Long-Acting Injectable Antipsychotic Use During Pregnancy:
A Brief Review and Concise Guide for Clinicians
Compared to the general population, women with histories of psychotic and affective illnesses are at increased risk of psychiatric symptoms during pregnancy.1,2 Although maintenance antipsychotic therapy is important to prevent relapses both in patients with schizophrenia3 and in many patients with bipolar disorder,4 clinicians are more likely to discontinue oral antipsychotic prescriptions for pregnant women as compared to nonpregnant women.5 This trend is likely due to concerns of teratogenicity5 even though current pregnancy safety data of oral antipsychotics are largely reassuring, with no significant increased risk for major congenital malformations.6
In the case of long-acting injectable antipsychotics (LAIs), we have observed an even greater tendency for clinicians either to not begin or to discontinue LAI prescriptions during pregnancy. This phenomenon occurs even in women with extremely high risk for psychiatric illness recurrence despite the established value of LAIs in the treatment of such individuals7 and despite the fact that use of these medications is supported by the current research, which has identified no clear contraindication to LAI use in pregnancy.8-10 In this article, we will discuss the judicious use of LAIs in pregnant women.
LAIs are injectable forms of antipsychotics designed to improve medication adherence and prevent psychiatric symptom recurrence. There are currently 6 antipsychotics available in LAI formulation in the US: aripiprazole, fluphenazine, haloperidol, olanzapine, paliperidone, and risperidone. LAI formulations differ in their dosing schedule, ranging from 2 weeks to several months for newer, extended release formulations. Compared to oral forms of antipsychotics, LAIs are superior in preventing psychiatric hospitalization.11 Other advantages of LAIs include increased clinician knowledge around medication adherence, reduced risk of overdose, and more frequent and standardized contact between clinicians and patients.7 Current data show that overall, the side effects of LAI medications reflect those of their oral counterparts.12 An exception to this finding is long-acting olanzapine, which requires a 3-hour observation period after administration to monitor for symptoms of post-injection sedation syndrome.13
As with all psychopharmacologic decisions in pregnant patients, when considering prescribing an LAI during pregnancy, it is important to identify the known and potential risks versus known and potential benefits associated with either providing or withholding psychopharmacologic treatment. Women with histories of severe psychiatric illness who are nonadherent with antipsychotic medication during the first trimester are almost twice as likely to relapse as compared to women who are adherent.14 These psychiatric relapses during the perinatal period are not insignificant, as severe psychiatric symptoms during pregnancy are associated with poor outcomes for mother and infant. Untreated schizophrenia or bipolar disorder may be an independent risk factor for congenital malformations in the newborn,15 and antepartum psychosis can lead to the psychotic denial of pregnancy.16
We suggest that the appropriate patient to receive an LAI during pregnancy should not differ significantly from the appropriate patient to receive an LAI when they are not pregnant. Women with histories of hospitalizations precipitated by medication nonadherence are appropriate candidates. Women with histories of frequent and extended psychiatric hospitalizations associated with schizophrenia, schizoaffective disorder, and, for some women, bipolar disorder should also be considered. Also, psychiatric decompensation during previous pregnancies or the immediate postpartum period or a history of illicit substance use are additional clinical risk factors that would tip clinical decision-making toward LAI use over an oral antipsychotic.
Prior to prescribing the LAI, the psychiatrist should establish that the patient has the capacity to engage in a discussion of informed consent. Ideally, this discussion would include the woman’s partner and should occur prior to conception. Known antipsychotic risks in nonpregnant populations in addition to pregnancy-related risks of oral antipsychotics should be reviewed together with the limited safety data for LAI formulations in pregnancy. For a balanced and comprehensive informed consent process to take place, the psychiatrist should also review and document the current limitations of known safety data and the risks of psychiatric decompensation from untreated or undertreated illness.
When choosing LAI formulations for the pregnant patient, it is important to first consider the same factors as when treating the nonpregnant patient. Important factors for the treating psychiatric clinician to consider include previous efficacy of the oral formulation, the patient’s ability to be compliant with the needed time for overlap with the oral formulation, potential side effects, and cost.17 When the symptoms are primarily affective in nature, a clinician should be reminded that the first line of treatment for bipolar disorder is lithium, with an antipsychotic recommended only as second-line therapy.18 If an LAI is indicated, the clinician should also consider that the FDA has only approved long-acting formulations of aripiprazole and risperidone for the treatment of bipolar disorder.
