsychopharmacol. 2014;24(1):51-64. PubMed doi:10.1016/j.euroneuro.2013.11.002
9. Davis WM, Hatoum NS. Neurobehavioral actions of cannabichromene and interactions with Δ9-tetrahydrocannabinol. Gen Pharmacol. 1983;14(2):247-252. PubMed doi:10.1016/0306-3623(83)90004-6
10. Valdeolivas S, Navarrete C, Cantarero I, et al. Neuroprotective properties of cannabigerol in Huntington’s disease: studies in R6/2 mice and 3-nitropropionate-lesioned mice. Neurotherapeutics. 2015;12(1):185-199. PubMed doi:10.1007/s13311-014-0304-z
11. Farrimond JA, Whalley BJ, Williams CM. Cannabinol and cannabidiol exert opposing effects on rat feeding patterns. Psychopharmacology (Berl). 2012;223(1):117-129. PubMed doi:10.1007/s00213-012-2697-x
12. Rey AA, Purrio M, Viveros MP, et al. Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology. 2012;37(12):2624-2634. PubMed doi:10.1038/npp.2012.123
13. Pertwee RG, Howlett AC, Abood ME, et al. International union of basic and clinical pharmacology, LXXIX: cannabinoid receptors and their ligands: beyond CBâ‚ and CBâ‚‚. Pharmacol Rev. 2010;62(4):588-631. PubMed doi:10.1124/pr.110.003004
14. Marrs WR, Blankman JL, Horne EA, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13(8):951-957. PubMed doi:10.1038/nn.2601
15. Müller-Vahl KR, Schneider U, Koblenz A, et al. Treatment of Tourette’s syndrome with Δ9-tetrahydrocannabinol (THC): a randomized crossover trial. Pharmacopsychiatry. 2002;35(2):57-61. PubMed doi:10.1055/s-2002-25028
16. Müller-Vahl KR, Schneider U, Prevedel H, et al. Δ9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. J Clin Psychiatry. 2003;64(4):459-465. PubMed doi:10.4088/JCP.v64n0417
17. Cameron C, Watson D, Robinson J. Use of a synthetic cannabinoid in a correctional population for posttraumatic stress disorder-related insomnia and nightmares, chronic pain, harm reduction, and other indications: a retrospective evaluation. J Clin Psychopharmacol. 2014;34(5):559-564. PubMed doi:10.1097/JCP.0000000000000180
18. Fraser GA. The use of a synthetic cannabinoid in the management of treatment-resistant nightmares in posttraumatic stress disorder (PTSD). CNS Neurosci Ther. 2009;15(1):84-88. PubMed doi:10.1111/j.1755-5949.2008.00071.x
19. Jetly R, Heber A, Fraser G, et al. The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology. 2015;51:585-588. PubMed
20. Roitman P, Mechoulam R, Cooper-Kazaz R, et al. Preliminary, open-label, pilot study of add-on oral Δ9-tetrahydrocannabinol in chronic post-traumatic stress disorder. Clin Drug Investig. 2014;34(8):587-591. PubMed doi:10.1007/s40261-014-0212-3
21. Mashiah M. Medical Cannabis as treatment for chronic combat PTSD: promising results in an open pilot study. Patients Out of Time Conference. 2012; Tucson, AZ.
22. Reznik I. Medical marijuana/cannabis use in patients with post-traumatic stress disorder. The International Conference on Integrative Medicine. 2011; Jerusalem, Israel.
