Objective: Despite abundant literature demonstrating increased metabolic syndrome (MetS) prevalence and important clinical correlates of MetS among middle-age adults with bipolar disorder, little is known about this topic among adolescents and young adults early in their course of bipolar disorder. We therefore examined this topic in the Course and Outcome of Bipolar Youth (COBY) study.
Methods: A cross-sectional, retrospective study was conducted of 162 adolescents and young adults (mean ± SD age = 20.8 ± 3.7 years; range, 13.6-28.3 years) with bipolar disorder (I, II, or not otherwise specified, based on DSM-IV) enrolled in COBY between 2000 and 2006. MetS measures (blood pressure, glucose, high-density lipoprotein cholesterol [HDL-C], triglycerides, and waist circumference), defined using the International Diabetes Federation criteria, were obtained at a single timepoint. Mood, comorbidity, and treatment over the 6 months preceding the MetS assessment were evaluated using the Longitudinal Interval Follow-Up Evaluation.
Results: The prevalence of MetS in the sample was 19.8% (32/162). Low HDL-C (56.5%) and abdominal obesity (46.9%) were the most common MetS criteria. MetS was nominally associated with lower lifetime global functioning at COBY intake (odds ratio [OR] = 0.97, P = .06). MetS was significantly associated with percentage of weeks in full-threshold pure depression (OR = 1.07, P = .02) and percentage of weeks receiving antidepressant medications (OR = 1.06, P = .001) in the preceding 6 months. MetS was not associated with manic symptoms or medications other than antidepressants.
Conclusions: The prevalence of MetS in this sample was at least double compared to the general population. Moreover, MetS is associated with increased burden of depression symptoms in this group. Management of early-onset bipolar disorder should integrate strategies focused on modifying MetS risk factors.’ ‹
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $10 processing fee will apply.
CME Objective
After studying this article, you should be able to:
‘ ¢Integrate strategies focused on modifying risk factors for metabolic syndrome in the management of bipolar disorder in young patients
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in July 2019 and is eligible for AMA PRA Category 1 Creditâ„¢ through August 31, 2021. The latest review of this material was July 2019.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
ABSTRACT
Objective: Despite abundant literature demonstrating increased metabolic syndrome (MetS) prevalence and important clinical correlates of MetS among middle-age adults with bipolar disorder, little is known about this topic among adolescents and young adults early in their course of bipolar disorder. We therefore examined this topic in the Course and Outcome of Bipolar Youth (COBY) study.
Methods: A cross-sectional, retrospective study was conducted of 162 adolescents and young adults (mean ± SD age = 20.8 ± 3.7 years; range, 13.6-28.3 years) with bipolar disorder (I, II, or not otherwise specified, based on DSM-IV) enrolled in COBY between 2000 and 2006. MetS measures (blood pressure, glucose, high-density lipoprotein cholesterol [HDL-C], triglycerides, and waist circumference), defined using the International Diabetes Federation criteria, were obtained at a single timepoint. Mood, comorbidity, and treatment over the 6 months preceding the MetS assessment were evaluated using the Longitudinal Interval Follow-Up Evaluation.
Results: The prevalence of MetS in the sample was 19.8% (32/162). Low HDL-C (56.5%) and abdominal obesity (46.9%) were the most common MetS criteria. MetS was nominally associated with lower lifetime global functioning at COBY intake (odds ratio [OR] = 0.97, P = .06). MetS was significantly associated with percentage of weeks in full-threshold pure depression (OR = 1.07, P = .02) and percentage of weeks receiving antidepressant medications (OR = 1.06, P = .001) in the preceding 6 months. MetS was not associated with manic symptoms or medications other than antidepressants.
Conclusions: The prevalence of MetS in this sample was at least double compared to the general population. Moreover, MetS is associated with increased burden of depression symptoms in this group. Management of early-onset bipolar disorder should integrate strategies focused on modifying MetS risk factors.
J Clin Psychiatry 2019;80(4):18m12422
To cite: Li C, Birmaher B, Rooks B, et al. High prevalence of metabolic syndrome among adolescents and young adults with bipolar disorder. J Clin Psychiatry. 2019;80(4):18m12422.
To share: https://doi.org/10.4088/JCP.18m12422
© Copyright 2019 Physicians Postgraduate Press, Inc.
aCentre for Youth Bipolar Disorder, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada
bUniversity of Toronto Faculty of Medicine, Toronto, Ontario, Canada
cDepartment of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh School of Medicine, Pittsburgh, Pennsylvania
dDepartment of Psychiatry and Human Behavior, Warren Alpert Medical School of Brown University, Providence, Rhode Island
eDepartment of Psychiatry, Nationwide Children’s Hospital and The Ohio State College of Medicine, Columbus, Ohio
fBradley Hospital, Riverside, Rhode Island
gButler Hospital, Riverside, Rhode Island
hDepartment of Statistics, University of Pittsburgh, Pittsburgh, Pennsylvania
iDepartment of Psychiatry and Biobehavioral Sciences, David Geffen School of Medicine, UCLA, Los Angeles, California
jMassachusetts Mental Health Center and Department of Psychiatry, Beth Israel Deaconess Medical Center, Boston, Massachusetts (current affiliations)
*Corresponding author: Benjamin I. Goldstein, MD, PhD, Centre for Youth Bipolar Disorder, Sunnybrook Health Sciences Centre, 2075 Bayview Ave, EG-48, Toronto, Ontario, Canada ([email protected]).
