
Demographic, Economic, and Clinical Correlates of Depression Treatment Response in an Underserved Primary Care Population

ABSTRACT
Objective: To examine demographic, economic, and clinical correlates of depression treatment outcomes in a rural low-income population served by federally qualified health centers (FQHCs).
Method: The current study utilized data collected during a pragmatic comparative effectiveness trial (N = 364) that was conducted at 9 FQHC clinics between November 2007 and June 2009. Participants were randomly assigned to either telemedicine-based collaborative care or practice-based collaborative care. Depression severity was measured at baseline and at 12-month follow-up using the Hopkins Symptom Checklist (SCL-20) and used to categorize outcomes as nonresponse, partial response, full response, and remission. The associations between demographic, economic, and clinical variables and outcomes were estimated using bivariate analyses and multinomial logistic regression.
Results: 287 participants (78.8%) completed the 12-month follow-up assessment. Among these, 127 participants (44.25%) did not respond to treatment, 53 (18.47%) experienced partial response, 47 (16.38%) experienced full response, and 60 (20.91%) experienced remission. Of the 7 demographic characteristics examined, only gender had a significant (P < .05) effect on outcomes. Of the 2 economic variables examined, income was not associated with outcomes, while individuals without health insurance reported higher response rates than those with public health insurance (P < .05). Among the 13 clinical variables examined, baseline depression severity, physical and mental health status, number of prior depression episodes, and comorbid generalized anxiety had a significant (P < .05) effect on outcomes.
Conclusions: Low treatment response rates and treatment response heterogeneity continue to be significant challenges to clinicians treating depression in low-income underserved populations facing multiple barriers to care. Baseline depression severity and chronicity, health status, and comorbid anxiety appear to have a consistent effect on treatment outcomes in depression.
Trial Registration: ClinicalTrials.gov identifier: NCT00439452
J Clin Psychiatry 2014;75(8):848-854
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: December 18, 2013; accepted April 7, 2014 (doi:10.4088/JCP.13m08954).
Corresponding author: Dinesh Mittal, MD, 2200 Fort Roots Dr, Bldg 58 (152/NLR), North Little Rock, AR 72114 ([email protected]).
Heterogeneity in depression treatment outcomes is common and poses a significant challenge to clinicians. Response rates with guideline-concordant antidepressant monotherapy are only about 50%.1,2 Individuals with residual symptoms are likely to have a more severe and chronic course of illness,3,4 impaired psychosocial functioning,5 and an increased frequency of suicidal ideations.6,7 Demographic and economic factors influencing depression treatment response have received heightened attention in recent years.8-10 Several studies have identified characteristics such as age,11-13 gender,12-15 race,2,8 marital status,12 level of education,2,12,16,17 employment status,2 and household income2,16 as associated factors that influence treatment outcomes in depression. Similarly, clinical characteristics such as severity of depression, age of depression onset, number of prior depression episodes, family history of depression, and comorbidities (such as panic disorder, generalized anxiety disorder [GAD], posttraumatic stress disorder, and substance use disorder)2,8 have also been described as risk factors for lower rate of treatment response.13,18
However, findings across studies are inconsistent,8,9 warranting further systematic research with regard to treatment response heterogeneity. Furthermore, most of the participants in the published clinical trials were recruited from urban centers predominantly serving individuals with private health insurance.2 Little is known about the factors that influence depression treatment outcomes in rural underserved populations facing major barriers to care, such as long travel distances, lack of health insurance, and stigmatizing attitudes,19,20 that may contribute to low response rates to depression treatment.
To address this knowledge gap, we examined the role of demographic, economic, and clinical factors in depression treatment response heterogeneity among individuals receiving depression care in federally qualified health centers (FQHCs).
METHOD
Study Setting and Participants
The current study utilized data collected during a pragmatic comparative effectiveness trial that was conducted at 9 FQHC clinics between November 2007 and June 2009. The study is registered with ClinicalTrials.gov (identifier: NCT00439452). All participants in the study were randomly assigned to either telemedicine-based collaborative care (TBCC) or practice-based collaborative care (PBCC). None of the participating clinics had on-site mental health specialists. For the purpose of this study, we included patients randomized to both arms (N = 364). Data were collected via blinded telephone interview with 364 participants at baseline and for 287 respondents (78.8%) at the 12-month follow-up. Details about the interventions and inclusion and exclusion criteria are described in a previously published article.21 Briefly, both PBCC and TBCC utilized a Depression Care Manager. In PBCC, the Depression Care Managers received no clinical supervision from a mental health specialist, whereas in TBCC, they received weekly supervision from the TBCC team. Specifically, in the TBCC arm, patients received stepped care, whereby treatment intensity was increased for patients failing treatment. If the patient did not respond to the initial antidepressant, the telephone pharmacist conducted a medication history and provided medication management as needed. If the patient did not respond to 2 trials, a telepsychiatry consultation was scheduled. At any time, patients had access to cognitive-behavioral therapy (CBT) delivered via interactive video, and patients failing an antidepressant trial were specifically encouraged to initiate and complete CBT.

