Objective: To investigate which neuropsychological tests can discriminate between behavioral variant frontotemporal dementia (bvFTD) and psychiatric disorders presenting with similar late-onset frontal behavioral changes, such as apathy, disinhibition, reduced empathy, or compulsive behavior.
Methods: Patients presenting with frontal behavioral changes in middle or late adulthood received extensive baseline examinations, including neuropsychological assessment and brain imaging. After 2 years, examinations were repeated and patients were diagnosed according to DSM-IV or international bvFTD consensus criteria. The study period was April 2011-June 2015. Two groups were selected: 32 patients with bvFTD and 53 patients with a psychiatric or psychological diagnosis. Associations between neuropsychological test scores and diagnostic group were investigated with logistic regression analyses, and diagnostic accuracy was investigated with a receiver operating characteristic curve.
Results: BvFTD patients scored lower on tests for confrontational naming, gestalt completion, and verbal abstraction compared to psychiatric patients (P < .01). The confrontational naming test (Boston Naming Test) showed the strongest association with diagnostic group: a lower score indicated a higher probability for a bvFTD diagnosis (P < .001). This test could discriminate between the groups with good diagnostic accuracy (area under the curve = 0.81). Tests for attention, memory, and executive functions showed no discriminative ability between the groups.
Conclusions: Although one of the criteria of bvFTD is low performance on executive tests, these tests are not useful in differentiating bvFTD from psychiatric disorders. We recommend administering language tests, especially an extensive confrontational naming test, to aid differentiation between bvFTD and a psychiatric disorder in patients presenting with late-onset frontal behavioral changes.
This CME activity is expired. For more CME activities, visit CMEInstitute.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $10 processing fee will apply.
CME Objective
After studying this article, you should be able to:
- Incorporate a naming test to aid differentiation between bvFTD and a psychiatric disorder in patients presenting with late-onset frontal behavioral changes
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in February 2020 and is eligible for AMA PRA Category 1 Credit™ through February 28, 2022. The latest review of this material was January 2020.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; has been a member of the Steering Committee for Educational Activities for Medscape; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
ABSTRACT
Objective: To investigate which neuropsychological tests can discriminate between behavioral variant frontotemporal dementia (bvFTD) and psychiatric disorders presenting with similar late-onset frontal behavioral changes, such as apathy, disinhibition, reduced empathy, or compulsive behavior.
Methods: Patients presenting with frontal behavioral changes in middle or late adulthood received extensive baseline examinations, including neuropsychological assessment and brain imaging. After 2 years, examinations were repeated and patients were diagnosed according to DSM-IV or international bvFTD consensus criteria. The study period was April 2011-June 2015. Two groups were selected: 32 patients with bvFTD and 53 patients with a psychiatric or psychological diagnosis. Associations between neuropsychological test scores and diagnostic group were investigated with logistic regression analyses, and diagnostic accuracy was investigated with a receiver operating characteristic curve.
Results: BvFTD patients scored lower on tests for confrontational naming, gestalt completion, and verbal abstraction compared to psychiatric patients (P < .01). The confrontational naming test (Boston Naming Test) showed the strongest association with diagnostic group: a lower score indicated a higher probability for a bvFTD diagnosis (P < .001). This test could discriminate between the groups with good diagnostic accuracy (area under the curve = 0.81). Tests for attention, memory, and executive functions showed no discriminative ability between the groups.
Conclusions: Although one of the criteria of bvFTD is low performance on executive tests, these tests are not useful in differentiating bvFTD from psychiatric disorders. We recommend administering language tests, especially an extensive confrontational naming test, to aid differentiation between bvFTD and a psychiatric disorder in patients presenting with late-onset frontal behavioral changes.
J Clin Psychiatry 2020;81(1):19m12811
To cite: Overbeek JM, Korten N, Gossink F, et al. The value of neuropsychological assessment in the differentiation between behavioral variant frontotemporal dementia and late-onset psychiatric disorders. J Clin Psychiatry. 2020;81(1):19m12811.
To share: https://doi.org/10.4088/JCP.19m12811
© Copyright 2020 Physicians Postgraduate Press, Inc.
aAlzheimer Center, Amsterdam UMC, Location VUmc, Amsterdam, The Netherlands
bDepartment of Old Age Psychiatry, GGZ inGeest, Amsterdam, The Netherlands
cAmsterdam Neuroscience, Amsterdam, The Netherlands
*Corresponding author: Jozefien M. Overbeek, MSc, Alzheimer Center, Postbus 7057, 1007 MB, Amsterdam, The Netherlands ([email protected]).
In middle or late adulthood, abnormal changes in personality and behavior can arise, with symptoms such as increased apathy, disinhibition, compulsive behavior, or reduced empathy. These symptoms reflect a dysfunction of the frontal lobes or the fronto-subcortical circuits of the brain and can be caused by a neurodegenerative disease or a psychiatric disorder such as depression.1 Patients are often referred to a psychiatrist or neurologist, who also receives the challenge to give the right diagnosis. An early and accurate diagnosis is crucial: while neurodegenerative diseases are progressive and, at present, incurable, most psychiatric disorders can be treated.
Several different neurodegenerative diseases can present with behavioral changes. Many of these can be diagnosed using nonbehavioral criteria. For instance, Alzheimer’s disease and cerebrovascular disease can be reliably diagnosed with biomarkers or brain imaging.2,3 Lewy body disease can be identified by several specific other symptoms, such as parkinsonism and REM sleep behavior disorder.4 When these neurodegenerative diseases have been ruled out, the distinction often remains between behavioral variant frontotemporal dementia (bvFTD) and psychiatric disorders.
