- Patients with obsessive-compulsive disorder (OCD) who do not respond adequately to a serotonin reuptake inhibitor (SRI) usually receive augmentation therapy with an atypical antipsychotic drug. Atypical antipsychotics, however, may not be appropriate for or acceptable to all patients.
- Two small uncontrolled trials in patients who responded poorly to ongoing treatments found that ondansetron (0.5-1.0 mg/d) modestly reduced OCD severity after 12 weeks of treatment. Two small randomized controlled trials in patients with an undocumented past treatment history found that ondansetron (4-8 mg/d) markedly reduced OCD severity after 8 weeks of treatment. Ondansetron was well tolerated in all studies.
- Ondansetron (1-8 mg/d) may be considered an experimental SRI augmentation agent in OCD patients for whom augmentation with an atypical antipsychotic drug is problematic.

ABSTRACT
Serotonin reuptake inhibitors (SRIs) are the mainstay in the treatment of obsessive-compulsive disorder (OCD). Patients who do not respond adequately to SRIs commonly receive augmentation therapy with another agent, usually an atypical antipsychotic drug. Atypical antipsychotics, however, may not be appropriate for or acceptable to all patients. Ondansetron is an experimental alternative for such patients. There have been at least 6 trials that have examined a short-term (8-12 weeks) role for ondansetron in patients with OCD. These include 1 placebo-controlled crossover trial (N = 11); 1 uncontrolled monotherapy trial (N = 8); 2 low-dose (0.5-1.0 mg/d), uncontrolled augmentation trials in patients who did not respond adequately to ongoing or earlier treatments (pooled N = 35); and 2 moderate- to high-dose (4-8 mg/d) randomized, placebo-controlled augmentation trials in patients with undocumented past treatment history (pooled N = 88). Ondansetron was modestly effective in the uncontrolled trials and strikingly effective in the controlled trials. Ondansetron was also very well tolerated in all of the studies. These enthusiastic observations must be tempered by the limitations of the reviewed data, such as small sample sizes, short study durations, lack of data on the effects of blinded ondansetron discontinuation, lack of long-term data, and study-specific limitations. At best, ondansetron (1-8 mg/d) may be considered an experimental SRI augmentation agent in OCD patients for whom augmentation with an atypical antipsychotic drug is problematic.
J Clin Psychiatry 2015;76(1):e72-e75 (doi:10.4088/JCP.14f09704.)
© Copyright 2015 Physicians Postgraduate Press, Inc.
Clinical Question
Ms Y is a 19-year-old woman with obsessive-compulsive disorder (OCD). She showed little improvement following 3- to 4-month monotherapy trials with sertraline (up to 300 mg/d) and fluoxetine (up to 60 mg/d) and suffered severe constipation with clomipramine (100 mg/d). She is presently on treatment with paroxetine (50 mg/d); the treatment response remains poor. She has not been able to successfully engage in cognitive-behavioral therapy and is reluctant to consider serotonin reuptake inhibitor (SRI) augmentation with an atypical antipsychotic drug because of concerns about antipsychotic-related weight gain. Some clinical data seem to suggest benefits with ondansetron augmentation. Might this be a strategy that she can usefully consider?
Introduction
Ondansetron is a 5-HT3 receptor antagonist that is primarily used to reduce nausea and vomiting associated with cancer chemotherapy, radiotherapy, and other conditions, including surgery and pregnancy. Through 5-HT3 receptor blockade, ondansetron has a weak, downstream, inhibitory effect on dopaminergic neurotransmission.1-3 This action has stimulated the study of several possible applications of the drug in psychiatry and neurology. Ondansetron has been used to treat schizophrenia both as monotherapy and as antipsychotic augmentation therapy.4,5 It has been used as a treatment for tic disorders,6 tardive dyskinesia,7 and alcoholism.8,9 It has also been successfully trialed in patients with tinnitus,10 fatigue related to chronic hepatitis,11 and opiate-related pruritus,12 but not benzodiazepine withdrawal.13
Given that antipsychotic drugs can augment the benefits of SRIs in patients with OCD, might ondansetron carry similar benefits? This question was examined in several uncontrolled and controlled trials during the past decade and a half. PubMed searches were conducted on November 30, 2014, using the search term ondansetron paired with obsessive-compulsive disorder, OCD, obsessions, and compulsions. Reference lists of identified articles were also searched. Uncontrolled and controlled clinical trials of ondansetron for OCD were identified through these searches and are summarized in the sections that follow.