Bipolar disorder (BD) is a recurrent and severe mood disorder that, in addition to the burden of depressive and manic symptoms, is associated with significant medical and psychiatric comorbidities.1 Numerous studies have demonstrated a high prevalence of metabolic syndrome (MetS) and MetS components (obesity, dyslipidemia, hypertension, and hyperglycemia) in adults with BD,2-9 with MetS prevalence ranging from about 10% to over 60%.2,10 MetS is a cluster of clinical and biochemical abnormalities that predispose individuals to cardiovascular disease (CVD) and diabetes mellitus.11 The International Diabetes Federation (IDF) criteria for MetS require the presence of central obesity (waist circumference > 37 in for men, > 31.5 in for women) plus any 2 of high triglycerides (≥ 150 mg/dL), low high-density lipoprotein cholesterol (HDL-C; < 40 mg/dL for men, < 50 mg/dL for women), high systolic (> 130 mm Hg) and/or diastolic (> 85 mm Hg) blood pressure, and high fasting glucose (≥ 100 mg/dL).12 A meta-analysis of 81 articles, including 6,983 adult participants, found 37.3% prevalence of MetS in BD, an odds ratio of 1.97 versus the general population, and increased risk of MetS among those currently treated with antipsychotics.10 Among individual MetS components, abdominal obesity is the most common criterion (48.7%-61% depending on the specific MetS definition), followed by high blood pressure (47.1%), low HDL-C (42.1%), and high triglycerides (39.3%), with high glucose being the least common (11.4%-17.3%, depending on the specific MetS definition).
CVD is a leading cause of increased mortality in individuals with BD.13 The presence of MetS and its components is associated with increasing age4,14-18 as well as with important clinical characteristics among adults with BD including antipsychotic medications,19-21 more psychiatric hospitalizations,22 and suicide attempts.23 Specific MetS components such as obesity have also been linked with proxies for greater BD severity including poorer treatment outcome,24 rapid cycling, chronic course,25 and lower functioning.22,25 Although most clinical studies include patients taking medications with known propensity for MetS criteria (eg, antipsychotics, lithium, divalproex), particularly obesity, epidemiologic studies that include BD samples with low rates of antimanic medication use have also reported increased rates of obesity in BD.26,27
Despite the substantial number of studies investigating MetS in adults with BD,2 little is known about MetS among adolescents and young adults with BD. One study of 200 Italian adults with BD included 22 subjects between the ages of 18 and 30 years. The prevalence of MetS was 9.1% in this age group, and the rate increased linearly with age.28 This value is higher than the MetS prevalence of 4.2% in the general pediatric and adolescent population in southern Italy.29 Other studies have investigated specific components of MetS30-33 or focused on the effects of antipsychotics on dimensional levels of MetS criteria (eg, changes in triglyceride levels) among youth with BD.34,35 For example, a study of 1,841 pediatric BD patients found that the BD cohort had increased rates of obesity and diabetes mellitus compared to healthy controls and that these higher rates are associated with more outpatient service use.31 Data from the Course and Outcome of Bipolar Illness in Youth (COBY) study revealed a 42% prevalence of overweight and obesity among pediatric BD subjects compared to 34% among the general youth population.30 Being overweight or obese was found to be most robustly associated with younger age, nonwhite race, lifetime physical abuse, substance use disorders, psychiatric hospitalizations, and exposure to ≥ 2 medication classes associated with weight gain.30 Another study of 1,848 BD subjects in Taiwan reported higher prevalence and incidence of hypertension in young adults with BD compared to the general population.33
Considering the findings of increased MetS prevalence and important clinical correlates of MetS among primarily middle-aged adults with BD, additional studies on MetS among youth and young adults with BD are indicated. The only prior study regarding MetS in this age range had a small number of subjects (N = 22),28 precluding an examination of clinical correlates. MetS confers significant risk for CVD, and the risk ratio for CVD mortality in BD compared to the general population is highest among young adults.36,37 For example, a study examining CVD mortality in Sweden reported that the CVD mortality rate ratios among BD patients between 25-34 years old is about 8, compared to 2 to 4 among older BD patients.36 Indeed, BD among adolescents was recently highlighted by the American Heart Association as a moderate-risk condition associated with accelerated atherosclerosis and early CVD.38 We therefore examined the prevalence of MetS and its components, as well as their clinical correlates, in a relatively large sample of adolescents and young adults with BD enrolled in the COBY study. We hypothesized that the prevalence of MetS in COBY would be greater than the general population and that MetS would be associated with exposure to antimanic medications, greater mood symptom burden, higher rates of suicide attempts and hospitalizations, and lower global functioning.
METHODS
Metabolic Syndrome
Metabolic syndrome was defined using the International Diabetes Federation (IDF) criteria,39 requiring the presence of central obesity (waist circumference > 37 in for men, > 31.5 in for women), plus any 2 of high triglycerides (≥ 150 mg/dL), low HDL-C (< 40 mg/dL for men, < 50 mg/dL for women), high systolic (> 130 mm Hg) and/or diastolic (> 85 mm Hg) blood pressure, and high fasting glucose (≥ 100 mg/dL). Waist circumference was measured with a SECA 201 girth measuring tape according to IDF guidelines.40 For subjects under the age of 16, waist circumference percentile values from IDF were used.39 Blood was drawn from each subject between 9:00 am-12:00 pm after a 10-hour fast and sent to the local hospital laboratory for analysis of glucose and lipids levels. Systolic and diastolic blood pressure was measured twice using Life Source automated blood pressure monitors, with analyses examining the mean measurements.
Participants
The methods for COBY have been described in detail elsewhere.41-43 In short, the study involved youths ages 7 to 17 years 11 months at intake, with Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) BD I or II or operationally defined BD not otherwise specified (NOS). Participants in the present cross-sectional, retrospective analysis included 162 adolescents and young adults, with a mean ± SD age of 20.8 ± 3.7 years (range, 13.6-28.3 years), 40.7% females, and 81.5% white race (similar to 45.5% females and 82.6% white race in the overall COBY sample), enrolled in COBY with BD I (69.1%), II (14.8%), or NOS (16%). Consecutive participants contacted for follow-up visits as part of the overall COBY study at the Pittsburgh and Brown sites were invited to participate. Participants completed a MetS visit 8.52 ± 1.60 years after enrollment in COBY. Participants are being followed prospectively, and future longitudinal studies will be forthcoming. Exclusion criteria were infectious illness within 14 days, known inflammatory or autoimmune illness, use of steroidal medication or insulin within 1 month of the MetS visit, self-reported alcohol or illicit drug use within 24 hours, and pregnancy.