- Despite demonstrated effectiveness of collaborative care management, depression response and remission rates continue to be low, posing significant challenges to mental health providers.
- Prior depression episodes, comorbid anxiety, and poor physical health status are significant prognostic predictors of depression response.
- Findings from this study suggest that federally qualified health centers are able to deliver equivalent depression outcomes to uninsured patients.
Measures
Depression severity (dependent variable) was measured at baseline and at 12-month follow-up using the Hopkins Symptom Checklist (SCL-20).22,23 SCL-20 scores range from 0 to 4, with higher scores indicating more severe depression. Using the baseline and 12-month follow-up SCL-20 scores, the dependent variable was specified as 4 mutually exclusive categories that reflect how clinicians typically judge the outcomes of an antidepressant trial: remission, full response, partial response, and no response.24 Remission was defined as a follow-up SCL-20 score < 0.5. Full response was defined as a ≥ 50% improvement in SCL-20 scores between baseline and follow-up and a follow-up SCL-20 score ≥ 0.5. Partial response was defined as an increase ≥ 25% but ≤ 50% in SCL-20 scores between baseline and follow-up, and a follow-up SCL-20 score ≥ 0.5. No response was defined as < 25% increase in SCL-20 scores between baseline and follow-up, and a follow-up SCL-20 score ≥ 0.5.
Demographic, economic, and clinical correlates were specified as explanatory variables. Baseline demographic, economic, and clinical prognostic factors were measured using the Depression Outcomes Module.25,26 Psychiatric comorbidity was measured with the Mini International Neuropsychiatric Interview.27,28 Social support was measured using the Duke Social Support and Stress Scale.29,30 Health status was measured using the 12-item Short Form Health Survey for Veterans and summarized into the physical component summary (PCS) and mental component summary (MCS) scores.31,32 In addition, because randomization to the TBCC group was such a strong predictor of outcomes,21 it was included as a covariate even though demographic, economic, and clinical variables did not differ significantly (P < .05) across study arms. The study was approved by the University of Arkansas for Medical Sciences Institutional Review Board. All participants were informed of the risks and benefits of their participation and provided written informed consent.
Statistical Analysis