BvFTD is characterized mostly by personality and behavioral changes, along with cognitive deterioration.5 Specifically, the behavioral criteria for bvFTD are disinhibition, apathy, reduced empathy, compulsive/stereotypical behavior, and hyperorality/diet change. All of these behavioral changes can also occur in psychiatric disorders, for instance, disinhibition in bipolar disorder and schizophrenia, apathy and reduced empathy in depression and schizophrenia, compulsive or stereotypical behavior in obsessive-compulsive disorder, and dietary changes in depression.5-7 All disorders can present with similar daily life dysfunction: social withdrawal, reduced self-care, and relationship problems.6 This large overlap in symptoms makes the diagnostic differentiation very difficult. In fact, a high proportion of bvFTD patients initially receive a psychiatric diagnosis (most often depression), and significant time can pass before the right diagnosis is given.8,9 The reverse also occurs: patients with a psychiatric disorder incorrectly receive bvFTD diagnoses,10 preventing patients from receiving appropriate treatment.
Despite the main identifying characteristics being behavioral, several biomarkers can assist in the diagnosis of bvFTD. Disproportional degrees of frontal and/or temporal atrophy on magnetic resonance imaging (MRI), hypometabolism of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) scan, and certain combinations of cerebrospinal fluid markers all have high sensitivity but moderate specificity for detecting bvFTD.11,12 Brain atrophy or hypometabolism is also found in depression.13 Approximately 10%-30% of bvFTD cases are familial and due to mutations in known genes.14 Genetic screening is reliable but is useful only in cases with a positive family history for bvFTD. Therefore, biomarkers can assist in the diagnosis of bvFTD, but they are not conclusive.
Another criterion of bvFTD is a neuropsychological profile with executive impairments and relative sparing of memory and visuospatial functions.5 Although this profile is typical for bvFTD, impairments have been reported in other cognitive domains such as memory15,16 and language.16-18

- Differentiating between behavioral variant frontotemporal dementia (bvFTD) and psychiatric disorders remains a diagnostic challenge. There is a large overlap of behavioral symptoms, such as apathy, disinhibition, reduced empathy, and compulsive behavior. Cognitive impairments occur in both patient groups, and it is unclear whether neuropsychological tests can aid in the differentiation.
- This study showed a good differentiating ability of a confrontational naming test: a high score indicated a high probability of a psychiatric diagnosis, and a low score indicated a high probability of a bvFTD diagnosis. Therefore, to aid differentiation between bvFTD and psychiatric disorder in patients presenting with late-onset frontal behavioral change, it is recommended to include a naming test in the neuropsychological assessment. Other cognitive tests showed less or no diagnostic value.
It is unclear whether bvFTD and psychiatric patients can be differentiated with a neuropsychological assessment. Psychiatric disorders can also cause cognitive impairments, for example, reduced memory and executive functions in patients with depression and bipolar disorder.19,20
There is little literature comparing neuropsychological functioning between patients with bvFTD and psychiatric disorders. One of our previous studies showed that bvFTD and psychiatric patients cannot be distinguished using the typical neuropsychological profile of bvFTD.21 BvFTD patients, however, scored lower on an emotion recognition test compared to psychiatric patients.22 Other studies show mixed results. In one study, bvFTD patients had higher executive and memory performance than patients with bipolar disorder or depression and lower verbal fluency performance than patients with bipolar disorder.23 Another study showed lower executive, but better memory performance of bvFTD patients compared to depressive patients.24 A review comparing studies with depressed patients and studies with FTD patients suggests worse performances by FTD patients on verbal fluency, semantic memory, and executive functions.25 Compared to patients with schizophrenia, bvFTD patients performed better or worse on executive tests23,26,27 and worse on verbal fluency tests.27
The most consistent of these results is better memory and lower verbal fluency performance of bvFTD patients compared to psychiatric patients. One caveat of many of these described studies, however, is that the patients were retrospectively selected and some studies included chronically ill psychiatric patients, who might have had more cognitive deficits. These patients may differ from undiagnosed patients with shorter symptom duration and therefore be less clinically representative.
Our aim was to investigate which neuropsychological tests can discriminate between bvFTD and psychiatric diagnoses in a clinically representative, prospective cohort of patients presenting with frontal behavioral changes.
METHODS
Study Sample
Data were derived from the Late-Onset Frontal lobe (LOF) study: a prospective, longitudinal study aimed at providing an early and accurate diagnosis in patients with late-onset frontal behavioral changes.28 Patients were recruited at the Alzheimer Center of the VU Medical Center and the Department of Old Age Psychiatry of GGZ InGeest between April 2011 and June 2013. The ethical review boards approved the study protocol, and written informed consent was obtained. The inclusion criteria were behavioral changes consisting of apathy, disinhibition, and/or compulsive/stereotypical behavior; age between 45-75 years with symptom onset between age 40-70 years; and a Frontal Behavior Inventory29 score of 11 or higher and/or Stereotypy Rating Inventory30 score of 10 or higher. The most important exclusion criteria were an established medical diagnosis that could explain the behavioral problems, such as dementia, a psychiatric disorder, or traumatic brain injury; Mini-Mental State Examination (MMSE) score < 18; and clinically apparent aphasia. These criteria resulted in a group with late-onset frontal behavioral changes of unknown cause.