23. Volicer L, Stelly M, Morris J, et al. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 1997;12(9):913-919. PubMed doi:10.1002/(SICI)1099-1166(199709)12:9<913::AID-GPS663>3.0.CO;2-D
24. Walther S, Mahlberg R, Eichmann U, et al. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology (Berl). 2006;185(4):524-528. PubMed doi:10.1007/s00213-006-0343-1
25. Walther S, Schüpbach B, Seifritz E, et al. Randomized, controlled crossover trial of dronabinol, 2.5 mg, for agitation in 2 patients with dementia. J Clin Psychopharmacol. 2011;31(2):256-258. PubMed doi:10.1097/JCP.0b013e31820e861c
26. Woodward MR, Harper DG, Stolyar A, et al. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am J Geriatr Psychiatry. 2014;22(4):415-419. PubMed doi:10.1016/j.jagp.2012.11.022
27. Curtis A, Clarke CE, Rickards HE. Cannabinoids for Tourette’s syndrome. Cochrane Database Syst Rev. 2009;(4):CD006565. PubMed
28. Müller-Vahl KR, Kolbe H, Schneider U, et al. Cannabinoids: possible role in patho-physiology and therapy of Gilles de la Tourette syndrome. Acta Psychiatr Scand. 1998;98(6):502-506. PubMed doi:10.1111/j.1600-0447.1998.tb10127.x
29. de Bitencourt RM, Pamplona FA, Takahashi RN. A current overview of cannabinoids and glucocorticoids in facilitating extinction of aversive memories: potential extinction enhancers. Neuropharmacology. 2013;64:389-395. PubMed doi:10.1016/j.neuropharm.2012.05.039
30. Bonn-Miller MO, Babson KA, Vandrey R. Using cannabis to help you sleep: heightened frequency of medical cannabis use among those with PTSD. Drug Alcohol Depend. 2014;136:162-165. PubMed doi:10.1016/j.drugalcdep.2013.12.008
31. Ahmed AI, van den Elsen GA, Colbers A, et al. Safety, pharmacodynamics, and pharmacokinetics of multiple oral doses of delta-9-tetrahydrocannabinol in older persons with dementia. Psychopharmacology (Berl). 2015;232(14):2587-2595. PubMed doi:10.1007/s00213-015-3889-y
32. Krishnan S, Cairns R, Howard R. Cannabinoids for the treatment of dementia. Cochrane Database Syst Rev. 2009;(2):CD007204. PubMed
33. Cougle JR, Bonn-Miller MO, Vujanovic AA, et al. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25(3):554-558. PubMed doi:10.1037/a0023076
34. Bonn-Miller MO, Vujanovic AA, Drescher KD. Cannabis use among military veterans after residential treatment for posttraumatic stress disorder. Psychol Addict Behav. 2011;25(3):485-491. PubMed doi:10.1037/a0021945
35. Greer GR, Grob CS, Halberstadt AL. PTSD symptom reports of patients evaluated for the New Mexico Medical Cannabis Program. J Psychoactive Drugs. 2014;46(1):73-77. PubMed doi:10.1080/02791072.2013.873843
36. Boden MT, Babson KA, Vujanovic AA, et al. Posttraumatic stress disorder and cannabis use characteristics among military veterans with cannabis dependence. Am J Addict. 2013;22(3):277-284. PubMed doi:10.1111/j.1521-0391.2012.12018.x
37. Bordieri MJ, Tull MT, McDermott MJ, et al. The moderating role of experiential avoidance in the relationship between posttraumatic stress disorder symptom severity and Cannabis dependence. J Contextual Behav Sci. 2014;3(4):273-278. PubMed doi:10.1016/j.jcbs.2014.08.005
38. Earleywine M, Bolles JR. Marijuana, expectancies, and post-traumatic stress symptoms: a preliminary investigation. J Psychoactive Drugs. 2014;46(3):171-177. PubMed doi:10.1080/02791072.2014.920118
39. Wilkinson ST, Stefanovics E, Rosenheck RA. Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry. 2015;76(9):1174-1180. PubMed doi:10.4088/JCP.14m09475