All patients completing the 12-month follow-up were included in the analysis (n = 287). Eleven percent of data were missing at baseline and were imputed using PROC MI in SAS 9.3 (SAS Institute Inc). Using no response as the reference category, bivariate analyses were used to examine differences in casemix factor means (t test) and proportions (χ2) across outcome categories. Multinomial logistic regression analysis was used for the multivariate analysis. Similar to logistic regression analysis, multinomial logistic regression analysis estimates odds ratios for mutually exclusive outcome states. We chose to base the analysis on a nominal distribution (which assumes nonordered outcome states) rather than an ordinal distribution (which assumes ordered/ranked outcome states) so that, for each casemix factor, separate odds ratios (ORs) would be estimated for the remission, full response, and partial remission outcome categories compared to the no response category. Ordered logistic regression would have yielded greater statistical power than the multinomial logistic regression. However, it relies on the proportional odds assumption, which restricts the effect of each casemix factor to be constant across all outcome categories. The proportional odds assumption was tested, and our data violated this assumption. The multinomial logistic regression was estimated using PROC LOGISTIC and PROC MIANALYZE in SAS 9.3.
RESULTS
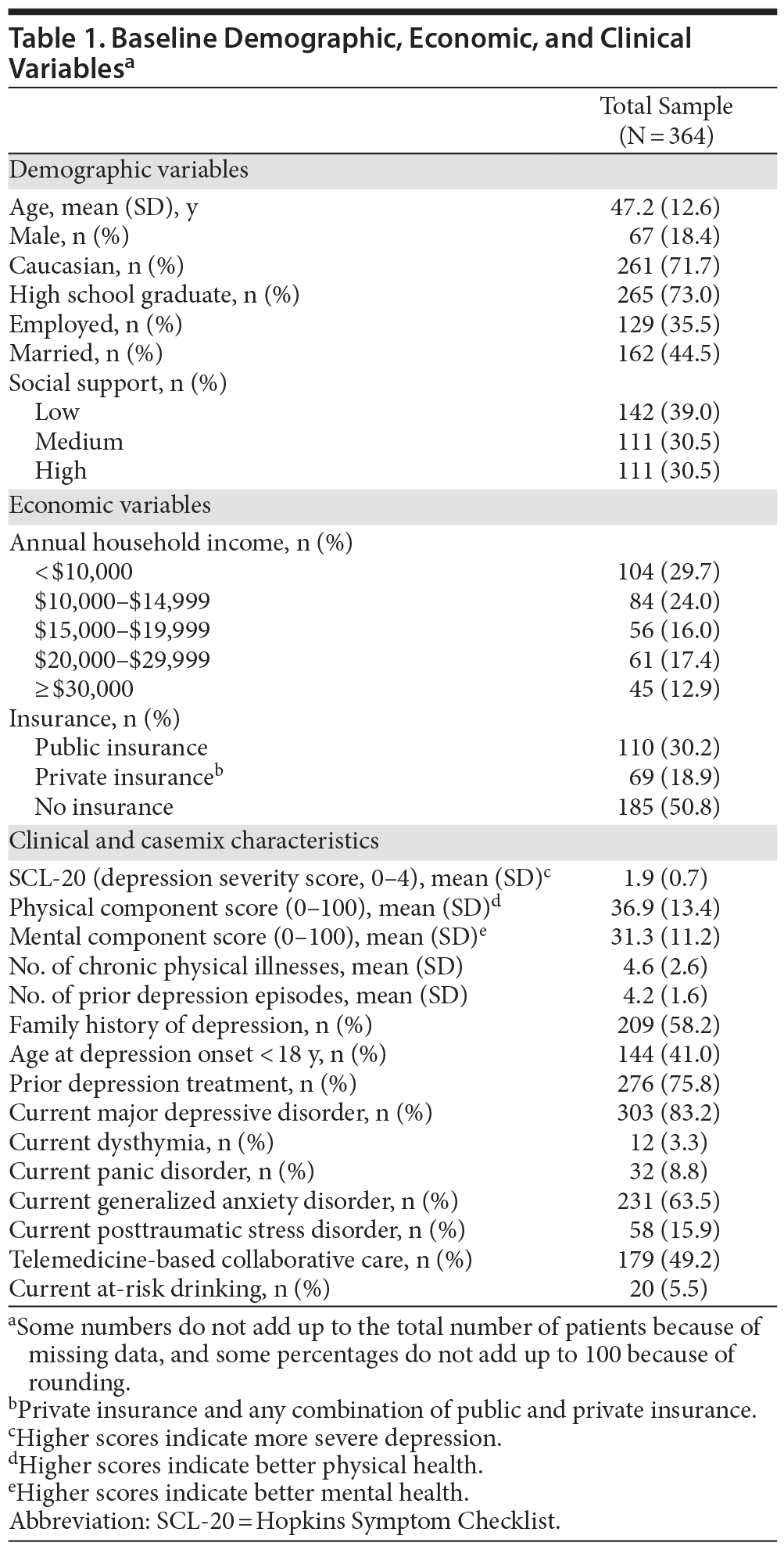
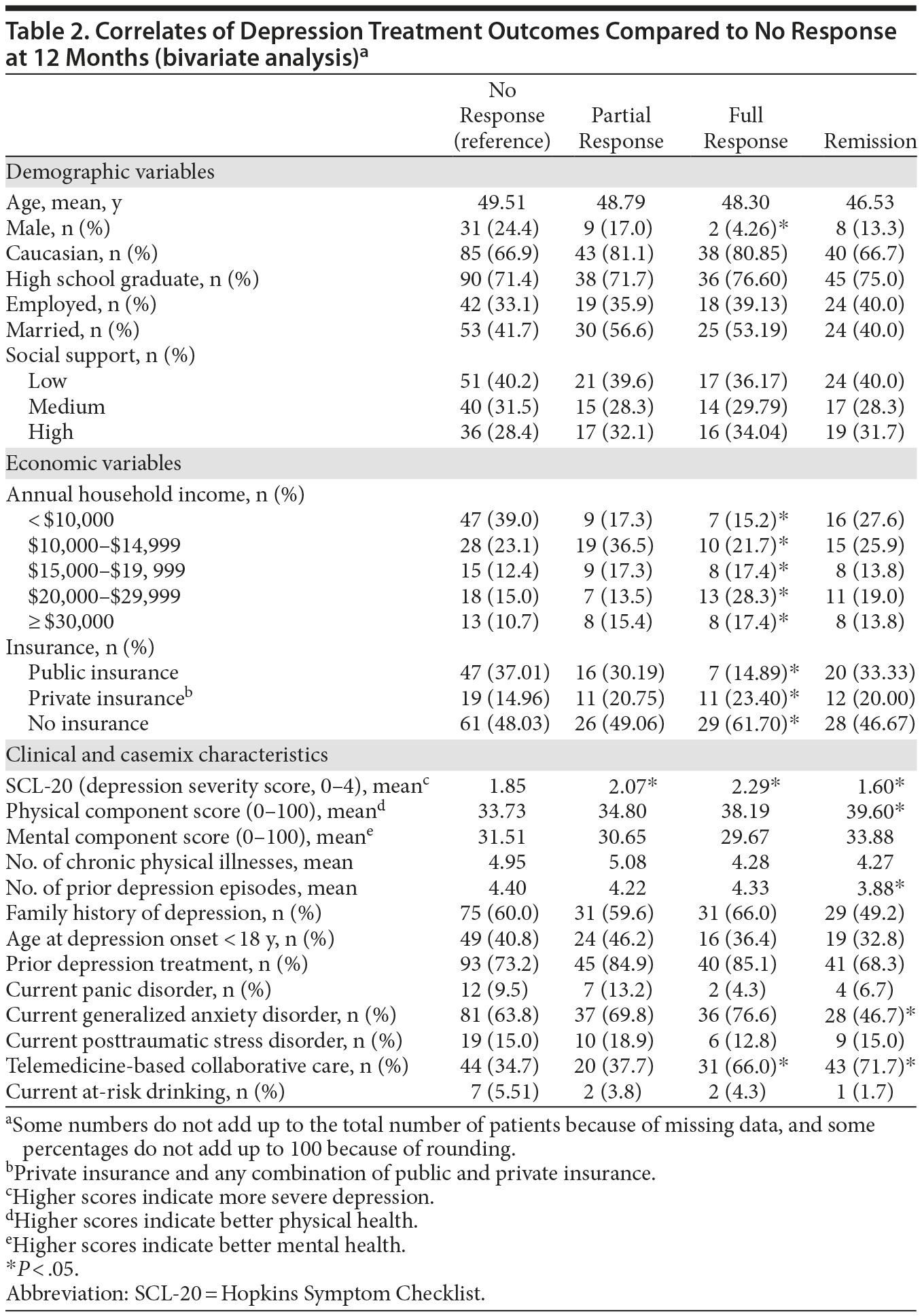
Demographic, economic, and clinical characteristics of the baseline sample are presented in Table 1. At the 12-month time point, 287 patients (78.8%) were retained; 177 (61.7%) were receiving antidepressants; 141 (84.9%) were adherent to antidepressants (taking antidepressants ≥ 80% of days during the last month); and 105 (61.4%) were receiving either usual or high doses of the antidepressant medications. Both the response and remission rates were higher at 12 months compared to 6 months. Specifically, response rate was 29.9% at 6 months and 36.2% at 12 months. The remission rate was 17.3% at 6 months and 20.9% at 12 months. Of those patients completing the 12-month follow-up, 127 participants (44.25%) did not report response to treatment, 53 (18.47%) experienced only partial response, 47 (16.38%) reported full response, and 60 (20.91%) experienced remission. Table 2 presents the results of the bivariate analysis. Only 1 demographic variable (gender) was significantly associated with treatment outcomes. A significantly lower percentage of males experienced a full response compared to nonresponse. Both economic factors were significantly associated with treatment outcomes. The proportion of individuals with public insurance