Procedures
Each participating university’s institutional review board approved the study. At enrollment, participants and parents gave informed consent and were directly interviewed for the presence of current and lifetime psychiatric illnesses in the youths.
Psychiatric and Anthropometric Measures
Psychiatric diagnoses were validated with the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL),44 the Kiddie Mania Rating Scale,45 and the depression section of the K-SADS-P.46 Psychiatric symptoms over the 6-month period preceding the MetS visit were evaluated using the Longitudinal Interval Follow-Up Evaluation (LIFE)47 and tracked on a week-by-week basis using this instrument’s Psychiatric Status Rating (PSR) scales. Analyses focused on PSR scores over the 6 months prior to the measurement of MetS components, as this was the target interval between COBY visits and associations between MetS and its predictors were anticipated to be strongest when examining the most proximal epoch of follow-up.
All assessments were performed by research staff trained to reliably administer the interviews. Interview results were presented to child psychiatrists or psychologists, who confirmed the diagnoses and PSR scores. κ values for psychiatric disorders on the K-SADS were ≥ 0.8, and intraclass correlation coefficients for syndromal/subsyndromal mood symptoms via the PSR were ≥ 0.75.
First- and second-degree family psychiatric history was ascertained using the Family History Screen.48 Socioeconomic status was ascertained at intake using the 4-factor Hollingshead scale.49 Abuse was ascertained using the KSADS-PL. Current and lifetime pharmacologic treatment exposure were obtained at the intake assessment. In addition, the Psychotropic Treatment Record and the Psychosocial Treatment Schedule of the LIFE were used to ascertain treatment exposure in the preceding 6-month period on a week-by-week basis. Weekly exposure was dichotomized (yes/no) for any psychotropic medication and for each of the following: antimanic anticonvulsants (ie, carbamazepine and/or divalproex sodium), lithium, second-generation antipsychotics, antidepressants, and stimulants. Weekly exposure to psychosocial treatments was likewise examined for 3 categories of intensity: inpatient hospitalization/residential treatment, specialized intensive services, and standard outpatient services. Global functioning was assessed at intake using the Children’s Global Assessment Scale (C-GAS).50
Statistical Analyses
Statistical analyses were performed using SAS (9.3) software. Correlations among the MetS components were examined with Pearson correlation coefficients. Comparisons of demographic and lifetime clinical characteristics by MetS group were performed using parametric and nonparametric tests where appropriate.
Logistic regression models were used to analyze the associations between presence of MetS and prospective course variables collected during the 6 months preceding blood draw. The percentages of weeks with sub- and full-threshold criteria for depression, mania/hypomania, and comorbid conditions were converted to number of weeks with sub- and full-threshold criteria, respectively, giving an associated odds ratio that reflects the expected percent increase in odds of having MetS for an additional week of symptoms. Demographic and/or lifetime clinical measures that exhibited significant associations with presence of MetS at the P ≤ .10 level were included in multiple logistic regression models as potential confounders. Given the hypothesis-generating (ie, exploratory) nature of the current study, we did not correct for multiple comparisons.
RESULTS

- Despite greatly increased cardiovascular risk in bipolar disorder, few studies have examined this topic early in life.
- There are increased rates of metabolic syndrome, a clustering of cardiovascular risk factors, in adolescents and young adults with bipolar disorder, especially those with more persistent depression.
- Improvements in cardiovascular health or depression may have reciprocal benefits for patients.
The overall prevalence of MetS in the sample was 19.8% (32/162). The prevalence of each MetS criterion was as follows: low HDL-C: 56.5%, abdominal obesity: 46.9%, high blood pressure: 24.2%, high triglycerides: 15.4%, and high glucose: 15.4%. The proportion of participants with varying counts of MetS components was as follows: 21.3% for 0 MetS components, 30% for 1 component, 28.1% for 2 components, and 20.6% for 3+ components; 78.8% of participants had at least 1 MetS component, while 48.8% had at least 2. The mean waist circumference was 35.3 inches (SD = 5.6; range, 23.8-56.8). The mean systolic blood pressure was 115.30 mm Hg (SD = 10.67; range, 90.0-142.5), and mean diastolic blood pressure was 77.09 mm Hg (SD = 9.54; range, 47.5-104.5). The mean triglyceride level was 100.68 mg/dL (SD = 79.4; range, 25.0-636.0). The mean glucose level was 93.17 mg/dL (SD = 14.93; range, 56.0-239.0). The mean HDL-C was 47.56 mg/dL (SD = 15.95; range, 21.0-101.0). We examined the co-occurrence of MetS components pairwise among participants with MetS. The most common co-occurrences were central obesity with low HDL-C (96.8%), central obesity with high diastolic blood pressure (51.6%), and low HDL-C with high diastolic blood pressure (50.0%).
For comparative reasons, we also examined MetS using the National Cholesterol Education Program (NCEP) definitions. The NCEP criteria are identical to the IDF criteria, except abdominal obesity is defined as waist circumference > 40 inches for men and > 35 inches for women, and MetS requires any 3 of central obesity, high triglycerides, low HDL-C, high systolic and/or diastolic blood pressure, and high fasting glucose.51 Using the NCEP criteria, the prevalence of MetS is 16.1% (26/162), compared to 19.8% using the IDF criteria. The prevalence of MetS components using the NCEP criteria are identical to those using IDF criteria, except the prevalence of abdominal obesity (30.4%) is lower than that of IDF criteria (46.9%).
