Objective: To evaluate efficacy, effect on mood, and safety of deep brain stimulation (DBS) for obsessive-compulsive disorder (OCD) at different target sites.
Data Sources: Electronic records from databases MEDLINE, EMBASE, and CENTRAL up to November 2019 were searched. Search terms included OCD, depression, and DBS.
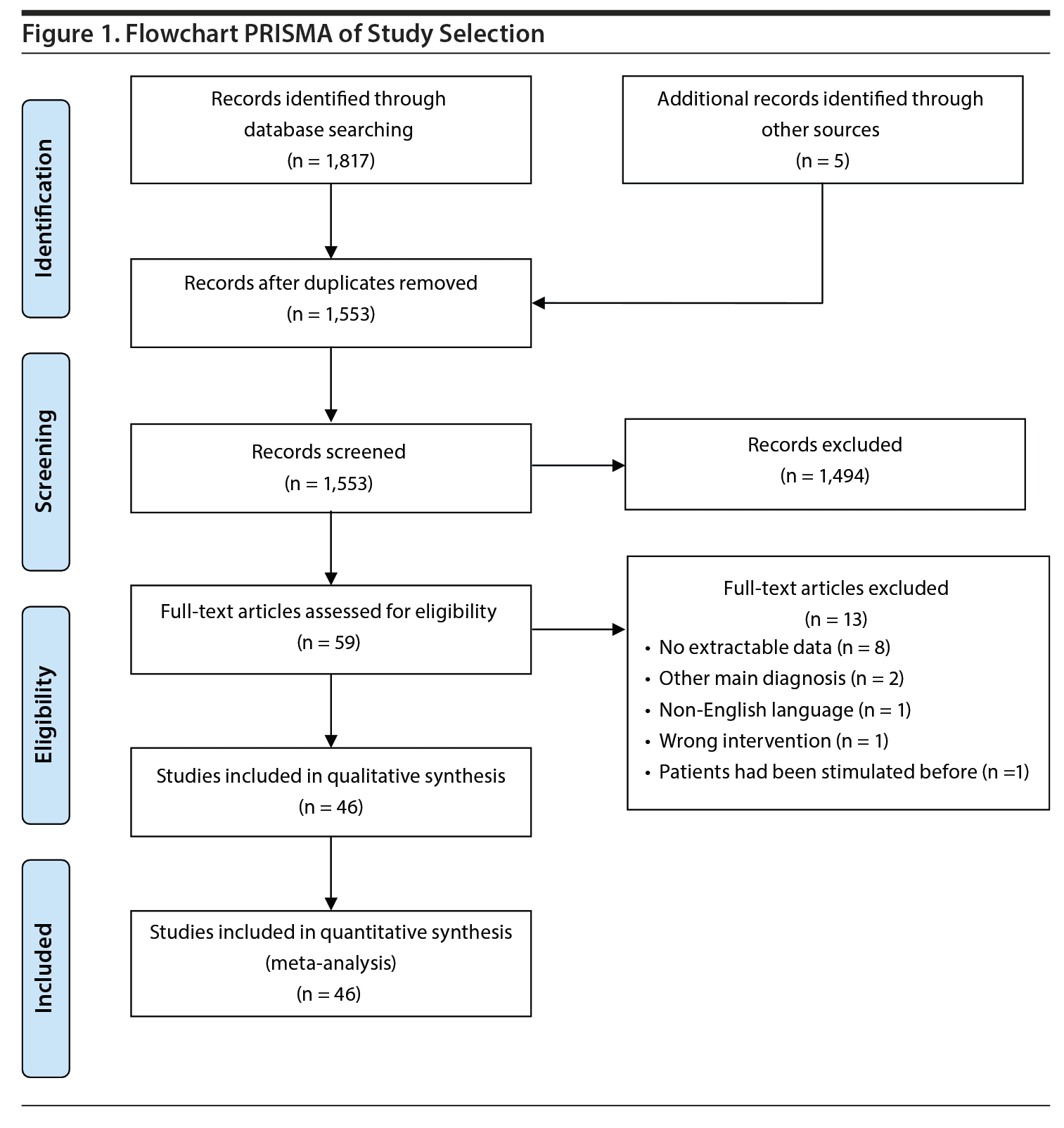
Study Selection: Eight randomized controlled trials (RCTs) (n = 85) and 38 observational studies (case reports and case series) (n = 225) were included.
Data Extraction: In RCTs, the differences in outcomes between sham and active stimulation for OCD and depression were evaluated and the proportion of responders was determined. In all included studies, at last follow-up, the improvement from baseline in OCD (Yale-Brown Obsessive Compulsive Scale [Y-BOCS score]) and a scale of weighted depression scores (WDS) were determined. Predictors of response (age, illness duration and severity, frequency parameters, and response in depression) were evaluated. The proportions of adverse events and dropouts were calculated.
Results: In RCTs, mean differences between sham and active stimulation in Y-BOCS and Hamilton Depression Rating Scale (HDRS) scores were −7.8 (95% CI = −11.2 to −4.3, I2 = 40%, P = .0001) and −7.3 (95% CI = −11.5 to −3.0, I2 = 0%, P = .0009), respectively. No differences between limbic and non-limbic targets were identified (χ2 = 0.21, I2 = 0%, P = .0006). At last follow-up, improvements in Y-BOCS and WDS were −15.0 (95% CI = −18.3 to −11.7, I2 = 90%, P < .001) and −13.7 (95% CI = -20.1 to −7.3, I2 = 76%, P < .001), respectively. No consistent predictors of response were found. There were 0.68 adverse events (95% CI = 0.59 to 0.78, I2 = 88%), 0.32 serious adverse events (95% CI = 0.12 to 0.62, I2 = 96%), and 0.13 dropouts (95% CI = 0.07 to 0.16, I2 = 16%) per treated patient.
Conclusions: DBS can significantly decrease Y-BOCS score and depressive symptoms in refractory OCD.
CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation. A $10 processing fee will apply.
CME Objective
After studying this article, you should be able to:
• Consider the use of deep brain stimulation for patients with severe, refractory obsessive-compulsive disorder and depressive symptoms
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
Release, Expiration, and Review Dates
This educational activity was published in May 2020 and is eligible for AMA PRA Category 1 Creditâ„¢ through June 30, 2022. The latest review of this material was May 2020.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; has been a member of the Steering Committee for Educational Activities for Medscape; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure appears at the end of the article.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
Objective: To evaluate efficacy, effect on mood, and safety of deep brain stimulation (DBS) for obsessive-compulsive disorder (OCD) at different target sites.
Data Sources: Electronic records from databases MEDLINE, EMBASE, and CENTRAL up to November 2019 were searched. Search terms included OCD, depression, and DBS.
Study Selection: Eight randomized controlled trials (RCTs) (n = 85) and 38 observational studies (case reports and case series) (n = 225) were included.
Data Extraction: In RCTs, the differences in outcomes between sham and active stimulation for OCD and depression were evaluated and the proportion of responders was determined. In all included studies, at last follow-up, the improvement from baseline in OCD (Yale-Brown Obsessive Compulsive Scale [Y-BOCS score]) and a scale of weighted depression scores (WDS) were determined. Predictors of response (age, illness duration and severity, frequency parameters, and response in depression) were evaluated. The proportions of adverse events and dropouts were calculated.
Results: In RCTs, mean differences between sham and active stimulation in Y-BOCS and Hamilton Depression Rating Scale (HDRS) scores were −7.8 (95% CI = −11.2 to −4.3, I2 = 40%, P = .0001) and −7.3 (95% CI = −11.5 to −3.0, I2 = 0%, P = .0009), respectively. No differences between limbic and non-limbic targets were identified (χ2 = 0.21, I2 = 0%, P = .0006). At last follow-up, improvements in Y-BOCS and WDS were −15.0 (95% CI = −18.3 to −11.7, I2 = 90%, P < .001) and −13.7 (95% CI = -20.1 to −7.3, I2 = 76%, P < .001), respectively. No consistent predictors of response were found. There were 0.68 adverse events (95% CI = 0.59 to 0.78, I2 = 88%), 0.32 serious adverse events (95% CI = 0.12 to 0.62, I2 = 96%), and 0.13 dropouts (95% CI = 0.07 to 0.16, I2 = 16%) per treated patient.