had a relatively low likelihood of experiencing a full response. Likewise, the individuals with annual household incomes less than $10,000 had a relatively low likelihood of experiencing a full response. Five of the 13 clinical variables were significantly associated with treatment outcomes. For baseline SCL-20 scores, there was a clear nonlinear effect on treatment outcomes. Mean baseline SCL-20 scores were significantly higher for those experiencing a partial and full response, yet significantly lower for those in remission, compared to patients in the nonresponse outcome category. In addition, compared to patients in the nonresponse outcome category, individuals reporting remission had significantly higher baseline PCS scores (indicating better physical health status) and a lower number of prior depression episodes. Compared to individuals in the nonresponse outcome category, a significantly lower percentage of individuals with comorbid GAD experienced full remission compared to those without GAD. A significantly higher percentage of participants randomized to the TBCC intervention experienced full response and remission compared to those randomized to PBCC.
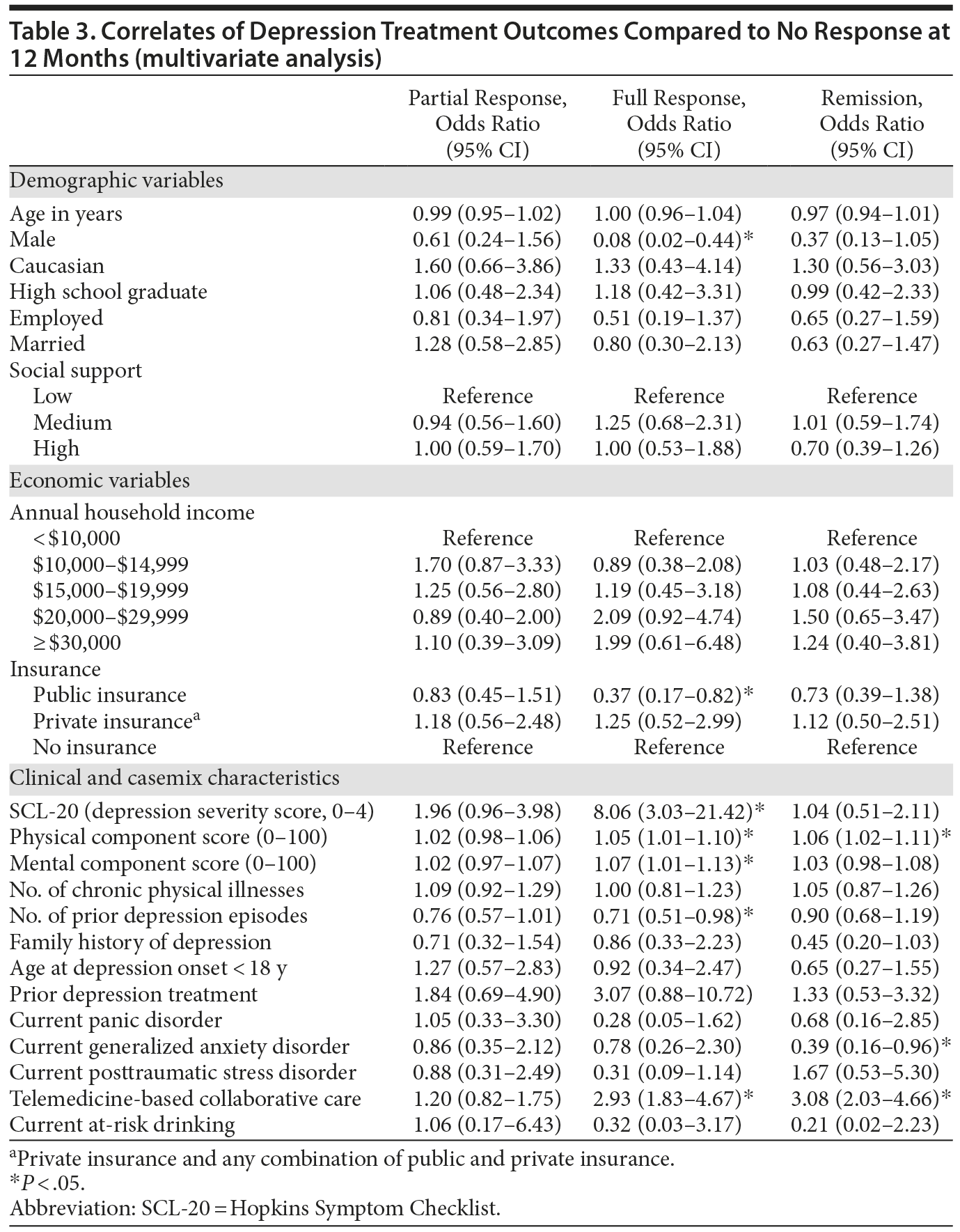
Results of multinomial logistic regression analysis are summarized in Table 3. When adjusting for all casemix factors simultaneously, only 2 nonclinical characteristics (insurance status and gender) were significantly correlated with treatment outcomes. Being male significantly and substantially lowered the likelihood of experiencing a full response to treatment (OR = 0.08; 95% CI, 0.02-0.44; P < .05). There was a similar trend for remission, but the effect did not reach statistical significance. Having public health insurance also significantly lowered the likelihood of experiencing a full response (OR = 0.37; 95% CI, 0.17-0.82; P < .05) compared to having no insurance. With regard to clinical correlates, the most robust effect on depression response was the baseline severity of depression. Having a higher severity of depression at baseline was associated with a greater likelihood of having a full response (OR = 8.06; 95% CI, 3.03-21.42; P < .05). In contrast, having a higher number of prior depression episodes was associated with a lower likelihood of experiencing a full response (OR = 0.71; 95% CI, 0.51-0.98; P < .05). Having higher baseline PCS scores (OR = 1.05, 95% CI, 1.01-1.10; P < .05) and MCS scores (OR = 1.07; 95% CI, 1.01-1.13; P < .05) was significantly associated with having a higher likelihood of a full response, and having higher baseline PCS scores was also significantly associated