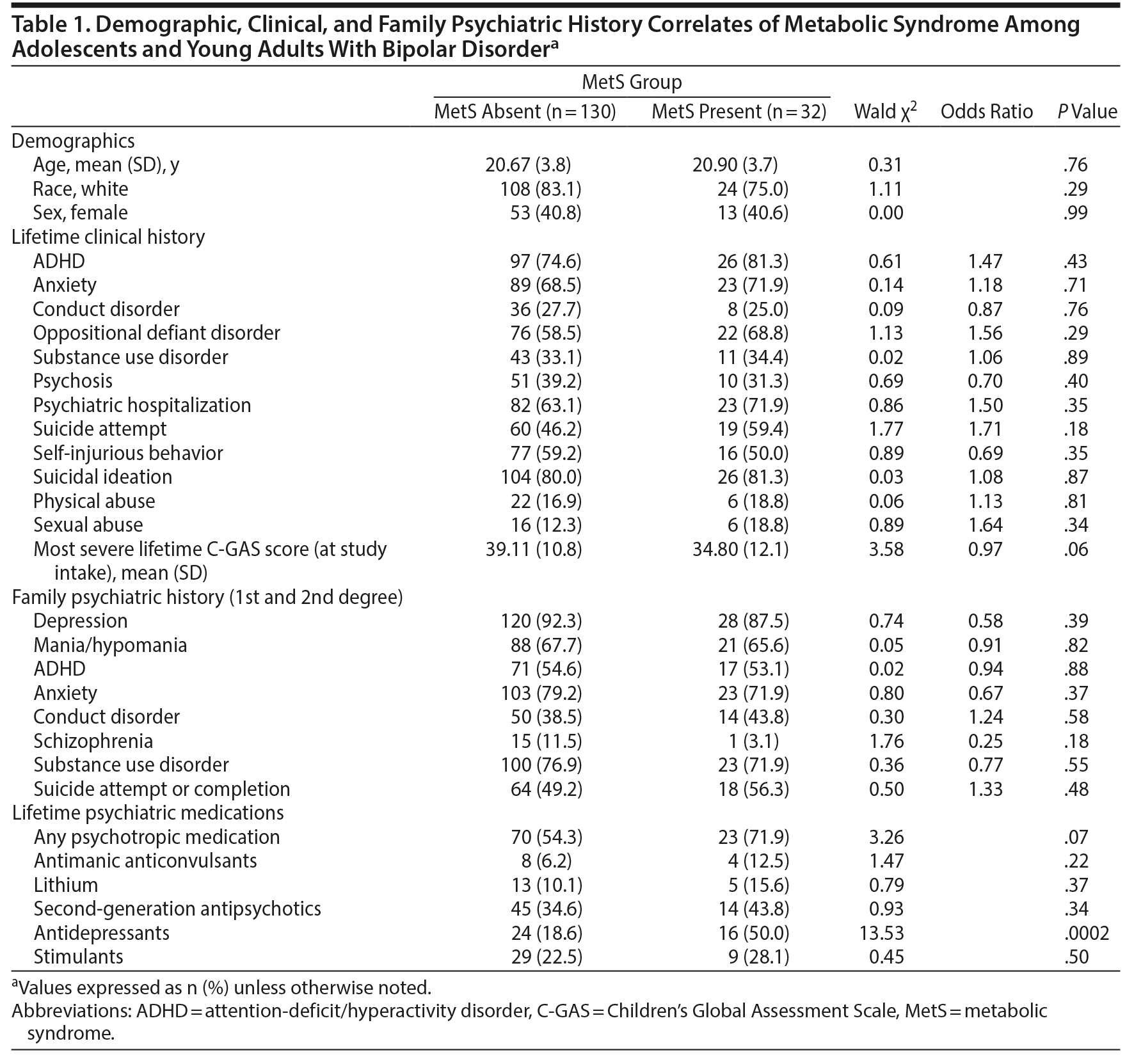
Table 1 presents the demographic, clinical, and family psychiatric history correlates of MetS. There was only a nonsignificant association (OR = 0.97, P = .06) between most severe lifetime C-GAS rating at intake and presence of MetS; this variable was therefore included as a covariate for subsequent analyses. We also evaluated in exploratory fashion whether atypical depression symptoms were associated with MetS. Because the PSR scale does not rate the severity of individual symptoms, we addressed this topic based on the presence of the following atypical depression symptoms from the K-SADS-P depression section at intake: increased sleep, fatigue, increased weight and/or appetite. Participants with, as compared to without, MetS were nominally more likely to have had increased sleep (28.1% vs 12.4%, P = .05) and nominally more likely to have all 3 of the atypical depression symptoms (12.5% vs 3.1%, P = .05).
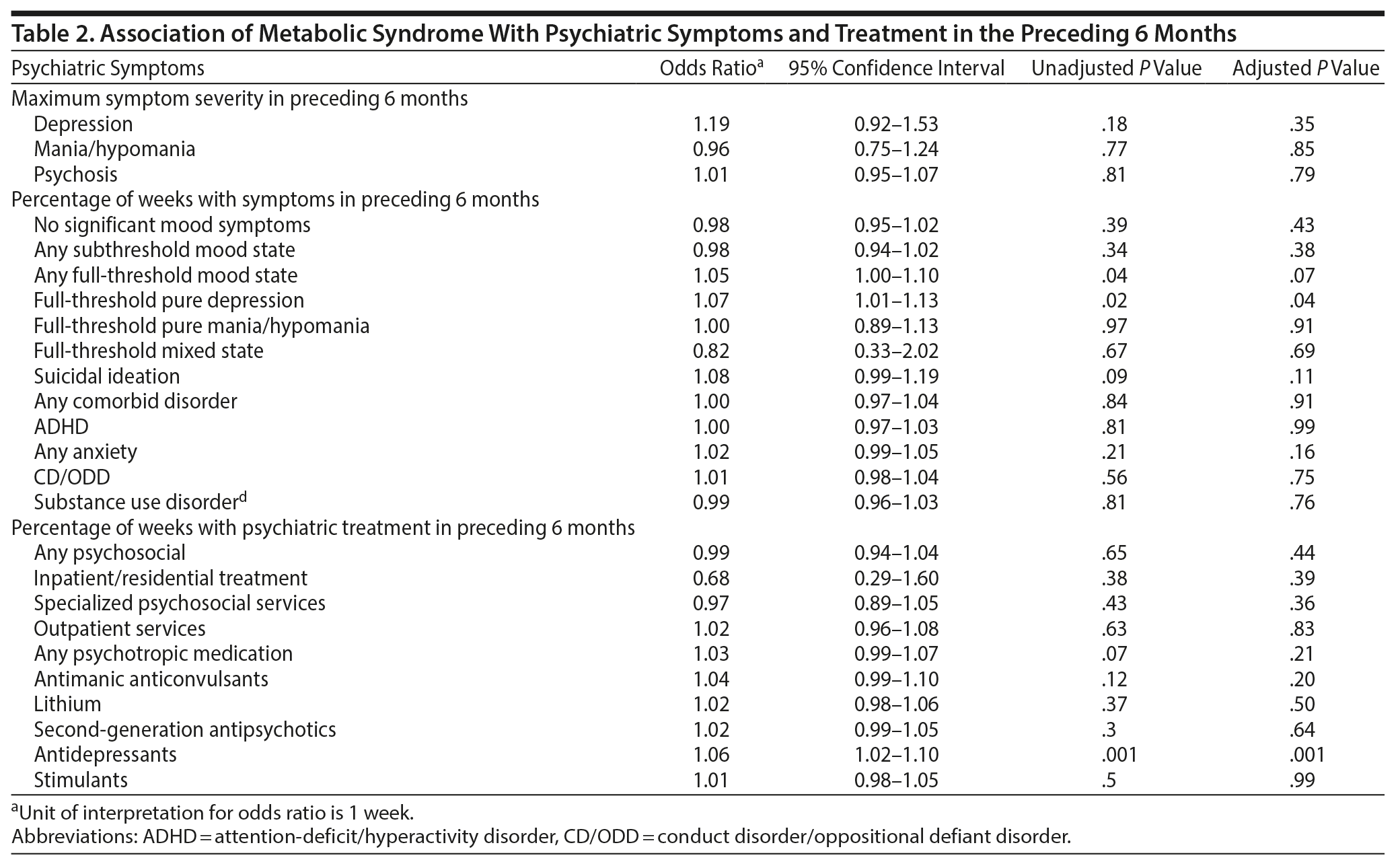
Table 2 presents the association between predictors of MetS in the 6 months preceding assessment of MetS. Presence of MetS was significantly associated with percentage of weeks in any full-threshold mood state (OR = 1.05, P = .04, 95% CI = 1.00-1.10), percentage of weeks in full-threshold pure depression (OR = 1.07, P = .02, 95% CI = 1.01-1.13), and percentage of weeks receiving antidepressant medications (OR = 1.06, P = .001, 95% CI = 1.02-1.10) in univariate analyses. Only the associations with depression symptoms and antidepressants remained significant after controlling for most severe lifetime C-GAS at intake.
To address the issue of concomitant medications, we undertook 2 sensitivity analyses. We first reran the logistic regression while sequentially covarying for each additional medication class, and odds ratio estimate for antidepressant use was virtually unchanged in each model (1.06-1.07, P values < .003). We next reran the logistic regression after sequentially excluding subjects using each of the other medication classes, and again the odds ratio estimate for antidepressant use was virtually unchanged (1.06-1.07, P values ≤ .02).
DISCUSSION
To our knowledge, this is the first study to focus on MetS among adolescents and young adults with BD. The overall prevalence of MetS, using IDF criteria, in the current study sample was 19.8%. Abdominal obesity and low HDL-C were the most common, whereas high triglycerides and elevated glucose were the least common criteria. MetS was significantly associated with most severe lifetime C-GAS rating at intake. Contrary to hypotheses, antimanic medications, and second-generation antipsychotics specifically, were not significantly associated with MetS. However, the burden of overall depression symptoms and of any full-threshold mood state over the preceding 6 months was greater, as was use of antidepressant medications, among participants with MetS. The prevalence of MetS in this sample was higher than the prevalence of 9.1% found among 22 young adults with BD in an Italian sample.28 In contrast, the prevalence of MetS in this study was lower than those found in most adult BD samples, in which the prevalence of MetS can exceed 60% (defined using various criteria).2,10 This finding is expected as MetS is generally less prevalent in youth and increases with age.28,52 By comparison to the current sample, the prevalence of IDF-defined MetS among adolescents in the general US population is 5.5%.53