Conclusions: DBS can significantly decrease Y-BOCS score and depressive symptoms in refractory OCD.
J Clin Psychiatry 2020;81(3):19r12821
To cite: Martinho FP, Duarte GS, Simíµes do Couto F. Efficacy, effect on mood symptoms, and safety of deep brain stimulation in refractory obsessive-compulsive disorder: a systematic review and meta-analysis. J Clin Psychiatry. 2020;81(3):19r12821.
To share: https://doi.org/10.4088/JCP.19r12821
© Copyright 2020 Physicians Postgraduate Press, Inc.
aFaculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal
bLaboratório de Farmacologia Clínica e Terapêutica, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal
cInstituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal
dPsychiatry and Psychology Department, Faculdade de Medicina, Universidade de Lisboa, Lisbon, Portugal
*Corresponding author: Filipe Peste Martinho, MD, Faculdade de Medicina, Universidade de Lisboa, Avenida Professor Egas Moniz, 1649-028, Lisboa, Portugal ([email protected]).
Obsessive-compulsive disorder (OCD) is characterized by the presence of obsessions (persistent and intrusive thoughts, urges, or impulses that cause marked anxiety and that the individual attempts to ignore, suppress, or neutralize) or compulsions (behaviors or mental acts that the individual feels driven to perform in response to an obsession in order to reduce anxiety).1 OCD has a lifetime prevalence of 2.3%.2
First-line therapeutic options for OCD include selective serotonin reuptake inhibitors (SSRIs) and cognitive-behavioral therapy (CBT) with exposure and response prevention, alone or in combination. Second-line options are heterogeneous but may include antidepressants or antipsychotics, among others.3,4 However, a fraction of people are refractory to all such options.5 Ablative neurosurgical procedures such as anterior capsulotomy, anterior cingulotomy, subcaudate tractotomy, and limbic leucotomy were developed in response to treatment-resistant disease, with promising results.6-8 These procedures are irreversible, which dissuades some patients. However, other patients also actually prefer the “one and done” approach of the ablative procedures as opposed to implanted hardware and clinical appointments for the rest of their lives with DBS.
Deep brain stimulation (DBS), a reversible and adaptable procedure that uses high frequency electrodes implanted in specific areas of the brain to promote electric and chemical changes,9,10 was initially used for the treatment of Parkinson’s disease. Its use in OCD patients was first published in 1999.11 DBS targets were chosen according to the knowledge of neural OCD basis, including results from lesions studies. Functionally, convergent findings implicate the cortico-striato-thalamo-cortical system (CSTC)12,13 in the pathophysiology of the disease. CSTC includes limbic structures such as the anterior limb of the internal capsule (ALIC),11 the nucleus accumbens (NAcc),14,15 the middle forebrain bundle,16 and the ventral capsule and ventral striatum (VC/VS, which includes the ventral portion of the internal capsule, the NAcc, the anteroventral portion of the putamen, and the transition between the NAcc and the head of the caudate nucleus),17,18 as well as non-limbic structures such as the medial dorsal and ventral anterior nuclei of the thalamus (MD/VA),19 the inferior thalamic peduncle (ITP),20-22 and the subthalamic nucleus (STN).23,24 The mechanism seems to be far more complex than initially thought, probably due to the integration of the loops, to the role of the amygdala and hippocampus, and to the distinct and disparate roles of the lateral and medial orbitofrontal cortices.25,26 Most targets belong to the CSTC pathway, and other targets, although not belonging to the CSTC, have intimate connections to it, such as the bed nucleus of stria terminalis (BST).27,28 The efficacy of DBS for refractory OCD has been shown in several studies.15,19-22,24,25,28-32 However, the magnitude of the effect, the different targets, and some studies with negative results highlight the need for further study. Of people who meet the criteria for OCD, 63.3% also meet criteria for a mood disorder, and 40.7% meet criteria for major depressive disorder (MDD).2 Multiple accounts of DBS for OCD have reported an improvement in mood symptoms.27,29,30,33-35 It is known that mood symptoms in patients with OCD may differ from those in patients with MDD,36 and neurobiological data seem to confirm that there are pathophysiologic differences between primary MDD and secondary depressive symptoms in OCD patients.37
The two meta-analyses performed so far38,39 have shown that there is a decrease in OCD symptoms with DBS but have not addressed mood. Furthermore, the last meta-analysis performed dates back to 2014 and does not include the largest randomized controlled trial (RCT) published to date.27 Although globally safe, DBS can have significant adverse events and is extremely expensive. Stronger evidence of its efficacy could help the involved subjects in the decision to choose DBS as a treatment, which provided encouragement to perform this study.
METHODS
This systematic review and meta-analysis was conducted according to the PRISMA guidelines.40
Eligibility Criteria
RCTs, either parallel or crossover, and observational studies that enrolled people with OCD treated with DBS were included. Only studies published in English were included. Patients required a main diagnosis of OCD of disabling severity, according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition or Fifth Edition.1,41 Studies were accepted regardless of participants’ comorbid conditions or age and publication year or publication status of the study.
Studies were required to report data on at least 1 of the following outcomes:
- Primary efficacy outcome: variation of obsessive and/or compulsive symptoms, measured by the Yale-Brown Obsessive Compulsive Scale (Y-BOCS).42
- Primary safety outcome: proportion of participants with serious adverse events.
- Secondary outcomes: proportion of patients with complete response (Y-BOCS improvement > 35%); proportion of patients in remission (Y-BOCS score < 6); variation of mood symptoms, measured by any validated instruments; proportion of participants with any adverse event; proportion of dropouts; and predictors of response.
Narrative or systematic reviews; articles on neurophysiological, neuropsychological, or functional imaging effects of DBS; or articles focused solely on acute effects were excluded.
Information Sources
MEDLINE, EMBASE, and CENTRAL were searched from inception to November 2019, as were WHO International Clinical Trials Registry Platform and ClinicalTrials.gov. Reference lists were cross-checked for additional references. Principal investigators of clinical trials with unpublished data were contacted for additional data.
Study Selection
Titles, abstracts, and full texts were screened independently by 2 reviewers. Disagreements were solved by consensus.
Data Collection Process
One reviewer extracted individual study data onto a piloted extraction sheet. Another reviewer confirmed the extracted data.
Data Items
The following data items were collected, when available: study design, duration, and country; inclusion and exclusion criteria; patient age and sex, duration of illness, and follow-up time; stimulation parameters (site, laterality, frequency, pulse width and voltage); (a) baseline, (b) ON-period and OFF-period outcomes (if RCT), and (c) longest follow-up outcomes (RCTs with open-label phase and non-RCTs) for (1) Y-BOCS and (2) depression score measured by the Hamilton Depression Rating Scale (HDRS), the Beck Depression Inventory (BDI), the Montgomery-Asberg Rating Scale (MADRS) and the Depression Anxiety Stress Scales-Depression (DASS-D); total and serious adverse events; and dropout rates. When possible, items were collected on an individual patient level. Included studies were cross-checked for duplicate patients (using available epidemiologic data such as age, gender, and OCD age at onset) and the latest and most detailed information was collected. When 2 or more studies reported on the same cohort of patients, the data from these studies were analyzed together on a single cohort.
Risk of Bias in Individual Studies
The Cochrane risk of bias tool43 was used to classify RCTs as being at low, high, or unclear risk of bias in the standard domains. Risk of bias in observational studies was evaluated with the Newcastle-Ottawa Scale,44 which awards 4 stars for selection of exposure and control groups, 2 stars for compatibility between exposure and control groups, and 3 stars for outcome evaluation. Two authors independently assessed risk of bias. Disagreements were solved by consensus.