Ondansetron for OCD: Preliminary Data
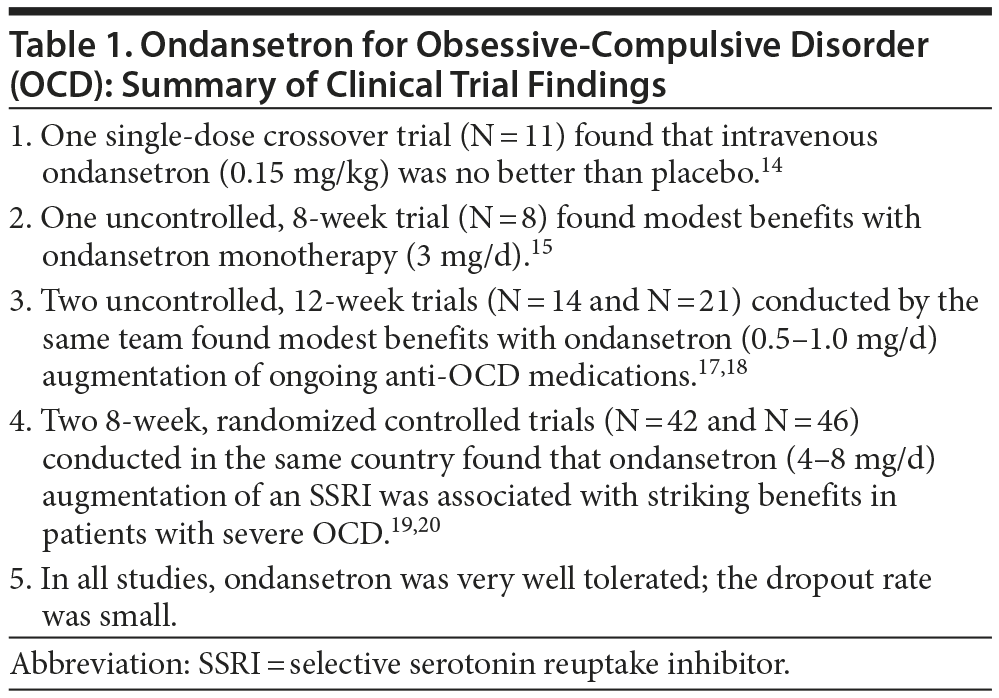
Acute, intravenous administration of ondansetron (0.15 mg/kg) was no better than placebo in a crossover study of 11 patients with OCD.14 In a pilot, 8-week, open-label trial conducted in 8 adults with OCD,15 ondansetron monotherapy (1 mg thrice per day) reduced Yale-Brown Obsessive Compulsive Scale (YBOCS) scores by 29%; in 3 patients deemed to have responded to treatment, YBOCS scores fell by a mean of 55%. In 6 patients who were followed up for 2 weeks after ondansetron discontinuation, there was a 45% worsening in YBOCS scores. A limitation of this study15 is that it was open label and uncontrolled, and so placebo mechanisms16 may have explained both onset and loss of benefits.
Low-Dose Ondansetron for OCD:Uncontrolled Trials
Pallanti et al17 specifically examined the benefits of low-dose ondansetron in OCD. The sample comprised 14 adults who had not responded to the combination of an SRI with an atypical antipsychotic drug. These patients received ondansetron augmentation in the dose of 0.25 mg twice daily for the first 6 weeks, up-titrated to 0.5 mg twice daily for the next 6 weeks. Midway through the study, only modest improvements were observed. However, by the 12-week treatment endpoint, 9 patients had experienced at least 25% improvement on YBOCS, with a Clinical Global Impression-Improvement (CGI-I) rating of much or very much improved. The group as a whole experienced a 23% decrease in YBOCS scores. Only a few minor adverse events were reported during the study.