At baseline, all patients underwent an extensive standardized assessment, including neurologic and psychiatric examinations, a clinical evaluation from a neurologist and a geriatric psychiatrist, neuropsychological assessment, laboratory tests, genetic screening, and MRI using a 3T-SignaHDxt scanner (GE Medical Systems, Milwaukee, Wisconsin). In case of insufficiently explanatory MRI results, an 18F-FDG PET scan was performed using an ECAT-EXACT-HR+ scanner (Siemens/CTI, Knoxville, Tennessee). This multidisciplinary assessment, including questionnaires, neuropsychological assessment, and brain imaging, was repeated after 2 years. Patients who were less testable due to disease progression received a shorter assessment. The diagnosis after 2 years was based mainly on progression in clinical symptoms and brain atrophy. The diagnoses were based on the international diagnostic criteria for bvFTD5 and the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision.7 The full selection criteria and the diagnostic procedure have been described in more detail previously.28 The final study sample consisted of 137 patients with different psychiatric and neurologic diagnoses.
For the present study, 2 patient groups were selected based on the diagnosis at year 2. The first group consisted of 32 patients with a diagnosis of probable (n = 28) or definite (n = 4) bvFTD. The second group consisted of 53 patients with a psychiatric or psychological diagnosis: depression (n = 14), minor depression (n = 4), bipolar disorder (n = 7), schizophrenia (n = 1), anxiety disorder (n = 1), obsessive-compulsive disorder (n = 1), autism (n = 3), personality disorder (n = 6), other psychiatric disorder (n = 5), subjective complaints (n = 5), or relational problems (n = 6). Not included were patients with possible bvFTD (n = 5) or other neurodegenerative or neurologic diagnoses (n = 42), patients for whom more than half of the neuropsychological tests were missing (n = 2), and patients with an unreliable diagnosis as result of limited or missing follow-up examinations (n = 3).
Measurements
Sample characteristics. In the baseline assessment, information was collected on age, gender, and years of education. Premorbid intelligence was estimated using the Dutch Reading Test for Adults.31 Global cognitive functioning was evaluated with the MMSE32 and frontal cognitive functioning with the Frontal Assessment Battery (FAB).33
Basic attention and processing speed. Verbal attention was measured with the test Digit Span Forward.34 Visual attention and processing speed were assessed by the Trail Making Test, Part A.35 Reading and color naming speed was measured by the Stroop test cards 1 and 2.36
Memory. Verbal memory was assessed with the Dutch version of the 15-word Auditory Verbal Learning Test.31 Associative memory was assessed with the Visual Association Test.31
Executive function. Working memory was assessed with the test Digit Span Backward.34 Inhibition was assessed with the Stroop test card 3.36 Mental flexibility was assessed with several different tests: the Meander test, a written alternating sequencing task31; the Rule Shift Cards Test37; and the Trail Making Test Part B.35 Phonemic fluency was assessed with the Dutch version of the Controlled Oral Word Association Test.31 Visual planning was measured with the Mazes Test.38 Estimation abilities were measured with the Cognitive Estimation Test.39 Finally, strategy forming was assessed with the Key Search Test.37
Language. The ability to retrieve information from semantic memory was assessed with the animal fluency test.31 Confrontational picture naming was assessed with the 29-item version of the Boston Naming Test.40 Verbal abstraction was assessed with the Similarities Test.34
Visuospatial functioning. Visual construction was assessed with the copy part of the Rey Complex Figure Test.41 Visual perception was assessed with a fragmented Figures Discovery Test.42
Social cognition. Social cognition was assessed with the Ekman Faces Test, an emotion recognition test, used in the additional analysis.43
Statistical Analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (IBM SPSS Statistics for Windows, Version 23). First, median group scores were compared to cutoff scores used in clinical practice.31 Subsequently, differences between the bvFTD and psychiatric groups in demographics and baseline test scores were compared using χ2 analysis, Student t test, or Mann-Whitney U test depending on the normality of the variables. Additionally, to investigate whether test scores at baseline are associated with diagnosis at year 2, univariate logistic regression analyses were performed for each test, with diagnostic group as dichotomous outcome variable. As age and gender might influence neuropsychological test performances, these variables were included as covariates. Subsequently, tests that were associated with diagnostic group with P < .05 were combined into a multivariate logistic regression model to investigate which tests show the highest association. Manual backward selection was used to obtain a model to which all variables contributed significantly. Potential multicollinearity was investigated by checking that the variance inflation factor was < 5 for all variables using linear regression analysis. For the tests in the resulting multivariate logistic regression model, the diagnostic accuracy was investigated by plotting a receiver operating characteristic (ROC) curve and calculating the area under the curve and the cutoff value with optimal sensitivity and specificity.
Two additional analyses were performed. First, a logistic regression analysis was done to investigate whether the best diagnostic test was still associated with diagnosis when controlling for the Ekman Faces Test, a discriminator found in a previous study.22 Second, the main analyses were repeated without the diagnoses subjective complaints and relationship problems in the psychiatric group.
The Benjamini and Hochberg procedure was used to correct for multiple comparisons, with a false discovery rate of 0.10.44 A P < .05 was used in comparing group characteristics to select possible confounders. Missing values were not included in the analyses. Most missing values were caused by organizational factors and were not related to patient characteristics. Missing values due to patient refusal or inability were evenly distributed among the diagnostic groups.