Summary Measures and Planned Method of Analysis
Because different depression instruments were used, depression scores were standardized by calculating the percentage of each patient’s score from the maximum score of the instrument used, and subsequent statistical analysis was performed with this value, which was named weighted depression score (WDS). Analyses of active vs sham stimulation data were performed using Review Manager 5.3 and SPSS v.23.45,46 Mean differences (MDs) and corresponding 95% confidence intervals (CIs) were calculated for Y-BOCS and WDS. Differences between active and sham stimulation were analyzed. A subgroup analysis for limbic versus non-limbic stimulation sites was conducted. Risk ratios (RRs) and number needed to treat (NNT) were calculated for dichotomous outcomes.
Analyses of last follow-up vs baseline data were performed with OpenMetaAnalyst47 and SPSS v.23. The Paule-Mandel random-effects method was used. MD from baseline was calculated for Y-BOCS and WDS. A Spearman rank-order correlation was used to study the correlation between decrease in Y-BOCS during RCT and patient age, duration of illness, stimulation frequency, pulse width and voltage, baseline Y-BOCS, and WDS improvement. Bonferroni correction was applied to all correlations. Heterogeneity was quantified using the I2 statistic.48 Safety outcomes were analyzed in OpenMetaAnalyst, by pooling Freeman-Tukey transformed proportions using Paule-Mandel random-effects model.
Analyses of epidemiologic data and stimulation parameters were performed on an individual patient level. Otherwise, cohort level analyses were performed, using the latest and most detailed information from each cohort.
Ethics
Because this research used anonymized data previously published in the literature, it is exempt from institutional review board approval.
RESULTS
Study Selection
Of the 1,817 articles whose abstracts were reviewed, 59 were selected for full-text assessment. Of these, 46 met eligibility criteria and were included in the meta-analysis (see Figure 1).
Study Characteristics
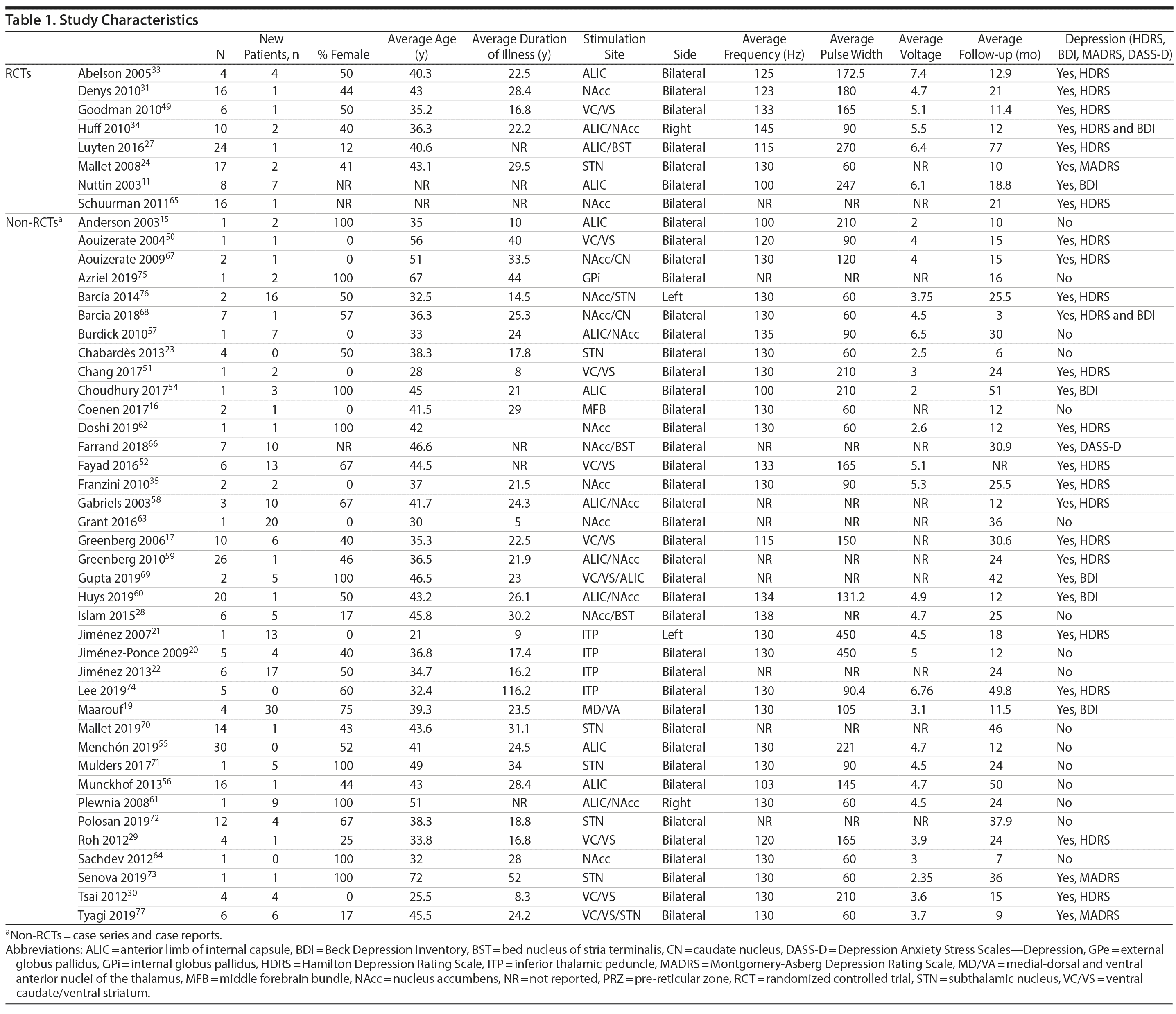
Eight studies were RCTs (2 of them reporting on the same cohort), and 38 were observational studies (13 case reports and 25 case series). Eighty-five patients were included in RCTs and 225 were included overall. The average patient age was 40 years (from 18 to 72 years), and 46% were female. The average duration of illness was 24 years (from 5 to 52 years). The average time of follow-up was 33 months (0.3 to 171 months). The most frequent stimulation sites were limbic (33 studies in total, 7 studies in VC/VS,17,29,30,49-52 6 studies in ALIC,15,33,53-56 6 studies in ALIC/NAcc,34,57-61 6 studies in NAcc,31,35,62-65 2 studies in NAcc/BST,28,66 2 studies in NAcc/CN,67,68 1 study in ALIC/BST,27 1 study in MFB,16 and 1 in VC/VS/ALIC69). Six studies reported stimulation in the STN,23,24,70-73 4 in ITP,20-22,74 1 in GPi,75 and 1 in MD/VA.19 Two studies reported mixed stimulation in limbic and non-limbic sites, 1 in NAcc/STN,76 and 1 in VC/VS/STN.77 Two studies reported stimulation in the left side and 2 in the right side, and the remainder 42 were bilateral. The average stimulation frequency used was 132 Hz (85 to 280 Hz), average pulse width was 143 ms (60 to 450 ms), and average voltage was 4.9 V (1.5 to 10.5 V). All studies collected Y-BOCS scores. Thirty-one collected data on depression (20 used HDRS, 5 BDI, 3 MADRS, 2 HDRS and BDI, and 1 DASS-D). A summary of study characteristics may be found in Table 1.