with a higher likelihood of experiencing remission (OR = 1.06; 95% CI, 1.02-1.11; P < .05). In contrast, having comorbid GAD at baseline was associated with a lower likelihood of experiencing remission (OR = 0.39; 95% CI, 0.16-0.96; P < .05). Finally, consistent with previous findings,21 controlling for clinical and sociodemographic factors, being randomized to the TBCC intervention increased the likelihood of experiencing a full response (OR = 2.93; 95% CI, 1.83-4.67; P < .05) and remission (OR = 3.08; 95% CI, 2.03-4.66; P < .05) compared to being randomized to PBCC.
DISCUSSION
The study revealed 4 significant findings about the outcomes of low-income individuals who received depression care in FQHCs. First, only about 35% of study participants experienced either full response or remission by the 12-month follow-up period, despite randomization to collaborative care. Second, among the demographic variables, only gender appeared to have a significant impact on the outcomes. Third, individuals with public insurance had significantly lower rates of response than individuals with no insurance. Fourth, clinical variables that were significantly associated with outcomes included baseline severity of depression, prior depression episodes, physical and mental health status, and presence of comorbid generalized anxiety.
The combined remission and response rates in this study were lower when compared to other pivotal studies of enhanced depression treatment such as the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial.2 The reasons for a considerably lower treatment response/remission rate may be multifold. Participants in this study were primarily recruited from a rural disadvantaged population that faced many barriers to treatment. Risk factors for nonresponse (eg, public health insurance, comorbid GAD, poor physical health and mental health status) were more prevalent in this rural sample compared with the more urban STAR*D sample.2
The results of this study indicate male gender as the most important demographic risk factor for nonresponse. This finding is consistent with findings from the STAR*D trial, which showed better treatment outcomes for females.2 However, a meta-analysis by Entsuah et al33 found gender to have a nonsignificant association with treatment outcomes, whereas other studies8 have reported that women have poorer outcomes compared to men. Even though the clinical relevance of this nonmodifiable risk factor is uncertain, the results from our study suggest a need for further research focused on identifying under what conditions gender is associated with depression outcomes.