RESULTS
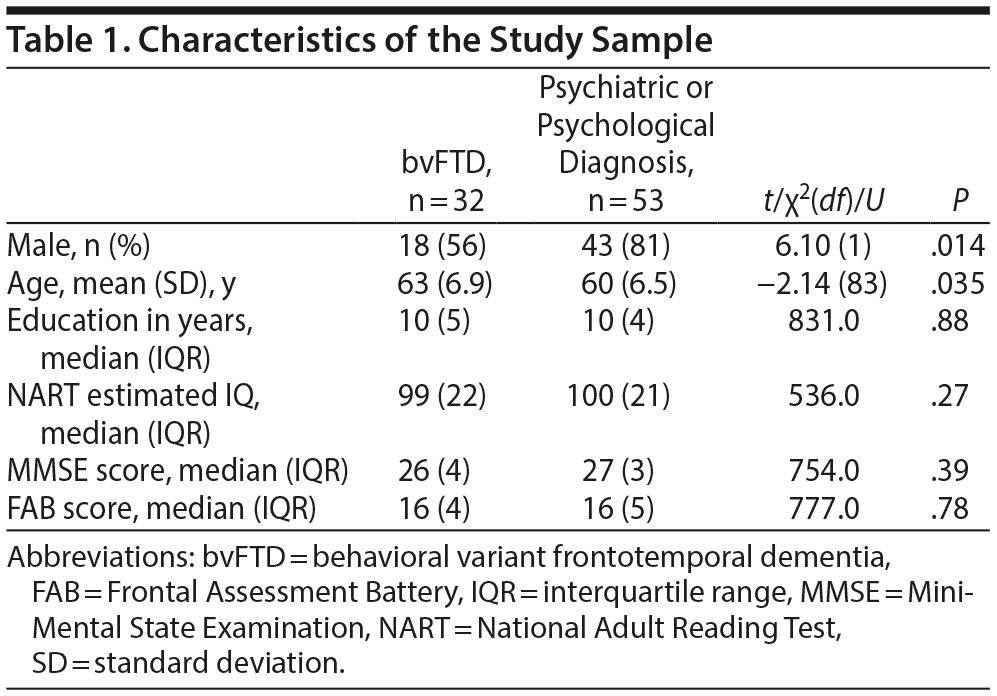
Table 1 shows the characteristics of the study sample. Patients with a psychiatric or psychological diagnosis were slightly younger and more often male compared to bvFTD patients. No significant differences were found in years of education, estimated IQ, MMSE scores, or FAB scores.
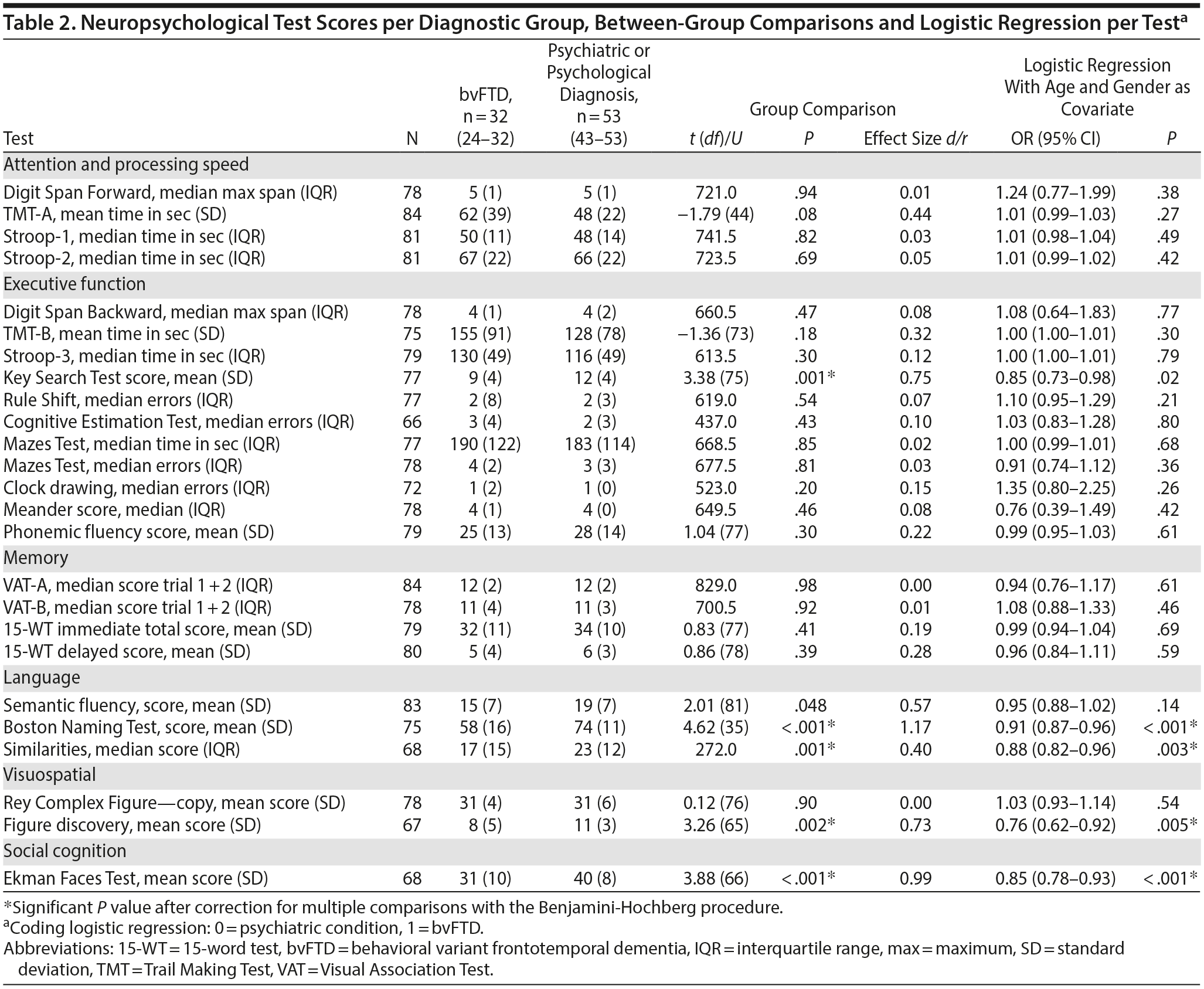
Table 2 shows the average or median test scores on each neuropsychological test for each diagnostic group. Compared to cutoff scores used in clinical practice, both groups scored average to below average on most tests, but the bvFTD group scored very low on the Boston Naming Test (z-score < 2)31 When the 2 patient groups were compared directly, the bvFTD group had significantly lower scores than the psychiatric group on the following tests: Similarities, Key Search Test, Boston Naming Test, and Figure Discovery (Table 2).
