Adulthood Self-Reported Cardiovascular Risk and ADHD Medications: Results From the 2004-2005 National Epidemiologic Survey on Alcohol and Related Conditions
To the Editor: During the past 2 decades, the number of children, adolescents, and adults receiving medications for attention-deficit/hyperactivity disorder (ADHD) has substantially increased and treatment duration has been lengthened.1 Although placebo-controlled trials have shown that these agents can increase heart rate and blood pressure,2 raising concerns about their cardiovascular safety,3-5 no causal relationship between ADHD medications and serious cardiovascular events has been found among children.6,7 However, few studies have been conducted in the adult population,8-10 and most of them relied on new ADHD medication users.9,10 In the largest study conducted among adults, Habel et al8 reported that current or new use of ADHD medications with a median exposure duration of 6 months, compared with nonuse or remote use, was not associated with an increased risk of myocardial infarction, sudden cardiac death, or stroke. However, this finding needs to be replicated in a large sample of adults with a DSM-IV diagnosis of ADHD to examine whether a longer exposure duration to ADHD medications may increase the adulthood risk of serious cardiovascular events.3 In addition, whether ADHD medications may increase the risk of angina pectoris has not been examined so far.
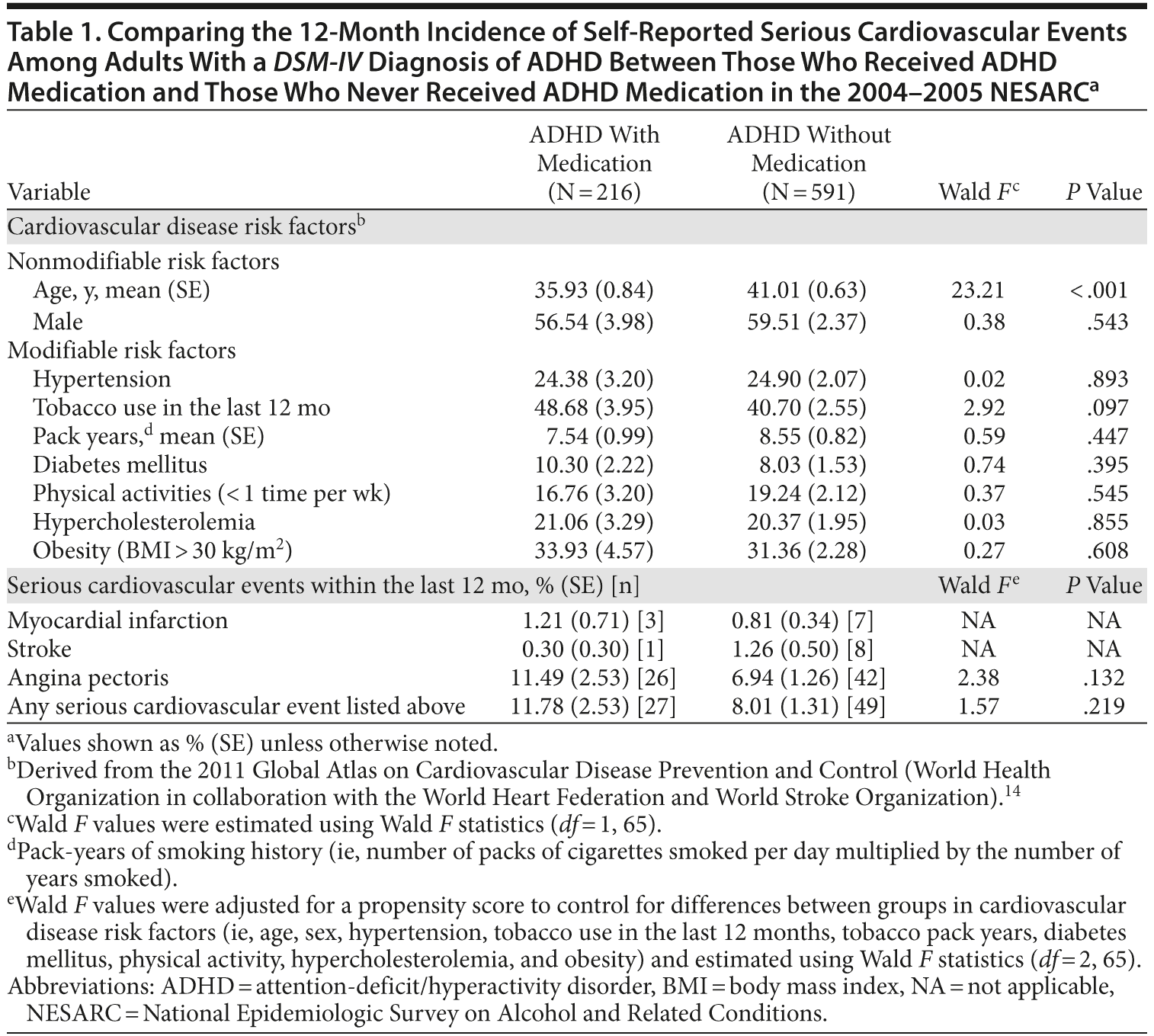
Method. On the basis of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC),11 a nationally representative survey of US civilian noninstitutionalized adult population, we investigated the association between ADHD medications and self-reported severe cardiovascular events in naturalistic conditions. Using data from the NESARC, Bernardi et al12 identified through a structured face-to-face diagnostic interview 807 subjects with a DSM-IV diagnosis of ADHD, among whom 27.6% reported having received specific medication for ADHD (N = 216). Peyre et al13 reported that, among these participants, the median treatment duration was 2.6 years (SE = 0.92), the median age at first prescription was 15.9 years, and 36.2% (SE = 0.81) of