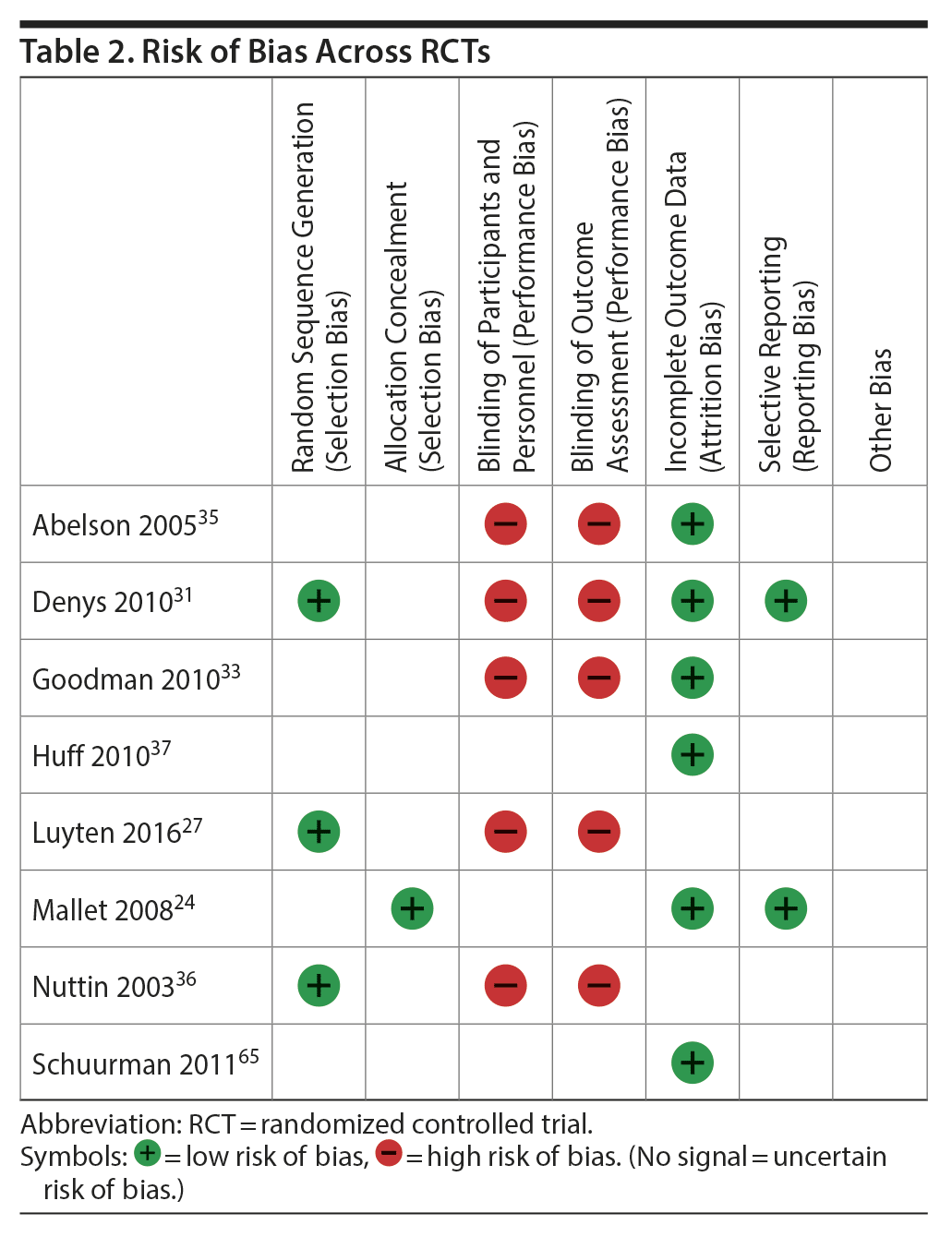
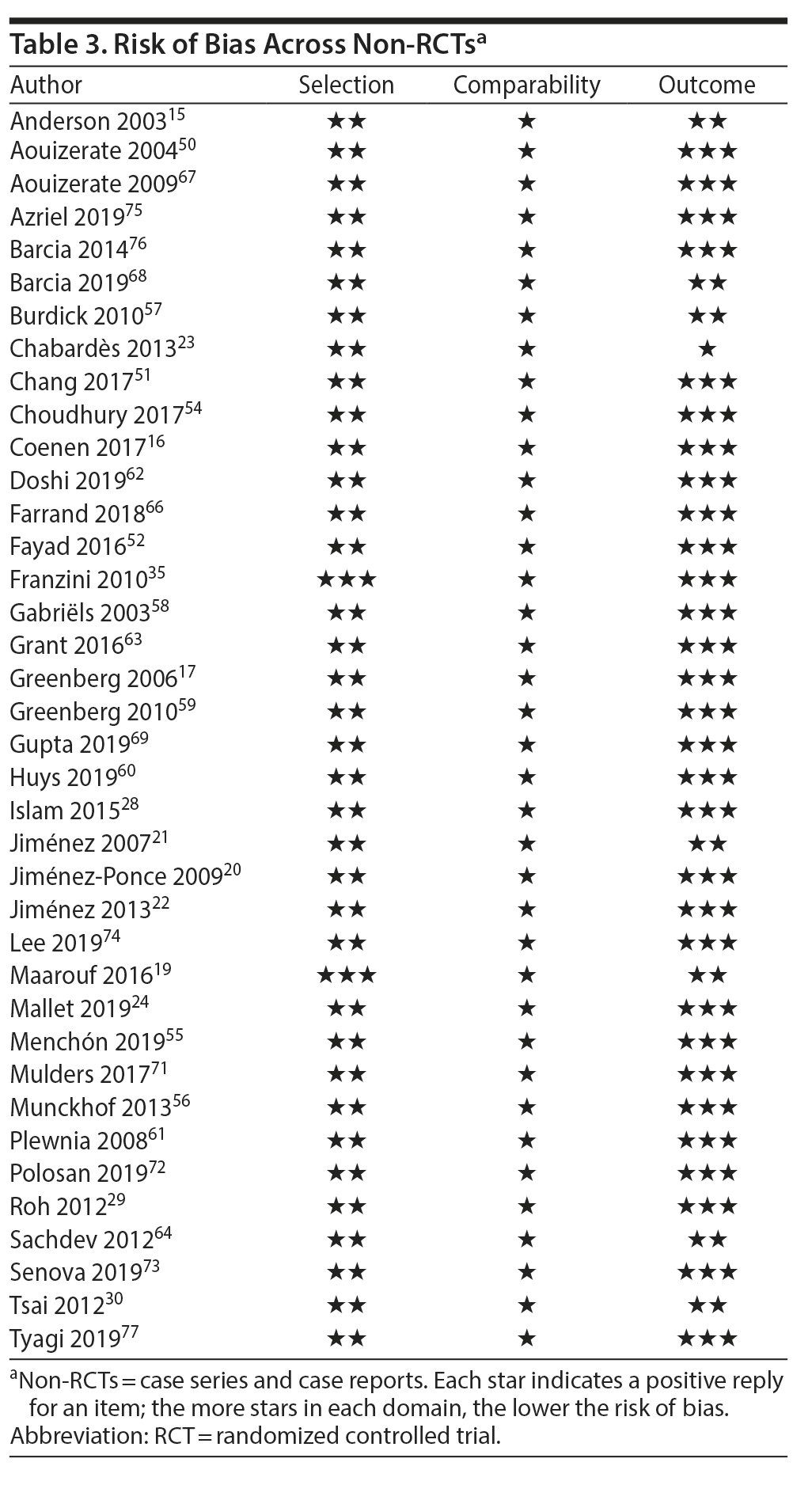
Risk of Bias Within Studies
In RCTs, risk of bias for selection, attrition, and reporting was considered low or uncertain in all studies. Risk bias in the performance and detection parameters was considered high in all but 2 studies, in which it was uncertain.24,34 Most non-RCTs were attributed 2 or 3 stars in patient selection and outcome assessment. Since no study had a comparison arm, comparability domain questions were not applicable, so all studies were awarded 1 star by default. Risk of bias within RCTs and non-RCTs may be found in Table 2 and Table 3, respectively.
Synthesis of Results
Analyses were performed on 2 aggregates of studies: (a) RCTs only for On and Off stimulation results and (b) all selected studies for baseline and last follow-up results. Analyses of absolute and percentage data were conducted and had similar results. Data from duplicate patients were merged, and of the 46 included studies, 39 cohorts were analyzed.