For all neuropsychological tests, a univariate logistic regression was performed with the test as independent variable and diagnostic group as dichotomous dependent variable (Table 2). After adjustment for age and gender, the tests Similarities, Figure Discovery, and Boston Naming Test were still significantly associated with diagnostic group, where a lower test score was associated with a higher probability of a bvFTD diagnosis.
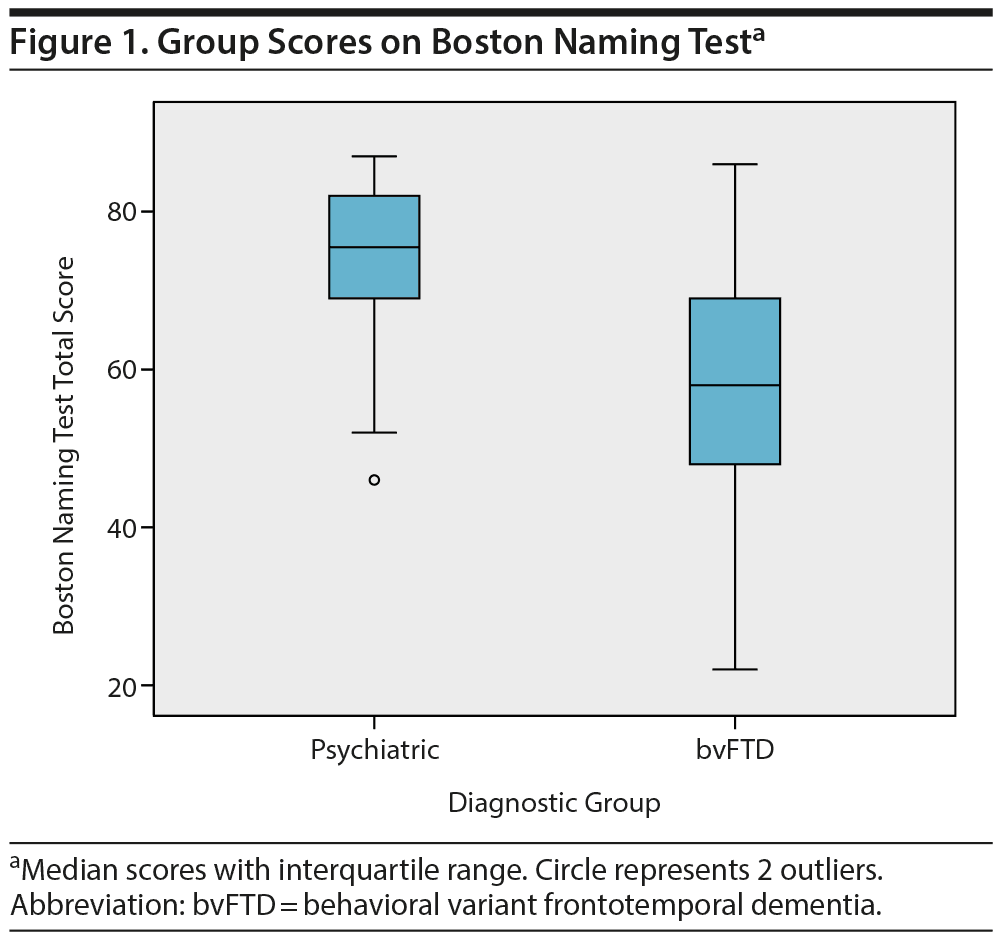
Subsequently, these neuropsychological tests were combined in a multivariate logistic regression analysis. After backward selection, a model remained with the Boston Naming Test as the only independent variable significantly associated with diagnosis (B = −0.094, SE = 0.024, P < .001). This finding indicates that a lower score on the Boston Naming Test at baseline corresponds with a higher probability of a bvFTD diagnosis at year 2. The median group scores on the Boston Naming Test are shown in Figure 1.