them received an ADHD medication during the past year. Participants were asked if during the past year they had a serious cardiovascular event (including myocardial infarction, angina pectoris, and stroke) that was confirmed by a doctor. Logistic regression analyses and a propensity score method were performed to adjust for self-reported risk factors for cardiovascular diseases14 using SUDAAN version 10 (RTI International, Research Triangle Park, North Carolina).
Results. Among ADHD participants, the 12-month incidence of self-reported serious cardiovascular events in those who received ADHD medication was 11.78% [N = 27] and 8.01% [N = 49] in those who were never treated (Table 1). There was no significant difference in serious cardiovascular events between groups (adjusted odds ratio [AOR] [95% CI]: 1.57 [0.79-2.72]; P = .219). In addition, there was no significant association between self-reported serious cardiovascular events and the exposure duration to ADHD medications (AOR: 1.05 [0.98-1.12]; P = .163) and between those who received an ADHD medication during the past year and those who received an ADHD medication before the past year (AOR: 0.72 [0.22-2.39]; P = .531).
After adjustments for cardiovascular risk factors were made, we found no significant association between the use of ADHD medications or the duration of use and the 12-month incidence of self-reported serious cardiovascular events in adults.
Our study has several limitations. First, cardiovascular risk factors and serious cardiovascular events were self-reported, possibly underestimating their incidence. Second, the present study could be underpowered to detect a small difference between groups. Last, data on certain risk factors (eg, unhealthy diet), other serious cardiovascular events (eg, sudden cardiac death), and daily dose of medications were not available.
Therefore, the findings from this study should be interpreted with caution. As ADHD medications may increase the risk for sudden death in patients with structural cardiac abnormalities, the current guidelines15 recommend a medical evaluation prior to starting stimulant treatment and periodic monitoring of pulse and blood pressure during treatment.