Efficacy
Baseline scores. The average Y-BOCS score at baseline was 33.8 (SD = 4.2) in RCTs and 33.7 (SD = 3.8) overall.
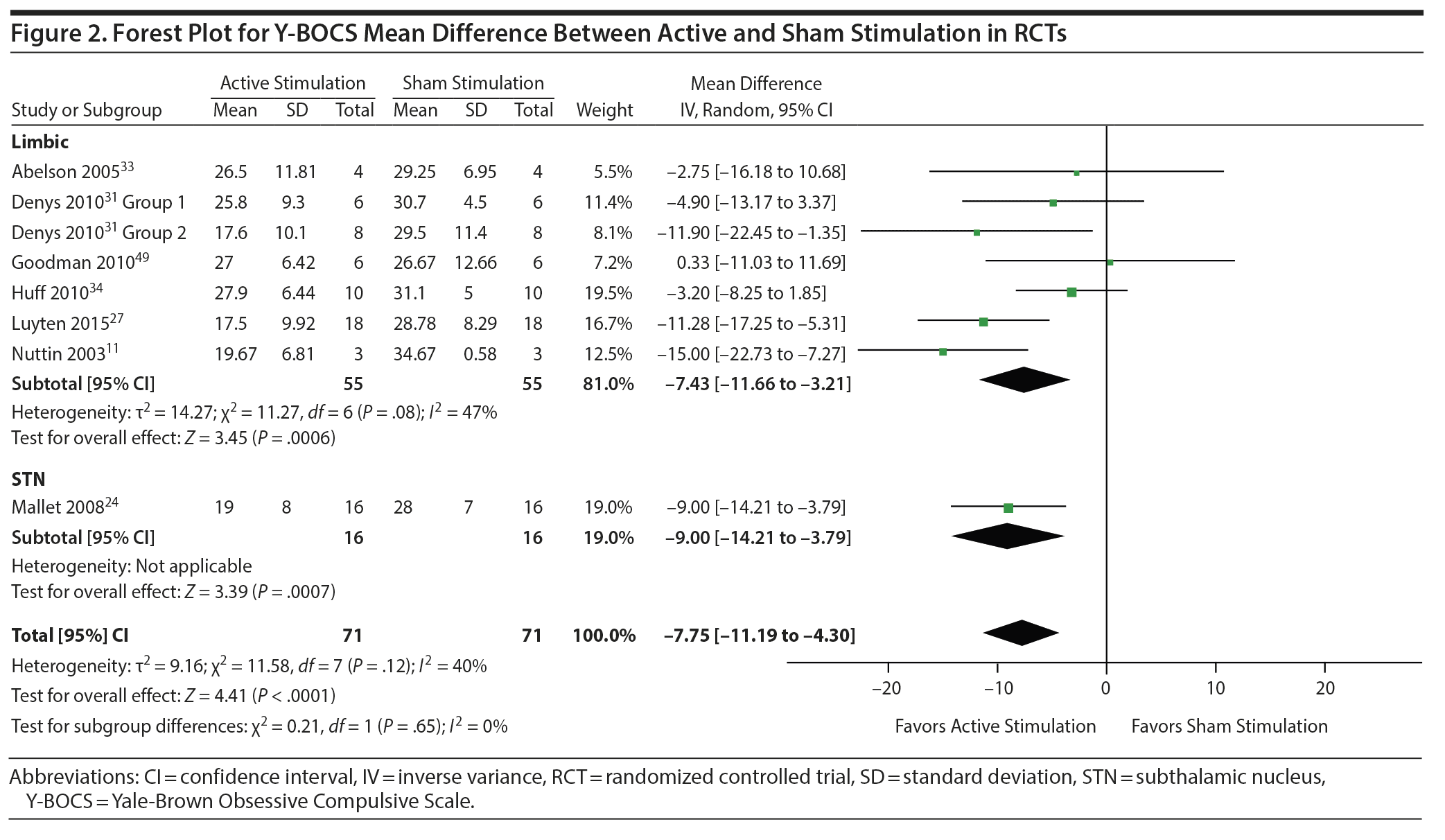
Decrease in Y-BOCS score. In RCTs, MD in Y-BOCS in sham versus active stimulation was −7.8 (95% CI = −11.2 to −4.3, I2 = 40%, P < .0001) (see Figure 2).
Complete response to treatment. Complete response to treatment (as defined by a decrease of > 35% in Y-BOCS score from sham to active stimulation) was analyzed. In RCTs, the percentage of patients that reached complete response to treatment during active stimulation was 51%, as opposed to 18% during sham stimulation (RR = 2.4 [95% CI = 1.3 to 4.3, I2 = 0%, P = .003], risk difference = 0.33 [95% CI = 0.16 to 0.49, I2 = 37%, P = .0001], NNT = 3.03). In all included studies, the percentage of patients that reached complete response to treatment at last follow-up was 57.9% (95% CI = 49.7 to 69.9%, I2 = 62%, P < .001).
Remission. Remission (as defined by Y-BOCS score < 6) was analyzed. In RCTs, the percentage of patients that reached remission was 8% during active stimulation and 5% during sham stimulation, but was not statistically significant (RR = 1.3 [95% CI = 0.2% to 10.55%, I2 = 26%, P = .80], NNT = 33.3). In all included studies, the percentage of patients that reached remission at last follow-up was 5.4% (95% CI = 2.4% to 8.4%, I2 = 0%, P = .92).
Subgroup analysis. Only 2 subgroups were included in subgroup analyses: the overall aggregate of limbic targets and STN (limbic targets subgroup: MD = −7.4, 95% CI = −11.7 to −3.2, I2 = 47%, P = .0006; STN subgroup: MD = −9.0, 95% CI = −14.2 to −3.8, P = .0007; test for subgroup differences: χ2 = 0.21, I2 for subgroup differences = 0%, P = .65). The STN subgroup included only 1 study. In all included studies, improvement in Y-BOCS from baseline was −15.0 (95% CI = −18.3 to −11.7, I2 = 90%, P < .001). Due to the fact that some RCTs optimized stimulation parameters before the RCT period24,34 and others did not, a post hoc subgroup sensitivity analysis was conducted, comparing efficacy between these two groups, and yielded no subgroup difference between the two groups of trials (P = .21).
Predictors of response. Age, duration of illness, stimulation frequency, pulse width and voltage, basal Y-BOCS, and depression response were evaluated, both in RCT data and at last follow-up, totaling 14 correlations. The most consistent correlation found was between response in Y-BOCS and response in depression, both in RCT data and at last follow-up (respectively, Spearman Ï = 0.989, P = .006 and Spearman Ï = 0.454, P = .000). Age was not a predictor of response either in RCTs or at last follow-up. Duration of illness was a positive predictor of response in RCT data (Spearman Ï = 0.377, P = .02) but not at last follow-up. Stimulation frequency was a negative predictor of response in RCT data (Spearman Ï = 0.416, P = .005) but a positive predictive factor at last follow-up (Spearman Ï = 0.195, P = .026). In RCT data, pulse width was a positive predictor of response (Spearman Ï = 0.416, P = .005), but not at last follow-up. Voltage was not a predictor of response at either stage. Illness severity at baseline as measured by Y-BOCS was not a predictor of response at baseline but was a negative predictor of response at last follow-up (Spearman Ï = −0.271, P = .001). However, since 14 correlations were performed, only the negative correlation between Y-BOCS response and baseline Y-BOCS and the positive correlation between Y-BOCS response and depression response would hold up to a Bonferroni correction.
Effect on mood. Effect of DBS on mood symptoms was reported in 31 studies. The average WDS basal score was 33.7 (SD = 39.8) in RCTs and 36.6 (SD = 17.0) overall.
Two studies were included in the analyses for RCTs. In RCTs, MD in the HDRS between sham and active stimulation was −7.3 (95% CI = −11.5 to −3.0, I2 = 0%, P = .0009). At last follow-up, absolute decrease of the average weighted depression score from baseline was −13.7 (95% CI = -20.1 to −7.3, I2 = 76%, P < .001).