Among the economic factors, results indicated that individuals with public health insurance (primarily Medicaid) had poorer outcomes than individuals with no insurance, which appears counterintuitive. The role of insurance in influencing treatment outcomes has received relatively little attention in the literature. Our findings are consistent with the findings from the STAR*D trial in that it also suggested a possible role for health insurance status in treatment outcomes. Persons with public health insurance had the lowest odds of response compared to those with no insurance and private insurance.2 Furthermore, studies have reported that individuals with public health insurance tend to have a longer duration of current depression episode, greater medical comorbidity,34 and higher treatment attrition rates.16 FQHCs have a mission to treat all patients regardless of ability to pay and to help their uninsured patients obtain free or discounted medications. Our results clearly indicate that FQHCs were able to deliver equivalent depression outcomes for their uninsured patients compared to their privately insured patients. The fact that patients with public health insurance have worse outcomes than the uninsured probably reflects the strict eligibility criteria for Arkansas Medicaid, which requires recipients to have a disability or other significant risk factor (eg, pregnancy, single parent, foster care).
The estimated impact of clinical casemix factors in the disadvantaged patients enrolled in this study was similar to those observed in other more studied populations.2,8 Of the 13 clinical characteristics that were analyzed in this study, 6 factors had a significant effect on treatment outcomes. The baseline severity of depression had the largest impact on treatment outcomes. The bivariate analysis revealed a significant nonlinear trend for baseline depression severity across the 3 outcome categories. In multivariate analysis, baseline severity was significantly and substantially correlated with treatment response. A 1-point increase in the SCL-20 score at baseline resulted in an 8-fold increase in the odds of experiencing full response compared to nonresponse. While these results seem inconsistent with the findings from the majority of the published studies,8 our definition of response differs from the definitions used in those studies in an important way. Because our outcome categories were specified to be mutually exclusive, those in remission were not included in the full response category. In contrast, most studies do include those patients experiencing remission in their full response outcome category.2 Thus, our findings are not necessarily inconsistent with the literature that suggests that a higher severity of depression at baseline is associated with lower response rates.8
Consistent with the literature, our results indicate that there is a positive association between physical and mental health status at baseline and depression treatment outcomes.8,35 Of the 4 psychiatric comorbidities we examined, only GAD had a significant negative impact on outcomes. This effect was moderately large with respect to remission. Thus, it appears that while GAD is not necessarily a risk factor for nonresponse, it can be a barrier to achieving full remission of symptoms. Finally, number of prior depression episodes was found to be a significant risk factor, with each additional episode lowering the odds of experiencing a full response. Although the literature is inconclusive, several studies have reported similar findings.8 The clinical relevance of this finding is paramount. Individuals with a high number of prior depression episodes at baseline may represent a treatment-refractory subgroup. Further research is needed to find out if early intervention in such individuals has a positive effect on the response rates.
In this study, nonmodifiable factors such as age and race were not associated with treatment outcomes. Published findings have largely been inconclusive about whether age is a risk factor for depression treatment, although more studies than not report poorer outcomes for those 50 years and older.8 The STAR*D trial2 reported significantly lower remission rates for African Americans compared with Caucasians. Even though race was not significantly associated with treatment outcomes in our study, Caucasians had a higher likelihood of response and remission (Table 3).
Socioeconomic status has also received considerable attention in published literature. For instance, findings from the STAR*D trial suggest that high level of education, employment, and high level of income are positive predictors of response.2 In this study, socioeconomic status showed no effect on outcomes. Again, our results indicate that FQHCs were able to deliver equivalent depression outcomes to their patients with lower socioeconomic status.
The findings of our study should be interpreted in light of certain limitations. This study used a secondary analysis of a relatively small sample, which resulted in marginal statistical power to detect small effect sizes. Even though this study adopted a well-recognized definition of the 4 outcome categories,24 there is no consensus on the use of this categorization, making it difficult to draw generalized comparisons across various studies that used different definitions. Furthermore, this study analyzed outcomes after a 12-month intervention period, which is substantially longer than other published studies in this area. Notwithstanding these limitations, this study adds to the literature by comprehensively examining a wide range of demographic, economic, and clinical variables that could have a potential impact on depression treatment response in an underserved population facing multiple barriers to care.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Author affiliations: Health Services Research and Development, Central Arkansas Veterans Healthcare System, North Little Rock; and South Central Mental Illness Research Education and Clinical Center, Central Arkansas Veterans Healthcare System, North Little Rock (Drs Mittal and Fortney); Department of Psychiatry, University of Arkansas for Medical Sciences, Little Rock (all authors); and Department of Applied Gerontology, University of North Texas, Denton (Dr Chekuri).