Diagnostic Value
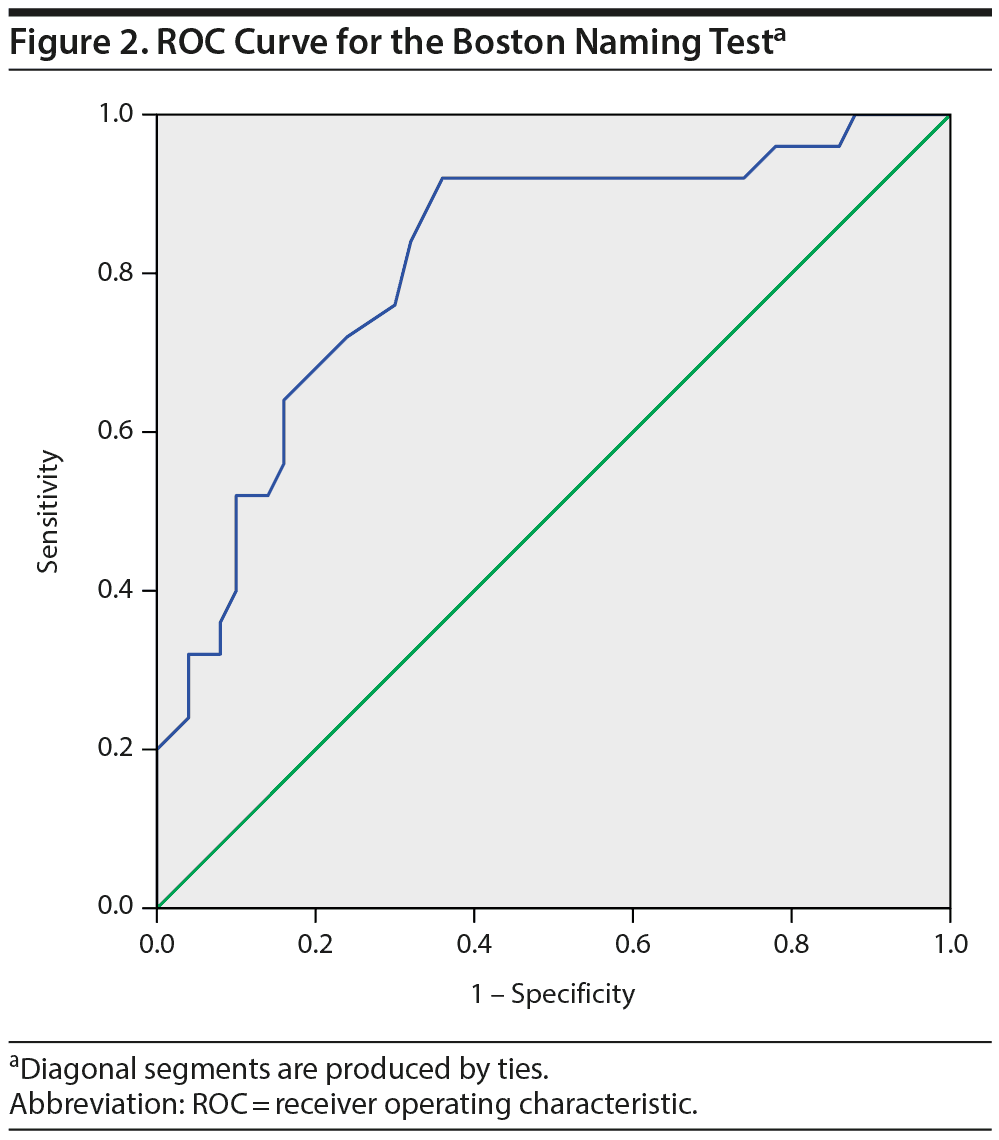
To explore the diagnostic value of the Boston Naming Test, a ROC curve was calculated that showed an area under the curve of 0.81 (95% CI = 0.71-0.92, P < .001; Figure 2), indicating a good ability of the test to differentiate the bvFTD and the psychiatric groups. The scoring of the Boston Naming Test is based on a 0-3 score for 29 items, with a maximum score of 87. A cutoff score of < 72 gives an optimal combination of a sensitivity of 92% and a specificity of 64%. However, in differential diagnosis, high specificity is more desirable than high sensitivity. Specificity for bvFTD is 90% at a cutoff score of < 60 (sensitivity = 48%), whereas the specificity of a psychiatric or psychological disorder is 90% at a cutoff score of > 71 (sensitivity = 64%). Thus, a score of 60 or lower corresponds to a high probability of bvFTD, and a score of 71 or higher corresponds to a high probability of a psychiatric disorder. Test scores between those values do not allow for reliable differentiation.
Additional Analyses
Previously, we found that an emotion recognition test, the Ekman Faces Test, discriminated between bvFTD and psychiatric disorders.22 Therefore, we performed an additional logistic regression analysis with the Boston Naming Test and Ekman Faces Test as covariates. The Boston Naming Test was still significantly associated to diagnostic group (Boston Naming Test: B = −0.095, SE = 0.032, P = .003), and the Ekman Faces Test was of borderline significance (Ekman Faces Test: B = −0.091, SE = 0.047, P = .052).
In addition, the logistic regression and ROC curve analyses were repeated with the psychiatric group without the patients with relationship problems or subjective complaints. Excluding these patients led to similar results. The logistic regression with Boston Naming Test as predictor was comparable (B = −0.090, SE = 0.026, P < .001), and the ROC curve had an area under the curve of 0.81 (95% CI = 0.70-0.92, P < .001).
CONCLUSION
The aim of our study was to investigate which neuropsychological tests are of value in differentiating between bvFTD and psychiatric disorders in patients presenting with late-onset frontal behavioral changes. Our main finding is that tests with a language component can differentiate between the groups, whereas executive tests cannot. Worse performance on a confrontational picture naming test (Boston Naming Test), a combined gestalt completion and naming test (Figure Discovery Test), and a verbal abstraction test (Similarities) corresponded to a higher probability of bvFTD diagnosis. Of these tests, the Boston Naming Test was most strongly associated to diagnostic group and could discriminate between the groups with good diagnostic accuracy. There were no differences between the groups on tests for attention, memory, and executive functions.
It is striking that all discriminative tests have a semantic language component. This corresponds with the deterioration of the anterior temporal lobe in bvFTD. The patients showed no apparent language disabilities yet, as this was an exclusion criterion. The tests might have picked up subtle language deficiencies that become more pronounced as the disease progresses. Survey questions for aphasia and amount of speech did not differ between groups, while bvFTD patients did have a higher score in literal thinking compared to psychiatric patients. This corresponds with the lower score on the verbal abstraction test.
Besides language functions, 2 of the discriminative tests (Figure Discovery and Boston Naming Test) also required visual recognition. It is unlikely that a problem in visual perception underlies the difference between the groups, as they showed no difference on another visual test (Rey Complex Figure—copy) and visuospatial functions remain intact in the early phase of bvFTD.16 Other required cognitive abilities were reasoning (Similarities Test) and looking in an exploratory, goal-directed way (Figure Discovery).