There was a correlation between response in Y-BOCS and response in WDS, both in RCTs and at last follow-up (Spearman Ï = 0.989, P = .006 and Spearman Ï = 0.454, P = .000).
Safety
A total of 814 adverse events were reported: 289 psychiatric adverse events (most commonly hypomania, sleep complaints, irritability, apathy, and depression), 215 medical adverse events (most commonly weight change, sexual complaints, infections, gastrointestinal symptoms, and orthopedic/musculoskeletal symptoms), 202 neurologic symptoms (most commonly paresthesias, cognitive complaints, headache, and sensorial complaints), 41 device-related symptoms (most commonly sensations with extension leads or stimulation), and 67 other. Of these, 66 adverse events were considered serious, of which 24 were medical, 19 neurologic, 13 psychiatric, and 10 device-related. There were 4 reported deaths; 1 due to breast cancer, 1 due to overdose, 1 due to tuberculosis, and 1 due to suicide. There were 0.68 adverse events per participant (95% CI = 0.59 to 0.78, I2 = 88%, cohort-level analysis, 30 included cohorts, 195 patients). There were 0.32 serious adverse events per participant (95% CI = 0.12 to 0.52, I2 = 96%, cohort-level analysis, 27 included cohorts, 158 included patients). There were 0.13 dropouts per participant (95% = CI 0.07 to 0.16, I2 = 16%, cohort-level analysis, 30 included cohorts, 175 included patients). There were no correlations between total adverse events, serious adverse events, or dropout rate and stimulation site or time of follow-up.
DISCUSSION
Efficacy
In this meta-analysis, we found a statistically significant decrease in Y-BOCS score of 7.8 from sham to active stimulation and a complete response probability 2.4 times higher in active vs sham stimulation, with an NNT of 3. At last follow-up, there was a decrease in Y-BOCS from basal of 15.0, and 57.9% reached complete response. These data are comparable to those of previous meta-analyses of DBS, in which the decrease in Y-BOCS score was 8.93 between active and sham stimulation39 and complete response rate was 60%.38 In comparison to surgical approaches, these results are slightly better than those of capsulotomy (52.9% of patients had complete response)78 and cingulotomy (47% of patients had complete response).79 Despite this, a recent meta-analysis80 found capsulotomy to have a greater utility than DBS; however, it used a measure of utility, which was different from the methodology used here.
In subgroup analysis for efficacy in different targets, there were no differences between limbic and non-limbic sites. A possible explanation for this is that OCD is due to a dysfunction of the CSTC network, and not of a specific nucleus or region, so the intervention over any part of the network will have some effect on symptoms. However, this analysis was limited because it was not possible to compare efficacy in different limbic sites, due to high variability in limbic stimulation targets between and within studies and considerable overlap in their stimulation, and only 1 non-limbic target RCT24 was included. A recent RCT77 comparing stimulation of VC/VS and STN showed that Y-BOCS improved similarly between the STN and the VC/VS group, confirming the data from this meta-analysis. On the other hand, stimulation of the STN (but not of VC/VS) improved cognitive flexibility, and stimulation of the VC/VS improved mood (to a greater degree than STN stimulation). This, along with tractography data from that trial showing connection of VC/VS and STN to different brain regions, suggests that despite both structures belonging to the CSTC pathway, stimulation of VC/VS and STN may affect different functional networks.
The most consistent results in the search for predictors of response were that decrease in depression symptoms correlated with Y-BOCS decrease and that age and voltage did not, contradicting a previous meta-analysis that found that older age at onset was a predictor of response.38 The remainder of analyses had inconsistent statistically significant results that lost significance after a Bonferroni correction.
Testing stimulation occurred prior to the blinding phase in most RCTs, in order to identify maximum efficacy and increase the study’s detection power. However, that may have led to the unblinding of the trial due to the patients’ knowledge of stimulation effects. So, in most RCTs there was almost certainly a high risk of detection bias. Despite this, most included studies reported symptom increase when the device battery became depleted, which was in effect a triple blinding situation, which favors therapeutic efficacy of the method. On the other hand, in order to avoid detection bias, 2 RCTs24,34 used low voltages during the pre-blinding phase, which might have decreased the detection power of the trials. For that reason, a post hoc analysis was conducted in order to compare efficacy between these two approaches, and no difference was detected.
A limiting factor in this review might have been the Y-BOCS itself. Because the scale attributes maximum score to obsessions that last for 8 hours a day, and any patients included in the review had very serious OCD with obsessions longer than 8 hours, this scale is not very sensitive to symptomatic improvement in the very severe extreme of the OCD symptom spectrum, even if that improvement is very significant. Additionally, the remission threshold was a Y-BOCS of 6, which is rather conservative. So, considering these aspects, the Y-BOCS improvement reported can be considered clinically significant.
Effect on Mood
There was a decrease in HDRS score of 7.3 between active and sham stimulation. At last follow-up, DBS led to a decrease of 13.7 in WDS. These results may have been limited by the fact that many reports excluded patients with a diagnosis of major depressive disorder, many reported this parameter incompletely or not at all, and different reports used different mood scales, which had to be standardized, possibly decreasing the quality of the analysis. There was a statistically significant correlation between Y-BOCS and WDS decreases. However, it is not possible with this review to determine whether this decrease indicates that (1) clinically severe OCD leads to depressive symptoms that remit once illness is treated or (2) there are pathological mechanisms of depression underpinning OCD, and the interference of OCD on these mechanisms leads to symptom improvement. Two reports31,51 suggest that symptomatic improvement happens sequentially: first, mood and anxiety within hours; next, obsessions within days; and finally, compulsions within weeks or months. This sequence appears to be in accordance with the hypothesis that there are pathological mechanisms of depression underpinning OCD.
Safety
There are significant proportions of adverse events and dropouts. This is consistent with previous reports38,39 and appears to be similar to adverse event rates in capsulotomy.81 A recent meta-analysis, however, found capsulotomy to have less adverse events than DBS.80 The high rate of adverse events found here may be due to overrepresentation of transient events, which were not possible to exclude. Furthermore, there were significant differences in adverse event reporting in the included reports. There was no association between adverse events and stimulation site or time of follow-up, which may be a suggestion that their incidence is limited to the perioperative time.
CONCLUSIONS
Our results showed that, including recent trials performed, DBS can significantly decrease YBOCS score and depressive symptoms in refractory OCD.
Submitted: March 8, 2019; accepted January 20, 2020.
Published online: May 26, 2020.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, use of deep brain stimulation devices outside of US Food and Drug Administration-approved labeling may have been performed in the studies evaluated in this meta-analysis. Please check indications on labeling provided by manufacturers.
Financial disclosure: Drs Martinho, Duarte, and Simíµes do Couto have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: The authors have no support or funding to report.
Clinical Points
- Deep brain stimulation has been shown to be effective for the treatment of patients with severe and refractory OCD. However, its costs, adverse events, diversity of targets, and limited use demand a clear analysis of efficacy.
- For a patient with severe and refractory OCD, DBS can significantly decrease depression and OCD symptoms.
REFERENCES
1.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
2.Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 2010;15(1):53-63. PubMed CrossRef
3.Baldwin DS, Anderson IM, Nutt DJ, et al; British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2005;19(6):567-596. PubMed CrossRef
4.Bandelow B, Zohar J, Hollander E, et al; WFSBP Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disoders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders—first revision. World J Biol Psychiatry. 2008;9(4):248-312. PubMed CrossRef
5.Schläepfer TE, George MS, Mayberg H; WFSBP Task Force on Brain Stimulation. WFSBP Guidelines on brain stimulation treatments in psychiatry. World J Biol Psychiatry. 2010;11(1):2-18. PubMed CrossRef
6.Pepper J, Hariz M, Zrinzo L. Deep brain stimulation versus anterior capsulotomy for obsessive-compulsive disorder: a review of the literature. J Neurosurg. 2015;122(5):1028-1037. PubMed CrossRef
7.Greenberg BD, Rauch SL, Haber SN. Invasive circuitry-based neurotherapeutics: stereotactic ablation and deep brain stimulation for OCD. Neuropsychopharmacology. 2010;35(1):317-336. PubMed CrossRef
8.Keen EC, Widge AS, Dougherty DD. Functional neurosurgery in severe and treatment-refractory OCD. In: Pittenger C, ed. Obsessive-Compulsive Disorder: Phenomenology, Pathophysiology, and Treatment. Oxford, UK: Oxford University Press; 1997:507-516.