The study has 3 primary limitations that should be considered before interpreting the findings. First, this study is based on a single measurement of MetS components, which precludes conclusions regarding causality and/or direction of the observed associations. Repeated-measures analyses will be informative in better understanding the associations between MetS and mood symptoms in BD. Second, the study did not include a healthy and/or clinical control group. Thus, it is not clear whether the associations observed in the current study are specific to BD. However, it is important to note the prevalence of MetS in the current study is substantially higher than that reported in the comparably aged general population. Third, the study is based on a clinical sample and may not be representative of untreated adolescents and young adults with BD.
The prevalence of hypertriglyceridemia (15.4%) among BD participants was similar to US adolescents in the general population (14.2%).53 BD participants had increased prevalence of abdominal obesity (46.9% vs 34.7%), low HDL-C (56.5% vs 21.6%), high blood pressure (24.2% vs 4.1%), and high glucose (15.4% vs 11.8%) compared to US adolescents in the general population. In addition, there was a greater proportion of participants with 3+ MetS components among BD adolescents (13.6% vs 5.5%), whereas the proportion with 2+ MetS components was similar (24.7% vs 21.3%).53
We found that the burden of depression and any full-threshold mood state symptoms in the preceding 6 months was greater among participants with MetS. Previous studies have reported associations between depression and higher prevalence of MetS.54 Putative links between mood symptoms and MetS include the direct effect of those symptoms (eg, sleep disturbance, sedentary lifestyle, increased appetite), the effects of treatments targeting those symptoms (as described below), and shared biological mechanisms such as inflammation. Indeed, a recent study regarding inflammation based on the COBY sample found that several MetS components were associated with increased levels of proinflammatory markers.43
Although antidepressants have been associated with weight gain, there is less evidence that modern antidepressants confer meaningfully increased risk of MetS.55-58 The maximum severity of depression symptoms in the preceding 6 months was not associated with MetS; however, it remains possible that this latter association is confounded by indication, whereby participants with more severe depression were more likely to receive antidepressant treatment. Indeed, among young adult women, history of major depression is associated with a 2-fold risk of MetS, independent of demographic characteristics, smoking, physical activity, nutrition, and alcohol use.59 Similar associations are observed for self-reported depression symptoms.60 Another recent study found that presence of major depression with anhedonia in a community sample of young adults is associated with increased prevalence of MetS, whereas this was not the case for major depression without anhedonia.61 Future studies are warranted to evaluate for sex differences and for specific symptom-related differences in terms of the link between MetS and depression in youth and young adults.