Financial disclosure: Drs Mittal, Chekuri, and Fortney and Ms Lu have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: This research was supported by a grant from the National Institute of Mental Health (R01 MH076908/MH076908-04S1) to Dr Fortney.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Acknowledgments: The authors gratefully acknowledge the patients and staff at the Boston Mountain Rural Health Center, Inc; Community Clinic NWA; Corning Area Healthcare Inc; East Arkansas Family Health Center, Inc; and Jefferson Comprehensive Care Systems, Inc, as well as staff at the Community Health Centers for Arkansas Inc. They also acknowledge the important contributions of project staff including Amanda Davis-Lunsford, MA (technical writing); Loretta Ducker, RN (care management); Debrah Hodges (data management); Choi Lai, MS (data analysis); Michael McCarther, BA (project coordinator); Camille Mack, MA (data collection); Jennifer Stephens (data collection); and Vera Tate, MD (chart reviews). The authors thank Tisha Deen, PhD, for helpful comments on the manuscript; Jamie Henry for assistance with citations; and Carrie Edlund, MS, for assistance with manuscript preparation. The acknowledged individuals report no potential conflict of interest.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? a meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009;19(1):34-40. PubMed doi:10.1016/j.euroneuro.2008.08.009
2. Trivedi MH, Rush AJ, Wisniewski SR, et al; STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28-40. PubMed doi:10.1176/appi.ajp.163.1.28
3. Judd LL, Paulus MJ, Schettler PJ, et al. Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? Am J Psychiatry. 2000;157(9):1501-1504. PubMed doi:10.1176/appi.ajp.157.9.1501
4. Paykel ES, Ramana R, Cooper Z, et al. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25(6):1171-1180. PubMed doi:10.1017/S0033291700033146
5. Miller IW, Keitner GI, Schatzberg AF, et al. The treatment of chronic depression, part 3: psychosocial functioning before and after treatment with sertraline or imipramine. J Clin Psychiatry. 1998;59(11):608-619. PubMed doi:10.4088/JCP.v59n1108
6. Papakostas GI, Petersen T, Pava J, et al. Hopelessness and suicidal ideation in outpatients with treatment-resistant depression: prevalence and impact on treatment outcome. J Nerv Ment Dis. 2003;191(7):444-449. PubMed doi:10.1097/01.NMD.0000081591.46444.97
7. Judd LL, Akiskal HS, Paulus MP. The role and clinical significance of subsyndromal depressive symptoms (SSD) in unipolar major depressive disorder. J Affect Disord. 1997;45(1-2):5-17, discussion 17-18. PubMed doi:10.1016/S0165-0327(97)00055-4
8. Carter GC, Cantrell RA, Victoria Zarotsky, et al. Comprehensive review of factors implicated in the heterogeneity of response in depression. Depress Anxiety. 2012;29(4):340-354. PubMed doi:10.1002/da.21918
9. Esposito K, Goodnick P. Predictors of response in depression. Psychiatr Clin North Am. 2003;26(2):353-365. PubMed doi:10.1016/S0193-953X(02)00104-1
10. Nierenberg AA. Predictors of response to antidepressants: general principles and clinical implications. Psychiatr Clin North Am. 2003;26(2):345-352, viii. PubMed doi:10.1016/S0193-953X(02)00105-3
11. Angstman KB, MacLaughlin KL, Rasmussen NH, et al. Age of depressed patient does not affect clinical outcome in collaborative care management. Postgrad Med. 2011;123(5):122-128. PubMed doi:10.3810/pgm.2011.09.2467
12. Hennings JM, Owashi T, Binder EB, et al. Clinical characteristics and treatment outcome in a representative sample of depressed inpatients—findings from the Munich Antidepressant Response Signature (MARS) project. J Psychiatr Res. 2009;43(3):215-229. PubMed doi:10.1016/j.jpsychires.2008.05.002
13. Bschor T, Canata B, Müller-Oerlinghausen B, et al. Predictors of response to lithium augmentation in tricyclic antidepressant-resistant depression. J Affect Disord. 2001;64(2-3):261-265. PubMed doi:10.1016/S0165-0327(00)00211-1
14. McGrath PJ, Stewart JW, Petkova E, et al. Predictors of relapse during fluoxetine continuation or maintenance treatment of major depression. J Clin Psychiatry. 2000;61(7):518-524. PubMed doi:10.4088/JCP.v61n0710
15. Kornstein SG. Gender differences in depression: implications for treatment. J Clin Psychiatry. 1997;58(suppl 15):12-18. PubMed
16. Warden D, Rush AJ, Wisniewski SR, et al. Income and attrition in the treatment of depression: a STAR*D report. Depress Anxiety. 2009;26(7):622-633. PubMed doi:10.1002/da.20541
17. Hirschfeld RM, Russell JM, Delgado PL, et al. Predictors of response to acute treatment of chronic and double depression with sertraline or imipramine. J Clin Psychiatry. 1998;59(12):669-675. PubMed doi:10.4088/JCP.v59n1205
18. Gildengers AG, Houck PR, Mulsant BH, et al. Trajectories of treatment response in late-life depression: psychosocial and clinical correlates. J Clin Psychopharmacol. 2005;25(suppl 1):S8-S13. PubMed doi:10.1097/01.jcp.0000161498.81137.12
19. Jones AR, Cook TM, Wang J. Rural-urban differences in stigma against depression and agreement with health professionals about treatment. J Affect Disord. 2011;134(1-3):145-150. PubMed doi:10.1016/j.jad.2011.05.013
20. Lane B, Roufeil LM, Williams S, et al. It’s just different in the country: postnatal depression and group therapy in a rural setting. Soc Work Health Care. 2001;34(3-4):333-348. PubMed doi:10.1300/J010v34n03_06
21. Fortney JC, Pyne JM, Mouden SB, et al. Practice-based versus telemedicine-based collaborative care for depression in rural federally qualified health centers: a pragmatic randomized comparative effectiveness trial. Am J Psychiatry. 2013;170(4):414-425. PubMed doi:10.1176/appi.ajp.2012.12050696
22. Derogatis LR, Lipman RS, Rickels K, et al. The Hopkins Symptom Checklist (HSCL): a measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry. 1974;7(0):79-110. PubMed
23. Derogatis LR, Lipman RS, Rickels K, et al. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19(1):1-15. PubMed doi:10.1002/bs.3830190102
24. Hirschfeld RM, Montgomery SA, Aguglia E, et al. Partial response and nonresponse to antidepressant therapy: current approaches and treatment options. J Clin Psychiatry. 2002;63(9):826-837. PubMed doi:10.4088/JCP.v63n0913
25. Smith GR Jr, Burnam A, Burns BJ, et al. Depression Outcomes Module (DOM). In: Rush Jr AJ, Pincus, HA, First MB, et al, eds. Handbook of Psychiatric Measures. Washington, DC: American Psychiatric Association: 2000;213-215.