In another study of low-dose ondansetron described by the same authors,18 the sample comprised 21 adults with OCD, all of whom had responded inadequately to an SRI. Ondansetron augmentation was begun at 0.25 mg twice daily for 2 weeks and was up-titrated to 0.5 mg twice daily for 10 further weeks. Response was defined as at least 25% improvement on YBOCS, a final YBOCS score of 24 or less, and a CGI-I score indicating much or very much improvement. Raters but not patients were blind to treatment status. One patient experienced constipation and dropped out of treatment at week 4. At the 12-week treatment endpoint, 12 patients (57%) were classified as responders; 8 of these patients met response criteria during the first 6 weeks. The YBOCS scores decreased by a mean of 27% in the whole sample and by 44% in responders. Improvements were observed in both obsessive and compulsive symptoms. Four weeks after the discontinuation of ondansetron, the YBOCS scores increased by a mean of 15% in the whole sample and by a mean of 38% in the responder sample. Low-dose ondansetron did not cause clinically significant adverse effects.
Both of these studies were open label and uncontrolled, and placebo mechanisms16 could have been responsible for the findings. Additionally, in the latter report18 there were inconsistencies between the abstract and text and also within the text itself. Also, surprisingly, although the study was uncontrolled, in one section the authors18 referred to experimental and control groups.
Moderate- to High-Dose Ondansetron for OCD: Controlled Trials
Two randomized controlled trials (RCTs), published from Iran, examined moderate to high doses of ondansetron in patients with OCD. Soltani et al19 recruited 42 adults with severe OCD. These patients were randomized to receive either ondansetron (4 mg/d) or placebo for 8 weeks; all patients also received fluoxetine. There were only 4 dropouts: 2 from each group. In the ondansetron augmentation group, mean YBOCS scores dropped from 35 at baseline to 6 at the 8-week study endpoint. In the placebo augmentation group, these scores dropped from 35 to 16. The advantage for ondansetron was statistically significant. Very few adverse effects were reported in the 2 groups, and only 1 patient, treated with ondansetron, dropped out because of adverse effects (headache). This study19 had some curiosities. The mean baseline YBOCS score was 35; that is, patients were severely ill. Yet, no patient had received any psychiatric medication for at least the previous 6 weeks. No information was provided about illness duration, exposure to previous treatments (if any) and response thereto, or other variables that might have helped the reader understand to what population the results of this study could be generalized. Lastly, treatment benefits were striking. In the fluoxetine group, patients improved by a mean of nearly 55% in just 8 weeks. In the fluoxetine plus ondansetron group, mean scores dropped from close to the top of the YBOCS range down into the remission range, again after just 8 weeks of treatment.
Heidari et al20 randomized 46 adults with severe OCD to receive either ondansetron (4 mg twice a day) or placebo for 8 weeks; all patients also received fluvoxamine 100 mg/d for the first 4 weeks and 200 mg/d for the rest of the study. The mean YBOCS score was approximately 30 at baseline, and the mean duration of illness was 4 years. Only 2 patients dropped out of the study, neither due to adverse events. Mean YBOCS scores dropped to 15 with ondansetron augmentation and 21 with placebo augmentation by the 8-week study endpoint; the advantage for ondansetron on YBOCS was statistically significant for obsessions and compulsions, as well as the total scale score. At the study endpoint, 86% of ondansetron patients versus 32% of placebo patients showed at least 35% improvement, and 64% of ondansetron patients versus 27% of placebo patients had YBOCS scores of 16 or less. Adverse events were few and comparable in the 2 groups. As with the only other RCT,19 this study20 was unusual in that patients were at least 6 weeks free of all psychotropic drugs at baseline. These authors20 also provided no details of previous treatments (if any) and response thereto.
One additional point is noteworthy. Soltani et al19 found the greatest separation between ondansetron and placebo at weeks 2 and 8; Heidari et al20 found the greatest separation at weeks 6-8.
Summary and Critique
The findings of the 6 ondansetron studies are summarized in Table 1. In brief, ondansetron was effective in the uncontrolled as well as in the placebo-controlled trials. In contrast with usual findings in psychopharmacology studies, ondansetron augmentation was more strikingly effective in the RCTs19,20 than in the uncontrolled studies.17,18 There are 2 possible explanations. One is that patients in the uncontrolled studies17,18 had a documented history of poor response to ongoing and/or previous anti-OCD medications; in contrast, previous medication history was not reported in the controlled trials,19,20 and patients were off all psychotropic medications for at least the previous 6 weeks. The other is that ondansetron was dosed at higher levels in the RCTs19,20 as compared with the uncontrolled trials. The issue of inadequate ondansetron dose versus diminished ondansetron efficacy in previously inadequately responding patients therefore needs clarification.