40. van den Elsen GA, Ahmed AI, Lammers M, et al. Efficacy and safety of medical cannabinoids in older subjects: a systematic review. Ageing Res Rev. 2014;14:56-64. PubMed doi:10.1016/j.arr.2014.01.007
41. Ahmed AI, van den Elsen GA, Colbers A, et al. Safety and pharmacokinetics of oral delta-9-tetrahydrocannabinol in healthy older subjects: a randomized controlled trial. Eur Neuropsychopharmacol. 2014;24(9):1475-1482. PubMed doi:10.1016/j.euroneuro.2014.06.007
42. Passmore MJ. The cannabinoid receptor agonist nabilone for the treatment of dementia-related agitation. Int J Geriatr Psychiatry. 2008;23(1):116-117. PubMed doi:10.1002/gps.1828
43. Maroof N, Pardon MC, Kendall DA. Endocannabinoid signalling in Alzheimer’s disease. Biochem Soc Trans. 2013;41(6):1583-1587. PubMed doi:10.1042/BST20130140
44. Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Exp Clin Psychopharmacol. 1994;2(3):244-268. doi:10.1037/1064-1297.2.3.244
45. Perkonigg A, Goodwin RD, Fiedler A, et al. The natural course of cannabis use, abuse and dependence during the first decades of life. Addiction. 2008;103(3):439-449, discussion 450-451. PubMed doi:10.1111/j.1360-0443.2007.02064.x
46. Volkow ND, Baler RD, Compton WM, et al. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219-2227. PubMed doi:10.1056/NEJMra1402309
47. Aceto MD, Scates SM, Lowe JA, et al. Cannabinoid precipitated withdrawal by the selective cannabinoid receptor antagonist, SR 141716A. Eur J Pharmacol. 1995;282(1-3):R1-R2. PubMed doi:10.1016/0014-2999(95)00447-S
48. Aceto MD, Scates SM, Martin BB. Spontaneous and precipitated withdrawal with a synthetic cannabinoid, WIN 55212-2. Eur J Pharmacol. 2001;416(1-2):75-81. PubMed doi:10.1016/S0014-2999(01)00873-1
49. Budney AJ, Moore BA, Vandrey RG, et al. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112(3):393-402. PubMed doi:10.1037/0021-843X.112.3.393
50. Kouri EM, Pope HG Jr. Abstinence symptoms during withdrawal from chronic marijuana use. Exp Clin Psychopharmacol. 2000;8(4):483-492. PubMed doi:10.1037/1064-1297.8.4.483
51. Hirvonen J, Goodwin RS, Li CT, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17(6):642-649. PubMed doi:10.1038/mp.2011.82
52. Sim-Selley LJ, Schechter NS, Rorrer WK, et al. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol Pharmacol. 2006;70(3):986-996. PubMed doi:10.1124/mol.105.019612
53. D’ Souza DC, Perry E, MacDougall L, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558-1572. PubMed doi:10.1038/sj.npp.1300496
54. Andréasson S, Allebeck P, Engström A, et al. Cannabis and schizophrenia: a longitudinal study of Swedish conscripts. Lancet. 1987;2(8574):1483-1486. PubMed doi:10.1016/S0140-6736(87)92620-1
55. Radhakrishnan R, Wilkinson ST, D’ Souza DC. Gone to pot—a review of the association between Cannabis and psychosis. Front Psychiatry. 2014;5:54. PubMed doi:10.3389/fpsyt.2014.00054
56. Large M, Sharma S, Compton MT, et al. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch Gen Psychiatry. 2011;68(6):555-561. PubMed doi:10.1001/archgenpsychiatry.2011.5
57. Lev-Ran S, Roerecke M, Le Foll B, et al. The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med. 2014;44(4):797-810. PubMed doi:10.1017/S0033291713001438
58. Pope HG Jr, Yurgelun-Todd D. The residual cognitive effects of heavy marijuana use in college students. JAMA. 1996;275(7):521-527. PubMed doi:10.1001/jama.1996.03530310027028