In contrast to contemporary antidepressants, antimanic medications in general, and second-generation antipsychotics in particular, are consistently associated with increased prevalence of MetS and its components in adults with BD.2,10 We previously reported that overweight/obesity among COBY participants at intake was associated with lifetime use of second-generation antipsychotics in univariate but not multivariate analyses.30 One may speculate that there are developmental differences in terms of risk factors for MetS in BD; for example, the impact of psychiatric symptoms and shared biology on MetS may be greater in youth, whereas the impact of psychiatric medications on MetS is greater in adults. Replication studies are warranted to evaluate this and other putative explanations for the lack of association between antimanic medications and MetS. Similarly, although we did not replicate previous studies that demonstrated associations between MetS and psychiatric hospitalization23 and suicide attempts,3,62 present findings were in the same direction, with numerically greater prevalence of MetS among COBY participants with lifetime history of psychiatric hospitalization (OR = 1.50, P = .35) and suicide attempt (OR = 1.71, P = .18).
In summary, this is the first study to examine the prevalence of MetS and its components, as well as their clinical correlates, in a relatively large sample of adolescents and young adults with BD. This study revealed that the prevalence of MetS among youth with BD is roughly quadruple that of the general population and that MetS is associated with increased burden of depression symptoms. Although results in the current study require replication in other samples with a direct comparison group, our findings suggest that excessive rates of MetS and its components, which are risk factors for CVD and diabetes, are already apparent among adolescents and young adults with BD. This phenomenon calls for the need to implement early screening, prevention, and intervention strategies for MetS and its components. Management of BD should ideally integrate medical and psychiatric care with attention on modifying MetS risk factors.63-65 Finally, the possibility that reducing MetS can reduce the burden of depression in BD remains, and studies addressing this topic are warranted.
Submitted: June 23, 2018; accepted April 17, 2019.
Published online: July 30, 2019.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, carbamazepine and divalproex are not approved by the US Food and Drug Administration for the treatment of bipolar disorder in youth, and lithium is approved only in youth 12 years and older for the treatment and prevention of mania.
Financial disclosure: Dr Axelson has served as a consultant for Janssen Research and received royalties from UpToDate. Dr Birmaher receives or will receive royalties for publications from Random House, Inc. (New Hope for Children and Teens with Bipolar Disorder), Lippincott Williams & Wilkins (Treating Child and Adolescent Depression), and UpToDate. He is employed by the University of Pittsburgh and the University of Pittsburgh Medical Center/Western Psychiatric Institute and Clinic and receives research funding from National Institute of Mental Health (NIMH). Dr Dickstein received grant support from NIMH and an independent investigator grant from the Brain and Behavior Research Foundation (NARSAD). Ms Gill receives grant support from NIMH. Dr B. I. Goldstein received grant or research support from NARSAD, Brain Canada, the Canadian Institutes of Health Research, the Heart and Stroke Foundation, NIMH, the Ontario Ministry of Research and Innovation, and University of Toronto Department of Psychiatry. Dr T. R. Goldstein receives grant support from NIMH, the American Foundation for Suicide Prevention, and the Brain & Behavior Research Foundation and royalties from Guilford Press. Ms Hower receives grant support from NIMH. Dr Hunt receives grant support from NIMH and honoraria from Wiley Publishers as a Senior Editor of the Brown University Child and Adolescent Psychopharmacology Update. Drs Iyengar, Keller, and Rooks and Ms Liao receive grant support from NIMH. Dr Keller receives grant support from the John J. McDonnell and Margaret T. O’ Brien Foundation. Dr Ryan received grant or research support from NIMH and served on the Scientific Advisory Board of the Child Mind Institute. Dr Strober receives grant support from NIMH and support from the Resnick Endowed Chair in Eating Disorders at UCLA. Dr Yen receives grant support from NIMH and American Foundation for Suicide Prevention and is a consultant at Janssen Research and Development, LLC. Dr Li has no personal affiliations or financial relationships with any commercial interest to disclose relative to this article.
Funding/support: This research was supported by the National Institute of Mental Health (NIMH) Course and Outcome of Bipolar Youth (COBY) study grants MH059929 (Dr Birmaher), MH59691 (Dr Keller/Dr Yen), and MH59977 (Dr Strober).
Role of the sponsor: No funding agency provided direct support in the conduct and/or publication of the study.