Limitations of the individual studies have already been listed in the previous sections. Other concerns are briefly described. Two of the open trials15,18 reported worsening of OCD symptoms after discontinuation of ondansetron; however, neither of the RCTs19,20 conducted posttreatment follow-up assessments under blinded conditions, and so the duration of persistence of benefits and the need for continuation therapy remain unresolved. In particular, the safety and efficacy of ondansetron in the long term require study if ondansetron is to be considered as a serious alternative to SRI augmentation with atypical antipsychotic drugs in poor responders. Finally, independent confirmation of ondansetron benefits is necessary. Both of the 12-week uncontrolled augmentation trials in inadequate responders were published by the same team,17,18 and both of the impressively positive RCTs19,20 in patients with undocumented past treatment history were conducted in the same country.
Conclusions
At best, ondansetron may be viewed as an experimental treatment, as follows:
- To treat OCD patients who do not respond to SRI treatment and in whom augmentation with an atypical antipsychotic drug is problematic (such as the patient described at the start of this article).
- To treat unselected and currently drug-naive patients with a view to eliciting an earlier and stronger treatment response.
When ondansetron is used as an experimental SRI augmentation agent, an 8- to 12-week trial appears reasonable, at a dose of 1-8 mg/d.
Parting Notes
- Many studies administered ondansetron in 2-3 doses per day because this drug has a short half-life of about 3-4 h.21
- On September 15, 2011, the US Food and Drug Administration22 issued a safety notification concerning ondansetron. The notification observed that ondansetron may increase the risk of QTc prolongation and the complications thereof. Ondansetron should therefore be avoided in patients with congenital long QT syndrome, and in those with cardiac arrhythmias, congestive heart failure, hypokalemia, or hypomagnesemia, all of which increase the risk of torsades de pointes. Ondansetron should also be avoided in patients who are receiving other medications that can prolong the QTc interval. ECG monitoring is desirable when ondansetron is used in patients at risk of cardiac arrhythmias. Interestingly, QTc prolongation with ondansetron was subsequently confirmed in some but not all studies.23,24 It is possible that the risk may be lower with low, oral doses, as might be used in psychiatry; however, there is no room for complacency.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Ye JH, Ponnudurai R, Schaefer R. Ondansetron: a selective 5-HT(3) receptor antagonist and its applications in CNS-related disorders. CNS Drug Rev. 2001;7(2):199-213. PubMed doi:10.1111/j.1527-3458.2001.tb00195.x
2. Thompson AJ, Lummis SC. 5-HT3 receptors. Curr Pharm Des. 2006;12(28):3615-3630. PubMed doi:10.2174/138161206778522029
3. Thompson AJ, Lummis SC. The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets. 2007;11(4):527-540. PubMed doi:10.1517/14728222.11.4.527
4. Bennett AC, Vila TM. The role of ondansetron in the treatment of schizophrenia. Ann Pharmacother. 2010;44(7-8):1301-1306. PubMed doi:10.1345/aph.1P008
5. Andrade C. Nonsteroidal anti-inflammatory drugs and 5-HT₃ serotonin receptor antagonists as innovative antipsychotic augmentation treatments for schizophrenia. J Clin Psychiatry. 2014;75(7):e707-e709. PubMed doi:10.4088/JCP.14f09292
6. Toren P, Weizman A, Ratner S, et al. Ondansetron treatment in Tourette’s disorder: a 3-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2005;66(4):499-503. PubMed doi:10.4088/JCP.v66n0413
7. Sirota P, Mosheva T, Shabtay H, et al. Use of the selective serotonin 3 receptor antagonist ondansetron in the treatment of neuroleptic-induced tardive dyskinesia. Am J Psychiatry. 2000;157(2):287-289. PubMed doi:10.1176/appi.ajp.157.2.287