9.Herrington TM, Cheng JJ, Eskandar EN. Mechanisms of deep brain stimulation. J Neurophysiol. 2016;115(1):19-38. PubMed CrossRef
10.Vitek JL. Mechanisms of deep brain stimulation: excitation or inhibition. Mov Disord. 2002;17(suppl 3):S69-S72. PubMed CrossRef
11.Nuttin BJ, Gabriels L, van Kuyck K, et al. Electrical stimulation of the anterior limbs of the internal capsules in patients with severe obsessive-compulsive disorder: anecdotal reports. Neurosurg Clin N Am. 2003;14(2):267-274. PubMed CrossRef
12.Nambu A. Somatotopic organization of the primate basal ganglia. Front Neuroanat. 2011;5:26. PubMed CrossRef
13.Rapoport JL. Obsessive compulsive disorder and basal ganglia dysfunction. Psychol Med. 1990;20(3):465-469. PubMed CrossRef
14.Sturm V, Lenartz D, Koulousakis A, et al. The nucleus accumbens: a target for deep brain stimulation in obsessive-compulsive and anxiety disorders. J Chem Neuroanat. 2003;26(4):293-299. PubMed CrossRef
15.Anderson D, Ahmed A. Treatment of patients with intractable obsessive-compulsive disorder with anterior capsular stimulation: case report. J Neurosurg. 2003;98(5):1104-1108. PubMed CrossRef
16.Coenen VA, Schlaepfer TE, Goll P, et al. The medial forebrain bundle as a target for deep brain stimulation for obsessive-compulsive disorder. CNS Spectr. 2017;22(3):282-289. PubMed CrossRef
17.Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384-2393. PubMed CrossRef
18.Heimer L. Basal forebrain in the context of schizophrenia. Brain Res Brain Res Rev. 2000;31(2-3):205-235. PubMed CrossRef
19.Maarouf M, Neudorfer C, El Majdoub F, et al. Deep brain stimulation of medial dorsal and ventral anterior nucleus of the thalamus in OCD: a retrospective case series. PLoS One. 2016;11(8):e0160750. PubMed CrossRef
20.Jiménez-Ponce F, Velasco-Campos F, Castro-Farfán G, et al. Preliminary study in patients with obsessive-compulsive disorder treated with electrical stimulation in the inferior thalamic peduncle. Neurosurgery. 2009;65(6 suppl):203-209, discussion 209. PubMed
21.Jiménez F, Velasco F, Sal×n-Pascual R, et al. Neuromodulation of the inferior thalamic peduncle for major depression and obsessive compulsive disorder. Acta Neurochir suppl (Wien). 2007;97(pt 2):393-398. PubMed CrossRef
22.Jiménez F, Nicolini H, Lozano AM, et al. Electrical stimulation of the inferior thalamic peduncle in the treatment of major depression and obsessive compulsive disorders. World Neurosurg. 2013;80(3-4):30.e17-30.e25, 30.e25. PubMed CrossRef
23.Chabardרs S, Polosan M, Krack P, et al. Deep brain stimulation for obsessive-compulsive disorder: subthalamic nucleus target. World Neurosurg. 2013;80(3-4):31.e1-31.e8. PubMed CrossRef
24.Mallet L, Polosan M, Jaafari N, et al; STOC Study Group. Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med. 2008;359(20):2121-2134. PubMed CrossRef
25.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16(1):43-51. PubMed CrossRef
26.Robbins TW, Vaghi MM, Banca P. Obsessive-compulsive disorder: puzzles and prospects. Neuron. 2019;102(1):27-47. PubMed CrossRef
27.Luyten L, Hendrickx S, Raymaekers S, et al. Electrical stimulation in the bed nucleus of the stria terminalis alleviates severe obsessive-compulsive disorder. Mol Psychiatry. 2016;21(9):1272-1280. PubMed CrossRef
28.Islam L, Franzini A, Messina G, et al. Deep brain stimulation of the nucleus accumbens and bed nucleus of stria terminalis for obsessive-compulsive disorder: a case series. World Neurosurg. 2015;83(4):657-663. PubMed CrossRef
29.Roh D, Chang WS, Chang JW, et al. Long-term follow-up of deep brain stimulation for refractory obsessive-compulsive disorder. Psychiatry Res. 2012;200(2-3):1067-1070. PubMed CrossRef
30.Tsai HC, Chang CH, Pan JI, et al. Pilot study of deep brain stimulation in refractory obsessive-compulsive disorder ethnic Chinese patients. Psychiatry Clin Neurosci. 2012;66(4):303-312. PubMed CrossRef
31.Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. PubMed CrossRef
32.Munckhof P, Denys D, Bosch D. Deep brain stimulation in obsessive compulsive disorder: double-blind crossover study. Acta Neurochir (Wien). 2011;153:667-761.
33.Abelson JL, Curtis GC, Sagher O, et al. Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry. 2005;57(5):510-516. PubMed CrossRef
34.Huff W, Lenartz D, Schormann M, et al. Unilateral deep brain stimulation of the nucleus accumbens in patients with treatment-resistant obsessive-compulsive disorder: Outcomes after one year. Clin Neurol Neurosurg. 2010;112(2):137-143. PubMed CrossRef
35.Franzini A, Messina G, Gambini O, et al. Deep-brain stimulation of the nucleus accumbens in obsessive compulsive disorder: clinical, surgical and electrophysiological considerations in two consecutive patients. Neurol Sci. 2010;31(3):353-359. PubMed CrossRef
36.Fineberg NA, Fourie H, Gale TM, et al. Comorbid depression in obsessive compulsive disorder (OCD): symptomatic differences to major depressive disorder. J Affect Disord. 2005;87(2-3):327-330. PubMed CrossRef
37.Saxena S, Brody A, Ho M, et al. Cerebral metabolism in major depression and obsessive-compulsive disorder occurring separately and concurrently.. Biol Psychiatry. 2001;50(3):159-170. PubMed CrossRef
38.Alonso P, Cuadras D, Gabri׫ls L, et al. Deep brain stimulation for obsessive-compulsive disorder: a meta-analysis of treatment outcome and predictors of response. PLoS One. 2015;10(7):e0133591. PubMed CrossRef
39.Kisely S, Hall K, Siskind D, et al. Deep brain stimulation for obsessive-compulsive disorder: a systematic review and meta-analysis. Psychol Med. 2014;44(16):3533-3542. PubMed CrossRef
40.Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed CrossRef
41.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
42.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006-1011. PubMed CrossRef
43.Higgins JPT, Green S, eds. Handbook for Systematic Reviews of Interventions, Version 5.1.0. London, UK: The Cochrane Collaboration; 2011.
44.Wells GA, Shea B, O’ Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. 2011.
45.Review Manager (RevMan), Version 5.3 [computer program]. Copenhagen, Denmark; The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
46.SPSS Statistics for Macintosh, Version 23.0 [computer program]. Armonk, NY: IBM Corp; 2015.