Acknowledgments: The authors thank the study participants and families for their participation, the COBY research team, and NIMH for their support.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1.Weber NS, Fisher JA, Cowan DN, et al. Psychiatric and general medical conditions comorbid with bipolar disorder in the National Hospital Discharge Survey. Psychiatr Serv. 2011;62(10):1152-1158. PubMed CrossRef
2.McElroy SL, Keck PE Jr. Metabolic syndrome in bipolar disorder: a review with a focus on bipolar depression. J Clin Psychiatry. 2014;75(1):46-61. PubMed CrossRef
3.McIntyre RS, Danilewitz M, Liauw SS, et al. Bipolar disorder and metabolic syndrome: an international perspective. J Affect Disord. 2010;126(3):366-387. PubMed CrossRef
4.Sicras A, Rejas J, Navarro R, et al. Metabolic syndrome in bipolar disorder: a cross-sectional assessment of a Health Management Organization database. Bipolar Disord. 2008;10(5):607-616. PubMed CrossRef
5.Guan N, Liu H, Diao F, et al. Prevalence of metabolic syndrome in bipolar patients initiating acute-phase treatment: a 6-month follow up. Psychiatry Clin Neurosci. 2010;64(6):625-633. PubMed CrossRef
6.Elmslie JL, Porter RJ, Joyce PR, et al. Comparison of insulin resistance, metabolic syndrome and adiponectin in overweight bipolar patients taking sodium valproate and controls. Aust N Z J Psychiatry. 2009;43(1):53-60. PubMed CrossRef
7.Lee NY, Kim SH, Cho B, et al. Patients taking medications for bipolar disorder are more prone to metabolic syndrome than Korea’s general population. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1243-1249. PubMed CrossRef
8.Bly MJ, Taylor SF, Dalack G, et al. Metabolic syndrome in bipolar disorder and schizophrenia: dietary and lifestyle factors compared to the general population. Bipolar Disord. 2014;16(3):277-288. PubMed CrossRef
9.Silarova B, Giltay EJ, Van Reedt Dortland A, et al. Metabolic syndrome in patients with bipolar disorder: comparison with major depressive disorder and non-psychiatric controls. J Psychosom Res. 2015;78(4):391-398. PubMed CrossRef
10.Vancampfort D, Vansteelandt K, Correll CU, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013;170(3):265-274. PubMed CrossRef
11.Miranda PJ, DeFronzo RA, Califf RM, et al. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J. 2005;149(1):33-45. PubMed CrossRef
12.Zimmet P, Alberti KG, Kaufman F, et al; IDF Consensus Group. The metabolic syndrome in children and adolescents—an IDF consensus report. Pediatr Diabetes. 2007;8(5):299-306. PubMed CrossRef
13.Roshanaei-Moghaddam B, Katon W. Premature mortality from general medical illnesses among persons with bipolar disorder: a review. Psychiatr Serv. 2009;60(2):147-156. PubMed CrossRef
14.Cardenas J, Frye MA, Marusak SL, et al. Modal subcomponents of metabolic syndrome in patients with bipolar disorder. J Affect Disord. 2008;106(1-2):91-97. PubMed CrossRef
15.Godin O, Etain B, Henry C, et al; FondaMental Advanced Centers of Expertise in Bipolar Disorders (FACE-BD) Collaborators. Metabolic syndrome in a French cohort of patients with bipolar disorder: results from the FACE-BD cohort. J Clin Psychiatry. 2014;75(10):1078-1085, quiz 1085. PubMed CrossRef
16.Grover S, Aggarwal M, Chakrabarti S, et al. Prevalence of metabolic syndrome in bipolar disorder: an exploratory study from North India. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36(1):141-146. PubMed CrossRef
17.Maina G, D’ Ambrosio V, Aguglia A, et al. Bipolar disorders and metabolic syndrome: a clinical study in 185 patients. Riv Psichiatr. 2010;45(1):34-40. PubMed
18.Salvi V, D’ Ambrosio V, Bogetto F, et al. Metabolic syndrome in Italian patients with bipolar disorder: a 2-year follow-up study. J Affect Disord. 2012;136(3):599-603. PubMed CrossRef
19.Yumru M, Savas HA, Kurt E, et al. Atypical antipsychotics related metabolic syndrome in bipolar patients. J Affect Disord. 2007;98(3):247-252. PubMed CrossRef
20.Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, et al. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20(3):131-137. PubMed CrossRef
21.Chang HH, Chou CH, Chen PS, et al. High prevalence of metabolic disturbances in patients with bipolar disorder in Taiwan. J Affect Disord. 2009;117(1-2):124-129. PubMed CrossRef
22.McIntyre RS, Woldeyohannes HO, Soczynska JK, et al. The rate of metabolic syndrome in euthymic Canadian individuals with bipolar I/II disorder. Adv Ther. 2010;27(11):828-836. PubMed CrossRef
23.Fagiolini A, Frank E, Scott JA, et al. Metabolic syndrome in bipolar disorder: findings from the Bipolar Disorder Center for Pennsylvanians. Bipolar Disord. 2005;7(5):424-430. PubMed CrossRef
24.Kemp DE, Gao K, Chan PK, et al. Medical comorbidity in bipolar disorder: relationship between illnesses of the endocrine/metabolic system and treatment outcome. Bipolar Disord. 2010;12(4):404-413. PubMed CrossRef
25.Ruzickova M, Slaney C, Garnham J, et al. Clinical features of bipolar disorder with and without comorbid diabetes mellitus. Can J Psychiatry. 2003;48(7):458-461. PubMed CrossRef
26.Goldstein BI, Liu SM, Zivkovic N, et al. The burden of obesity among adults with bipolar disorder in the United States. Bipolar Disord. 2011;13(4):387-395. PubMed CrossRef
27.McIntyre RS, Konarski JZ, Wilkins K, et al. Obesity in bipolar disorder and major depressive disorder: results from a national community health survey on mental health and well-being. Can J Psychiatry. 2006;51(5):274-280. PubMed CrossRef
28.Salvi V, D’ Ambrosio V, Rosso G, et al. Age-specific prevalence of metabolic syndrome in Italian patients with bipolar disorder. Psychiatry Clin Neurosci. 2011;65(1):47-54. PubMed CrossRef
29.Martino F, Puddu PE, Pannarale G, et al. Metabolic syndrome among children and adolescents from Southern Italy: contribution from the Calabrian Sierras Community Study (CSCS). Int J Cardiol. 2014;177(2):455-460. PubMed CrossRef
30.Goldstein BI, Birmaher B, Axelson DA, et al. Preliminary findings regarding overweight and obesity in pediatric bipolar disorder. J Clin Psychiatry. 2008;69(12):1953-1959. PubMed CrossRef
31.Jerrell JM, McIntyre RS, Tripathi A. A cohort study of the prevalence and impact of comorbid medical conditions in pediatric bipolar disorder. J Clin Psychiatry. 2010;71(11):1518-1525. PubMed CrossRef
32.Chien IC, Chang KC, Lin CH, et al. Prevalence of diabetes in patients with bipolar disorder in Taiwan: a population-based national health insurance study. Gen Hosp Psychiatry. 2010;32(6):577-582. PubMed CrossRef
33.Chien IC, Lin CH, Chou YJ, et al. Risk of hypertension in patients with bipolar disorder in Taiwan: a population-based study. Compr Psychiatry. 2013;54(6):687-693. PubMed CrossRef
34.McIntyre RS, Jerrell JM. Metabolic and cardiovascular adverse events associated with antipsychotic treatment in children and adolescents. Arch Pediatr Adolesc Med. 2008;162(10):929-935. PubMed CrossRef
35.Moreno C, Merchán-Naranjo J, Alvarez M, et al. Metabolic effects of second-generation antipsychotics in bipolar youth: comparison with other psychotic and nonpsychotic diagnoses. Bipolar Disord. 2010;12(2):172-184. PubMed CrossRef
36.Westman J, Hפllgren J, Wahlbeck K, et al. Cardiovascular mortality in bipolar disorder: a population-based cohort study in Sweden. BMJ Open. 2013;3(4):e002373. PubMed CrossRef
37.Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58(9):844-850. PubMed CrossRef
38.Goldstein BI, Carnethon MR, Matthews KA, et al; American Heart Association Atherosclerosis; Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132(10):965-986. PubMed CrossRef
39.International Diabetes Federation. IDF consensus definition of metabolic syndrome in children and adolescents. International Diabetes Federation website. https://www.idf.org/e-library/consensus-statements/61-idf-consensus-definition-of-metabolic-syndrome-in-children-and-adolescents.html. 2007.