The results conflict with literature showing executive impairments in bvFTD patients compared to healthy controls. It should be noted that this study included only patients with unclear diagnoses at baseline. These patients might differ in severity from the general groups of bvFTD and psychiatric patients. More complex executive tests might show more impairments.45
This study is the first to compare extensive neuropsychological assessment of patients with bvFTD and psychiatric disorders presenting with similar frontal behavioral symptoms. As the inclusion process was symptom-based and prospective, the study sample is a heterogeneous, highly clinically representative group of patients. In addition, the diagnoses are based on a multidisciplinary, extensive diagnostic process and a follow-up period of 2 years.
A few limitations also apply. First, the heterogeneous nature of the psychiatric group does not allow translation of the findings to general groups of psychiatric patients, since the subsamples of the various diagnoses are small. However, the focus of the study was mainly on differentiating the neurodegenerative (bvFTD) from the non-neurodegenerative diagnoses (psychiatric). Second, although the diagnosis after 2 years of follow-up gives a fair diagnostic certainty, ideally, autopsy-proven diagnoses would be used. Furthermore, one might argue that there is circular reasoning because the neuropsychological assessment at baseline was used in the diagnostic procedure. However, the final diagnoses were based mainly on the progression in clinical symptoms and brain atrophy during the 2-year follow-up period. A final consideration is that the sample size was relatively small. Replication of these results in other cohorts would strengthen the diagnostic validity. A recommendation for future studies is the addition of more basic visual perception tests and a description-based verbal naming test to better differentiate between components of visual perception and naming in picture naming tests.
In conclusion, although executive tests are able to differentiate bvFTD from healthy controls, they are not useful in differentiating bvFTD from psychiatric disorders. We recommend administering language tests, especially an extensive confrontational naming test, to aid differentiation between bvFTD and a psychiatric disorder in patients presenting with late-onset frontal behavioral changes.
Submitted: March 1, 2019; accepted November 15, 2019.
Published online: February 4, 2020.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents or device therapies that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Financial disclosure: Dr Pijnenburg received a personal fellowship from the Dutch Brain Foundation. Mss Overbeek, Fieldhouse, van de Beek, and Reus and Drs Korten, Gossink, Dols, and Schouws have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: There was no direct funding for this study; the test procedures were part of regular patient care. In general, research of the Alzheimer Center Amsterdam is part of the neurodegeneration research program of the Neuroscience Campus Amsterdam. The Alzheimer Center Amsterdam is supported by Alzheimer Nederland and Stichting VUmc funds.
Previous presentation: 11th International Conference on Frontotemporal Dementias, November 11-14, 2018; Sydney, Australia ▪ Annual Congress of the Dutch Psychiatry Association, Maastricht, The Netherlands; April 3-5, 2019.
Acknowledgments: The authors are grateful to Ina Foy and Alex Leighton, MSc, for assisting with editing. They have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
REFERENCES
1.Bonelli RM, Cummings JL. Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci. 2007;9(2):141-151. PubMed
2.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. PubMed CrossRef
3.Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology. 1993;43(2):250-260. PubMed CrossRef
4.McKeith IG, Boeve BF, Dickson DW, et al. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88-100. PubMed CrossRef
5.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(pt 9):2456-2477. PubMed CrossRef
6.Pose M, Cetkovich M, Gleichgerrcht E, et al. The overlap of symptomatic dimensions between frontotemporal dementia and several psychiatric disorders that appear in late adulthood. Int Rev Psychiatry. 2013;25(2):159-167. PubMed CrossRef
7.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
8.Woolley JD, Khan BK, Murthy NK, et al. The diagnostic challenge of psychiatric symptoms in neurodegenerative disease: rates of and risk factors for prior psychiatric diagnosis in patients with early neurodegenerative disease. J Clin Psychiatry. 2011;72(2):126-133. PubMed CrossRef
9.Pijnenburg YAL, Dols A. Meaning of psychiatric symptoms in frontotemporal dementia. J Neurol Neurosurg Psychiatry. 2018;89(5):448. PubMed CrossRef
10.Shinagawa S, Catindig JA, Block NR, et al. When a little knowledge can be dangerous: false-positive diagnosis of behavioral variant frontotemporal dementia among community clinicians. Dement Geriatr Cogn Disord. 2016;41(1-2):99-108. PubMed CrossRef
11.Vijverberg EGB, Wattjes MP, Dols A, et al. Diagnostic accuracy of MRI and additional [18F]FDG-PET for behavioral variant frontotemporal dementia in patients with late onset behavioral changes. J Alzheimers Dis. 2016;53(4):1287-1297. PubMed CrossRef
12.Vijverberg EGB, Dols A, Krudop WA, et al. Cerebrospinal fluid biomarker examination as a tool to discriminate behavioral variant frontotemporal dementia from primary psychiatric disorders. Alzheimers Dement (Amst). 2017;7:99-106. PubMed