59. Pope HG Jr, Gruber AJ, Yurgelun-Todd D. Residual neuropsychologic effects of cannabis. Curr Psychiatry Rep. 2001;3(6):507-512. PubMed doi:10.1007/s11920-001-0045-7
60. Bolla KI, Brown K, Eldreth D, et al. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337-1343. PubMed doi:10.1212/01.WNL.0000031422.66442.49
61. Meier MH, Caspi A, Ambler A, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci U S A. 2012;109(40):E2657-E2664. PubMed doi:10.1073/pnas.1206820109
62. Solowij N, Battisti R. The chronic effects of cannabis on memory in humans: a review. Curr Drug Abuse Rev. 2008;1(1):81-98. PubMed doi:10.2174/1874473710801010081
63. Meier MH, Hill ML, Small PJ, et al. Associations of adolescent cannabis use with academic performance and mental health: a longitudinal study of upper middle class youth. Drug Alcohol Depend. 2015;156:207-212. PubMed doi:10.1016/j.drugalcdep.2015.09.010
64. Brady JE, Li G. Trends in alcohol and other drugs detected in fatally injured drivers in the United States, 1999-2010. Am J Epidemiol. 2014;179(6):692-699. PubMed doi:10.1093/aje/kwt327
65. Asbridge M, Hayden JA, Cartwright JL. Acute cannabis consumption and motor vehicle collision risk: systematic review of observational studies and meta-analysis. BMJ. 2012;344:e536. PubMed doi:10.1136/bmj.e536
66. Sewell RA, Poling J, Sofuoglu M. The effect of cannabis compared with alcohol on driving. Am J Addict. 2009;18(3):185-193. PubMed doi:10.1080/10550490902786934
67. Ronen A, Gershon P, Drobiner H, et al. Effects of THC on driving performance, physiological state and subjective feelings relative to alcohol. Accid Anal Prev. 2008;40(3):926-934. PubMed doi:10.1016/j.aap.2007.10.011
68. Ramaekers JG, Robbe HW, O’ Hanlon JF. Marijuana, alcohol and actual driving performance. Hum Psychopharmacol. 2000;15(7):551-558. PubMed doi:10.1002/1099-1077(200010)15:7<551::AID-HUP236>3.0.CO;2-P
69. Lee MH, Hancox RJ. Effects of smoking cannabis on lung function. Expert Rev Respir Med. 2011;5(4):537-546, quiz 547. PubMed doi:10.1586/ers.11.40
70. Pletcher MJ, Vittinghoff E, Kalhan R, et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA. 2012;307(2):173-181. PubMed doi:10.1001/jama.2011.1961
71. Yamreudeewong W, Wong HK, Brausch LM, et al. Probable interaction between warfarin and marijuana smoking. Ann Pharmacother. 2009;43(7):1347-1353. PubMed doi:10.1345/aph.1M064
72. Kosel BW, Aweeka FT, Benowitz NL, et al. The effects of cannabinoids on the pharmacokinetics of indinavir and nelfinavir. AIDS. 2002;16(4):543-550. PubMed doi:10.1097/00002030-200203080-00005
73. Hadland SE, Knight JR, Harris SK. Medical marijuana: review of the science and implications for developmental-behavioral pediatric practice. J Dev Behav Pediatr. 2015;36(2):115-123. PubMed doi:10.1097/DBP.0000000000000129
74. Wang GS, Roosevelt G, Heard K. Pediatric marijuana exposures in a medical marijuana state. JAMA Pediatr. 2013;167(7):630-633. PubMed doi:10.1001/jamapediatrics.2013.140
75. Ware MA, Fitzcharles MA, Joseph L, et al. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg. 2010;110(2):604-610. PubMed doi:10.1213/ANE.0b013e3181c76f70
76. Miller FG, Wendler D, Swartzman LC. Deception in research on the placebo effect. PLoS Med. 2005;2(9):e262. PubMed doi:10.1371/journal.pmed.0020262
<!–a–>
*SR = supplementary reference, eg, SR1 means supplementary reference 1; these references are available in the supplementary material at PSYCHIATRIST.COM.
This PDF is free for all visitors!