47.Wallace B, Dahabreh I, Trikalinos T, et al. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. 2012;49(5):1-15. CrossRef
48.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. PubMed CrossRef
49.Goodman WK, Foote KD, Greenberg BD, et al. Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry. 2010;67(6):535-542. PubMed CrossRef
50.Aouizerate B, Cuny E, Martin-Guehl C, et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression: case report. J Neurosurg. 2004;101(4):682-686. PubMed CrossRef
51.Chang CH, Chen SYS, Tsai ST, et al. Compulsive skin-picking behavior after deep brain stimulation in a patient with refractory obsessive-compulsive disorder: a case report. Medicine (Baltimore). 2017;96(36):e8012. PubMed
52.Fayad SM, Guzick AG, Reid AM, et al. Six-nine year follow-up of deep brain stimulation for obsessive-compulsive disorder. PLoS One. 2016;11(12):e0167875. PubMed CrossRef
53.Nuttin BJ, Gabri׫ls LA, Cosyns PR, et al. Long-term electrical capsular stimulation in patients with obsessive-compulsive disorder. Neurosurgery. 2003;52(6):1263-1272, discussion 1272-1274. PubMed CrossRef
54.Choudhury TK, Davidson JE, Viswanathan A, et al. Deep brain stimulation of the anterior limb of the internal capsule for treatment of therapy-refractory obsessive compulsive disorder (OCD): a case study highlighting neurocognitive and psychiatric changes. Neurocase. 2017;23(2):138-145. PubMed CrossRef
55.Menchón JM, Real E, Alonso P, et al. A prospective international multi-center study on safety and efficacy of deep brain stimulation for resistant obsessive-compulsive disorder [published online ahead of print October 29, 2019]. Mol Psychiatry. PubMed CrossRef
56.van den Munckhof P, Bosch DA, Mantione MHM, et al. Active stimulation site of nucleus accumbens deep brain stimulation in obsessive-compulsive disorder is localized in the ventral internal capsule. Acta Neurochir suppl (Wien). 2013;117:53-59. PubMed
57.Burdick A, Foote KD, Goodman W, et al. Lack of benefit of accumbens/capsular deep brain stimulation in a patient with both tics and obsessive-compulsive disorder. Neurocase. 2010;16(4):321-330. PubMed CrossRef
58.Gabri׫ls L, Cosyns P, Nuttin B, et al. Deep brain stimulation for treatment-refractory obsessive-compulsive disorder: psychopathological and neuropsychological outcome in three cases. Acta Psychiatr Scand. 2003;107(4):275-282. PubMed CrossRef
59.Greenberg BD, Gabriels LA, Malone DA Jr, et al. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry. 2010;15(1):64-79. PubMed CrossRef
60.Huys D, Kohl S, Baldermann JC, et al. Open-label trial of anterior limb of internal capsule-nucleus accumbens deep brain stimulation for obsessive-compulsive disorder: insights gained. J Neurol Neurosurg Psychiatry. 2019;90(7):805-812. PubMed CrossRef
61.Plewnia C, Schober F, Rilk A, et al. Sustained improvement of obsessive-compulsive disorder by deep brain stimulation in a woman with residual schizophrenia. Int J Neuropsychopharmacol. 2008;11(8):1181-1183. PubMed CrossRef
62.Doshi PK, Hegde A, Desai A. Nucleus accumbens deep brain stimulation for obsessive-compulsive disorder and aggression in an autistic patient: a case report and hypothesis of the role of nucleus accumbens in autism and comorbid symptoms. World Neurosurg. 2019;125:387-391. PubMed CrossRef
63.Grant JE, Odlaug BL, Chamberlain SR. Long-term deep-brain stimulation treatment for obsessive-compulsive disorder. J Clin Psychiatry. 2016;77(1):132-133. PubMed CrossRef
64.Sachdev PS, Cannon E, Coyne TJ, et al. Bilateral deep brain stimulation of the nucleus accumbens for comorbid obsessive compulsive disorder and Tourette’s syndrome. BMJ Case Rep. 2012;2012:bcr2012006579. PubMed CrossRef
65.Schuurman PR, Munckhof P, Denys D, et al. Deep brain stimulation in obsessive compulsive disorder: double-blind crossover study. Acta Neurochir (Wien). 2011;153(3):729-730.
66.Farrand S, Evans AH, Mangelsdorf S, et al. Deep brain stimulation for severe treatment-resistant obsessive-compulsive disorder: an open-label case series. Aust N Z J Psychiatry. 2018;52(7):699-708. PubMed CrossRef
67.Aouizerate B, Cuny E, Bardinet E, et al. Distinct striatal targets in treating obsessive-compulsive disorder and major depression. J Neurosurg. 2009;111(4):775-779. PubMed CrossRef
68.Barcia JA, Avecillas-Chas×n JM, Nombela C, et al. Personalized striatal targets for deep brain stimulation in obsessive-compulsive disorder. Brain Stimul. 2019;12(3):724-734. PubMed CrossRef
69.Gupta A, Khanna S, Jain R. Deep brain stimulation of ventral internal capsule for refractory obsessive-compulsive disorder. Indian J Psychiatry. 2019;61(5):532-536. PubMed CrossRef
70.Mallet L, Du Montcel ST, Clair AH, et al; STOC Long-term Study Group. Long-term effects of subthalamic stimulation in obsessive-compulsive disorder: follow-up of a randomized controlled trial. Brain Stimul. 2019;12(4):1080-1082. PubMed CrossRef
71.Mulders AEP, Leentjens AFG, Schruers K, et al. Choreatic side effects of deep brain stimulation of the anteromedial subthalamic nucleus for treatment-resistant obsessive-compulsive disorder. World Neurosurg. 2017;104:1048.e9-1048.e13. PubMed CrossRef
72.Polosan M, Droux F, Kibleur A, et al. Affective modulation of the associative-limbic subthalamic nucleus: deep brain stimulation in obsessive-compulsive disorder. Transl Psychiatry. 2019;9(1):73. PubMed CrossRef
73.Senova S, Mallet L, Gurruchaga JM, et al. Severe obsessive-compulsive disorder secondary to neurodegeneration with brain iron accumulation: complete remission after subthalamic nuclei deep brain stimulation [published online ahead of print August 28, 2019]. Biol Psychiatry. PubMed CrossRef
74.Lee DJ, Dallapiazza RF, De Vloo P, et al. Inferior thalamic peduncle deep brain stimulation for treatment-refractory obsessive-compulsive disorder: a phase 1 pilot trial. Brain Stimul. 2019;12(2):344-352. PubMed CrossRef
75.Azriel A, Farrand S, Di Biase M, et al. Tractography-guided deep brain stimulation of the anteromedial globus pallidus internus for refractory obsessive-compulsive disorder: case report [published online ahead of print July 17, 2019]. Neurosurgery. PubMed CrossRef
76.Barcia JA, Reyes L, Arza R, et al. Deep brain stimulation for obsessive-compulsive disorder: is the side relevant? Stereotact Funct Neurosurg. 2014;92(1):31-36. PubMed CrossRef
77.Tyagi H, Apergis-Schoute AM, Akram H, et al. A randomized trial directly comparing ventral capsule and anteromedial subthalamic nucleus stimulation in obsessive-compulsive disorder: clinical and imaging evidence for dissociable effects. Biol Psychiatry. 2019;85(9):726-734. PubMed CrossRef
78.Oliver B, Gascón J, Aparicio A, et al. Bilateral anterior capsulotomy for refractory obsessive-compulsive disorders. Stereotact Funct Neurosurg. 2003;81(1-4):90-95. PubMed CrossRef
79.Jung HH, Kim CH, Chang JH, et al. Bilateral anterior cingulotomy for refractory obsessive-compulsive disorder: long-term follow-up results. Stereotact Funct Neurosurg. 2006;84(4):184-189. PubMed CrossRef
80.Kumar KK, Appelboom G, Lamsam L, et al. Comparative effectiveness of neuroablation and deep brain stimulation for treatment-resistant obsessive-compulsive disorder: a meta-analytic study. J Neurol Neurosurg Psychiatry. 2019;90(4):469-473. PubMed CrossRef
81.D’ Astous M, Cottin S, Roy M, et al. Bilateral stereotactic anterior capsulotomy for obsessive-compulsive disorder: long-term follow-up. J Neurol Neurosurg Psychiatry. 2013;84(11):1208-1213. PubMed CrossRef
This PDF is free for all visitors!