References
1. Vitiello B. Understanding the risk of using medications for attention deficit hyperactivity disorder with respect to physical growth and cardiovascular function. Child Adolesc Psychiatr Clin N Am. 2008;17(2):459-474, xi. PubMed doi:10.1016/j.chc.2007.11.010
2. Mick E, McManus DD, Goldberg RJ. Meta-analysis of increased heart rate and blood pressure associated with CNS stimulant treatment of ADHD in adults. Eur Neuropsychopharmacol. 2013;23(6):534-541. PubMed doi:10.1016/j.euroneuro.2012.06.011
3. Vasan RS, Larson MG, Leip EP, et al. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;345(18):1291-1297. PubMed doi:10.1056/NEJMoa003417
4. Nissen SE. ADHD drugs and cardiovascular risk. N Engl J Med. 2006;354(14):1445-1448. PubMed doi:10.1056/NEJMp068049
5. Hammerness PG, Perrin JM, Shelley-Abrahamson R, et al. Cardiovascular risk of stimulant treatment in pediatric attention-deficit/hyperactivity disorder: update and clinical recommendations. J Am Acad Child Adolesc Psychiatry. 2011;50(10):978-990. PubMed doi:10.1016/j.jaac.2011.07.018
6. Winterstein AG, Gerhard T, Kubilis P, et al. Cardiovascular safety of central nervous system stimulants in children and adolescents: population based cohort study. BMJ. 2012;345:e4627-e4627. PubMed doi:10.1136/bmj.e4627
7. Cooper WO, Habel LA, Sox CM, et al. ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med. 2011;365(20):1896-1904. PubMed doi:10.1056/NEJMoa1110212
8. Habel LA, Cooper WO, Sox CM, et al. ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA. 2011;306(24):2673-2683. PubMed doi:10.1001/jama.2011.1830
9. Schelleman H, Bilker WB, Kimmel SE, et al. Methylphenidate and risk of serious cardiovascular events in adults. Am J Psychiatry. 2012;169(2):178-185. PubMed doi:10.1176/appi.ajp.2011.11010125
10. Holick CN, Turnbull BR, Jones ME, et al. Atomoxetine and cerebrovascular outcomes in adults. J Clin Psychopharmacol. 2009;29(5):453-460. PubMed doi:10.1097/JCP.0b013e3181b2b828
11. Grant BF, Goldstein RB, Chou SP, et al. Sociodemographic and psychopathologic predictors of first incidence of DSM-IV substance use, mood and anxiety disorders: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. Mol Psychiatry. 2009;14(11):1051-1066. PubMed doi:10.1038/mp.2008.41
12. Bernardi S, Faraone SV, Cortese S, et al. The lifetime impact of attention deficit hyperactivity disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Psychol Med. 2012;42(4):875-887. PubMed doi:10.1017/S003329171100153X
13. Peyre H, Hoertel N, Cortese S, et al. Long-term effects of ADHD medication on adult height: results from the NESARC. J Clin Psychiatry. 2013;74(11):1123-1124. PubMed doi:10.4088/JCP.13l08580
14. World Health Organization. Cardiovascular Diseases (CVDs). Fact Sheet N_317. http://www.who.int/mediacentre/factsheets/fs317/en/index.html Updated March 2013. Accessed July 9, 2013.
15. CADDRA. Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA). 3rd ed. Toronto, ON: Canadian ADHD Practice Guidelines; 2011.
Author affiliations: Assistance Publique H×´pitaux de Paris (APHP), Robert Debré Hospital, Child and Adolescent Psychiatry Department (Drs Peyre, Hatteea, Dubuc, and Delorme); Cognitive Sciences and Psycholinguistic Laboratory, Ecole Normale Supérieure (Dr Peyre); Assistance Publique-H×´pitaux de Paris (APHP), Corentin Celton Hospital, Department of Psychiatry, 92130 Issy-les-Moulineaux; Paris Descartes University, PRES Sorbonne Paris Cité; INSERM UMR 894, Psychiatry and Neurosciences Center; Paris Descartes University, PRES Sorbonne Paris Cité (Drs Hoertel and Limosin); and Human Genetics and Cognitive Functions, Pasteur Institute (Dr Delorme), Paris, France.
Potential conflicts of interest: Dr Limosin has served as scientific consultant for Lundbeck and Eutherapy. Drs Peyre, Hoertel, Hatteea, Dubuc, and Delorme report no conflicts of interest.
Funding/support: None reported.
J Clin Psychiatry 2014;75(2):181-182 (doi:10.4088/JCP.13l08736).
© Copyright 2014 Physicians Postgraduate Press, Inc.