13.Drevets WC, Price JL, Simpson JR Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824-827. PubMed CrossRef
14.Seelaar H, Rohrer JD, Pijnenburg YAL, et al. Clinical, genetic and pathological heterogeneity of frontotemporal dementia: a review. J Neurol Neurosurg Psychiatry. 2011;82(5):476-486. PubMed CrossRef
15.Hornberger M, Piguet O, Graham AJ, et al. How preserved is episodic memory in behavioral variant frontotemporal dementia? Neurology. 2010;74(6):472-479. PubMed CrossRef
16.Smits LL, van Harten AC, Pijnenburg YAL, et al. Trajectories of cognitive decline in different types of dementia. Psychol Med. 2015;45(5):1051-1059. PubMed CrossRef
17.Harciarek M, Cosentino S. Language, executive function and social cognition in the diagnosis of frontotemporal dementia syndromes. Int Rev Psychiatry. 2013;25(2):178-196. PubMed CrossRef
18.Hardy CJD, Buckley AH, Downey LE, et al. The language profile of behavioral variant frontotemporal dementia. J Alzheimers Dis. 2016;50(2):359-371. PubMed CrossRef
19.Rock PL, Roiser JP, Riedel WJ, et al. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. 2014;44(10):2029-2040. PubMed CrossRef
20.Meesters PD, Schouws S, Stek M, et al. Cognitive impairment in late life schizophrenia and bipolar I disorder. Int J Geriatr Psychiatry. 2013;28(1):82-90. PubMed CrossRef
21.Vijverberg EGB, Gossink F, Krudop W, et al. The diagnostic challenge of the late-onset frontal lobe syndrome: clinical predictors for primary psychiatric disorders versus behavioral variant frontotemporal dementia. J Clin Psychiatry. 2017;78(9):e1197-e1203. PubMed CrossRef
22.Gossink F, Schouws S, Krudop W, et al. Social cognition differentiates behavioral variant frontotemporal dementia from other neurodegenerative diseases and psychiatric disorders. Am J Geriatr Psychiatry. 2018;26(5):569-579. PubMed CrossRef
23.Vijverberg EGB, Schouws S, Meesters PD, et al. Cognitive deficits in patients with neuropsychiatric symptoms: a comparative study between behavioral variant frontotemporal dementia and primary psychiatric disorders. J Clin Psychiatry. 2017;78(8):e940-e946. PubMed CrossRef
24.Braaten AJ, Parsons TD, McCue R, et al. Neurocognitive differential diagnosis of dementing diseases: Alzheimer’s dementia, vascular dementia, frontotemporal dementia, and major depressive disorder. Int J Neurosci. 2006;116(11):1271-1293. PubMed CrossRef
25.Elderkin-Thompson V, Boone KB, Hwang S, et al. Neurocognitive profiles in elderly patients with frontotemporal degeneration or major depressive disorder. J Int Neuropsychol Soc. 2004;10(5):753-771. PubMed CrossRef
26.Zakzanis KK, Kielar A, Young DA, et al. Neuropsychological differentiation of late onset schizophrenia and frontotemporal dementia. Cogn Neuropsychiatry. 2001;6(1):63-77. CrossRef
27.Ziauddeen H, Dibben C, Kipps C, et al. Negative schizophrenic symptoms and the frontal lobe syndrome: one and the same? Eur Arch Psychiatry Clin Neurosci. 2011;261(1):59-67. PubMed CrossRef
28.Krudop WA, Kerssens CJ, Dols A, et al. Building a new paradigm for the early recognition of behavioral variant frontotemporal dementia: Late Onset Frontal Lobe Syndrome study. Am J Geriatr Psychiatry. 2014;22(7):735-740. PubMed CrossRef
29.Kertesz A, Nadkarni N, Davidson W, et al. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. J Int Neuropsychol Soc. 2000;6(4):460-468. PubMed CrossRef
30.Shigenobu K, Ikeda M, Fukuhara R, et al. The Stereotypy Rating Inventory for frontotemporal lobar degeneration. Psychiatry Res. 2002;110(2):175-187. PubMed CrossRef
31.Bouma A, Mulder J, Lindeboom J, et al. Handboek Neuropsychologische Diagnostiek. Amsterdam, The Netherlands: Pearson Assessment and Information B.V.; 2012.
32.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. PubMed CrossRef
33.Dubois B, Slachevsky A, Litvan I, et al. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55(11):1621-1626. PubMed CrossRef
34.Wechsler D. WAIS-III: Wechsler Adult Intelligence Scale Administration and Scoring Manual. 3rd ed. New York, NY: Psychological Corporation; 1997.
35.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203-214. PubMed CrossRef
36.Stroop JR. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18(6):643-662. CrossRef
37.Wilson B, Alderman N, Burgess P, et al. Behavioural Assessment of the Dysexecutive Syndrome (BADS). Bury St Edmunds, UK: Thames Valley Test Company; 1996.
38.Wechsler D. WISC-III: The Wechsler Intelligence Scale for Children Manual. 3rd ed. San Antonio, TX: Psychological Corporation; 1991.
39.Gansler DA, Varvaris M, Swenson L, et al. Cognitive estimation and its assessment. J Clin Exp Neuropsychol. 2014;36(6):559-568. PubMed CrossRef
40.Lansing AE, Ivnik RJ, Cullum CM, et al. An empirically derived short form of the Boston Naming Test. Arch Clin Neuropsychol. 1999;14(6):481-487. PubMed CrossRef
41.Osterrieth P. Le test de copie d’ une figure complex: Contribution a l’ etude de la perception et de la memoire. Arch Psychol. 1944;30:206-356.
42.Luteijn F, Barelds D. Handleiding Groninger Intelligentie Test 2. Amsterdam, NL: Harcourt Test Publishers; 2004.
43.Ekman P, Friesen W. Pictures of Facial Affect. Palo Alto, CA: Consulting Psychologists Press; 1976.
44.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B (Methodological). 1995;57(1):289-300.
45.Torralva T, Roca M, Gleichgerrcht E, et al. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain. 2009;132(pt 5):1299-1309. PubMed CrossRef
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Geriatric Psychiatry section. Please contact Jordan F. Karp, MD, at [email protected], or Gary W. Small, MD, at [email protected].
This PDF is free for all visitors!