26. Kramer TL, Smith GR, D’ Arezzo KW, et al. Depression Outcomes Module. In: The Guide to Behavioral Health Outcomes Management Systems. Little Rock, AR: University of Arkansas for Medical Sciences; 2000: 71-83.
27. Lecrubier Y, Sheehan DV, Weiller E, et al. The Mini International Neuropsychiatric Interview (MINI). A short diagnostic structured interview: reliability and validity according to the CIDI. Eur Psychiatry. 1997;12(5):224-231. doi:10.1016/S0924-9338(97)83296-8
28. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The validity of the Mini International Neuropsychiatric Interview (MINI) according to the SCID-P and its reliability. Eur Psychiatry. 1997;12(5):232-241. doi:10.1016/S0924-9338(97)83297-X
29. Parkerson GR Jr, Michener JL, Wu LR, et al. Associations among family support, family stress, and personal functional health status. J Clin Epidemiol. 1989;42(3):217-229. PubMed doi:10.1016/0895-4356(89)90058-9
30. Parkerson GR Jr, Broadhead WE, Tse CK. Quality of life and functional health of primary care patients. J Clin Epidemiol. 1992;45(11):1303-1313. PubMed doi:10.1016/0895-4356(92)90171-I
31. Jones D, Kazis L, Lee A, et al. Health status assessments using the Veterans SF-12 and SF-36: methods for evaluating outcomes in the Veterans Health Administration. J Ambul Care Manage. 2001;24(3):68-86. PubMed doi:10.1097/00004479-200107000-00011
32. Kazis LE, Miller DR, Clark J, et al. Health-related quality of life in patients served by the Department of Veterans Affairs: results from the Veterans Health Study. Arch Intern Med. 1998;158(6):626-632. PubMed doi:10.1001/archinte.158.6.626
33. Entsuah AR, Huang H, Thase ME. Response and remission rates in different subpopulations with major depressive disorder administered venlafaxine, selective serotonin reuptake inhibitors, or placebo. J Clin Psychiatry. 2001;62(11):869-877. PubMed doi:10.4088/JCP.v62n1106
34. Lesser IM, Leuchter AF, Trivedi MH, et al. Insured and non-insured depressed outpatients: how do they compare? Ann Clin Psychiatry. 2007;19(2):73-82. PubMed doi:10.1080/10401230701334671
35. Rush AJ, Warden D, Wisniewski SR, et al. STAR*D: revising conventional wisdom. CNS Drugs. 2009;23(8):627-647. PubMed
<!–

Posttest
1. Which demographic variable was associated with a significantly lower percentage of patients experiencing a full response to depression treatment compared with no response?
a. Younger age
b. Male sex
c. High school completion
d. Current employment
2. Among patients experiencing no response to depression treatment, the greatest proportion had which annual household income level?
a. < $10,000
b. $10,000 to $14,999
c. $15,000 to $19,999
d. $20,000 to $29,999
3. Among patients experiencing depression remission, the greatest proportion had which insurance?
a. Public insurance only
b. Private insurance only or both private and public insurance
c. No insurance
4. Both Ms A and Mr B earn more than $20,000 per year and have private insurance. Which patient will probably require more intensive treatment for the current episode of major depressive disorder (moderate severity) than the other patient?
a. Ms A, who is experiencing her first depressive episode, is in good physical health, and has no comorbid psychiatric illnesses
b. Mr B, who has had several depressive episodes, has poor physical health, and has comorbid generalized anxiety disorder
–>

 (Keyword: August)to take this Posttest and complete the Evaluation.
(Keyword: August)to take this Posttest and complete the Evaluation.