40.International Diabetes Federation. The IDF Consensus Worldwide Definition of the Metabolic Syndrome. https://www.pitt.edu/~super1/Metabolic/IDF1.pdf. Published 2006.
41.Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175-183. PubMed CrossRef
42.Birmaher B, Axelson D, Goldstein B, et al. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. Am J Psychiatry. 2009;166(7):795-804. PubMed CrossRef
43.Goldstein BI, Lotrich F, Axelson DA, et al. Inflammatory markers among adolescents and young adults with bipolar spectrum disorders. J Clin Psychiatry. 2015;76(11):1556-1563. PubMed CrossRef
44.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980-988. PubMed CrossRef
45.Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13(4):463-470. PubMed CrossRef
46.Chambers WJ, Puig-Antich J, Hirsch M, et al. The assessment of affective disorders in children and adolescents by semistructured interview: test-retest reliability of the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present Episode Version. Arch Gen Psychiatry. 1985;42(7):696-702. PubMed CrossRef
47.Keller MB, Lavori PW, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation. a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44(6):540-548. PubMed CrossRef
48.Weissman MM, Wickramaratne P, Adams P, et al. Brief screening for family psychiatric history: the Family History Screen. Arch Gen Psychiatry. 2000;57(7):675-682. PubMed CrossRef
49.Hollingshead AA. Four factor index of social status. Yale J Sociol. 1975;8:21-52.
50.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS). Arch Gen Psychiatry. 1983;40(11):1228-1231. PubMed CrossRef
51.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421. PubMed
52.Ford ES, Li C, Zhao G, et al. Prevalence of the metabolic syndrome among US adolescents using the definition from the International Diabetes Federation. Diabetes Care. 2008;31(3):587-589. PubMed CrossRef
53.Park J, Hilmers DC, Mendoza JA, et al. Prevalence of metabolic syndrome and obesity in adolescents aged 12 to 19 years: comparison between the United States and Korea. J Korean Med Sci. 2010;25(1):75-82. PubMed CrossRef
54.Pan A, Keum N, Okereke OI, et al. Bidirectional association between depression and metabolic syndrome: a systematic review and meta-analysis of epidemiological studies. Diabetes Care. 2012;35(5):1171-1180. PubMed CrossRef
55.Correll CU. Weight gain and metabolic effects of mood stabilizers and antipsychotics in pediatric bipolar disorder: a systematic review and pooled analysis of short-term trials. J Am Acad Child Adolesc Psychiatry. 2007;46(6):687-700. PubMed CrossRef
56.Jerrell JM, McIntyre RS, Tripathi A. Childhood treatment with psychotropic medication and development of comorbid medical conditions in adolescent-onset bipolar disorder. Hum Psychopharmacol. 2011;26(7):451-459. PubMed CrossRef
57.Geller B, Luby JL, Joshi P, et al. A randomized controlled trial of risperidone, lithium, or divalproex sodium for initial treatment of bipolar I disorder, manic or mixed phase, in children and adolescents. Arch Gen Psychiatry. 2012;69(5):515-528. PubMed CrossRef
58.Mansoor B, Rengasamy M, Hilton R, et al. The bidirectional relationship between body mass index and treatment outcome in adolescents with treatment-resistant depression. J Child Adolesc Psychopharmacol. 2013;23(7):458-467. PubMed CrossRef
59.Kinder LS, Carnethon MR, Palaniappan LP, et al. Depression and the metabolic syndrome in young adults: findings from the Third National Health and Nutrition Examination Survey. Psychosom Med. 2004;66(3):316-322. PubMed CrossRef
60.Pulkki-Rץback L, Elovainio M, Kivimפki M, et al. Depressive symptoms and the metabolic syndrome in childhood and adulthood: a prospective cohort study. Health Psychol. 2009;28(1):108-116. PubMed CrossRef
61.Moreira FP, Jansen K, Cardoso TA, et al. Metabolic syndrome, depression and anhedonia among young adults. Psychiatry Res. 2019;271:306-310. PubMed CrossRef
62.Fagiolini A, Chengappa KN, Soreca I, et al. Bipolar disorder and the metabolic syndrome: causal factors, psychiatric outcomes and economic burden. CNS Drugs. 2008;22(8):655-669. PubMed CrossRef
63.Katon WJ, Lin EH, Von Korff M, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363(27):2611-2620. PubMed CrossRef
64.Druss BG, von Esenwein SA, Compton MT, et al. A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry. 2010;167(2):151-159. PubMed CrossRef
65.Kilbourne AM, Post EP, Nossek A, et al. Improving medical and psychiatric outcomes among individuals with bipolar disorder: a randomized controlled trial. Psychiatr Serv. 2008;59(7):760-768. PubMed CrossRef
This PDF is free for all visitors!