8. Johnson BA, Roache JD, Javors MA, et al. Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA. 2000;284(8):963-971. PubMed doi:10.1001/jama.284.8.963
9. Johnson BA, Ait-Daoud N, Seneviratne C, et al. Pharmacogenetic approach at the serotonin transporter gene as a method of reducing the severity of alcohol drinking. Am J Psychiatry. 2011;168(3):265-275. PubMed doi:10.1176/appi.ajp.2010.10050755
10. Taslimi S, Vahidi H, Pourvaziri A, et al. Ondansetron in patients with tinnitus: randomized double-blind placebo-controlled study. Eur Arch Otorhinolaryngol. 2013;270(5):1635-1641. PubMed doi:10.1007/s00405-012-2179-0
11. Piche T, Vanbiervliet G, Cherikh F, et al. Effect of ondansetron, a 5-HT3 receptor antagonist, on fatigue in chronic hepatitis C: a randomised, double blind, placebo controlled study. Gut. 2005;54(8):1169-1173. PubMed doi:10.1136/gut.2004.055251
12. Charuluxananan S, Somboonviboon W, Kyokong O, et al. Ondansetron for treatment of intrathecal morphine-induced pruritus after cesarean delivery. Reg Anesth Pain Med. 2000;25(5):535-539. PubMed doi:10.1097/00115550-200009000-00017
13. Romach MK, Kaplan HL, Busto UE, et al. A controlled trial of ondansetron, a 5-HT3 antagonist, in benzodiazepine discontinuation. J Clin Psychopharmacol. 1998;18(2):121-131. PubMed doi:10.1097/00004714-199804000-00004
14. Broocks A, Pigott TA, Hill JL, et al. Acute intravenous administration of ondansetron and m-CPP, alone and in combination, in patients with obsessive-compulsive disorder (OCD): behavioral and biological results. Psychiatry Res. 1998;79(1):11-20. PubMed doi:10.1016/S0165-1781(98)00029-8
15. Hewlett WA, Schmid SP, Salomon RM. Pilot trial of ondansetron in the treatment of 8 patients with obsessive-compulsive disorder. J Clin Psychiatry. 2003;64(9):1025-1030. PubMed doi:10.4088/JCP.v64n0907
16. Andrade C. There’s more to placebo-related improvement than the placebo effect alone. J Clin Psychiatry. 2012;73(10):1322-1325. PubMed doi:10.4088/JCP.12f08124
17. Pallanti S, Bernardi S, Antonini S, et al. Ondansetron augmentation in treatment-resistant obsessive-compulsive disorder: a preliminary, single-blind, prospective study. CNS Drugs. 2009;23(12):1047-1055. PubMed doi:10.2165/11530240-000000000-00000
18. Pallanti S, Bernardi S, Antonini S, et al. Ondansetron augmentation in patients with obsessive-compulsive disorder who are inadequate responders to serotonin reuptake inhibitors: improvement with treatment and worsening following discontinuation. Eur Neuropsychopharmacol. 2014;24(3):375-380. PubMed doi:10.1016/j.euroneuro.2013.12.003
19. Soltani F, Sayyah M, Feizy F, et al. A double-blind, placebo-controlled pilot study of ondansetron for patients with obsessive-compulsive disorder. Hum Psychopharmacol. 2010;25(6):509-513. PubMed doi:10.1002/hup.1145
20. Heidari M, Zarei M, Hosseini SM, et al. Ondansetron or placebo in the augmentation of fluvoxamine response over 8 weeks in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2014;29(6):344-350. PubMed doi:10.1097/YIC.0000000000000043
21. Roila F, Del Favero A. Ondansetron clinical pharmacokinetics. Clin Pharmacokinet. 1995;29(2):95-109. PubMed doi:10.2165/00003088-199529020-00004
22. US Food and Drug Administration. Zofran (ondansetron): drug safety communication—risk of abnormal heart rhythms. http://www.fda.gov/Safety/MedWatch/
SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm272041.htm. Accessed December 1, 2014.
23. Ganjare A, Kulkarni AP. Comparative electrocardiographic effects of intravenous ondansetron and granisetron in patients undergoing surgery for carcinoma breast: a prospective single-blind randomised trial. Indian J Anaesth. 2013;57(1):41-45. PubMed doi:10.4103/0019-5049.108560
24. Obal D, Yang D, Sessler DI. Perioperative doses of ondansetron or dolasetron do not lengthen the QT interval. Mayo Clin Proc. 2014;89(1):69-80. PubMed doi:10.1016/j.mayocp.2013.10.008

 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.