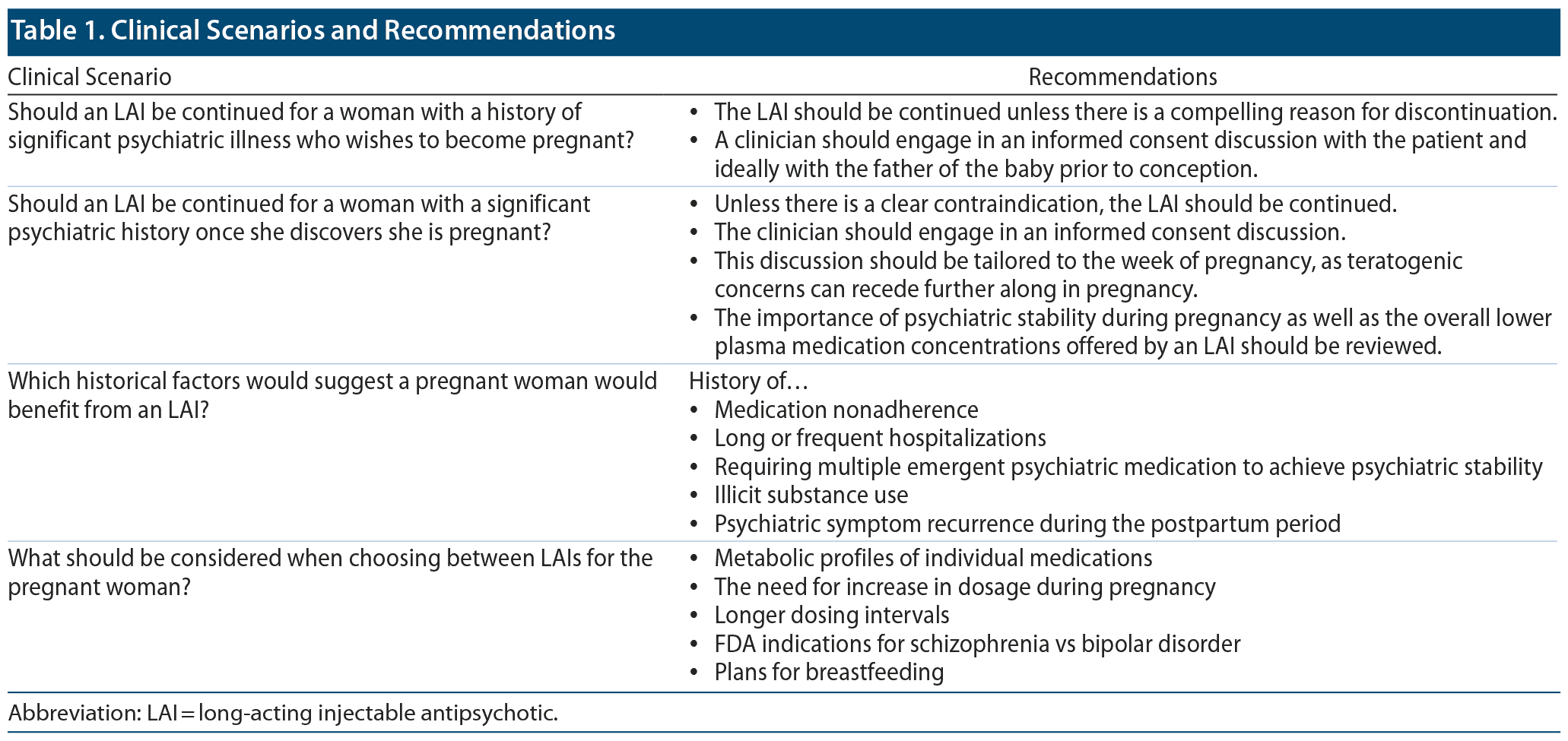
There are, however, several additional factors to consider when administering an LAI to a pregnant woman. Table 1 lists several clinical scenarios and recommendations. In general, LAI use, which is associated with a more constant plasma drug level, may reduce fetal exposure to the highly fluctuating plasma drug levels associated with maternal oral antipsychotic use.19 During pregnancy, the clinician should monitor for the need of a temporarily increased dosage of some antipsychotics given pregnancy-associated pharmacokinetic changes.20 These pharmacokinetic effects are particularly relevant to aripiprazole due to its metabolism by CYP2D6, an enzyme whose expression and activity are known to increase during pregnancy.20 Given the association of some antipsychotics with higher rates of metabolic complications during pregnancy that may have adverse effects on both mother and baby,21 including the development of preeclampsia and eclampsia,22 LAIs with more favorable metabolic profiles should be selected. A clinician should also consider using an LAI with a longer dosing interval. Women often have difficulty attending appointments in the postpartum period, with up to 40% of women not attending their postpartum obstetric follow-up appointment.23 Ensuring that women with significant psychiatric histories are maintained on psychiatric medication during the postpartum period is crucial given their high risk of psychiatric symptom recurrence during that time.24
When discussing the administration of an LAI to a pregnant woman, her future desire to breastfeed should also be considered. Though the current safety data on antipsychotics and lactation are limited, they are largely reassuring.25 For women with healthy pregnancies who are expected to carry to term, the benefits of breastfeeding may outweigh potential risks of antipsychotic exposure via breastmilk. However, in cases of women at risk for preterm delivery, a clinician may consider avoiding administering an LAI to women who strictly plan to breastfeed as preterm infants are at risk for elevated plasma medication concentrations due to immature hepatic and renal systems.26 A clinician should also consider the pharmacologic properties of antipsychotics and their impact on prolactin, a key hormone in the lactation process. Aripiprazole is a partial agonist of the dopamine receptor and often reduces prolactin levels.27 This reduction in prolactin may negatively impact lactation. In contrast, other antipsychotics are dopamine antagonists and can cause increased prolactin levels. Though the increased prolactin level may not impair lactation, the rare possibility of overproduction leading to mastitis should be discussed. This is relevant, as antipsychotic-induced mastitis has been described even in nonpregnant and nonlactating women.28
Given the relevance of psychiatric medications to the obstetric treatment of the pregnant patient, close collaboration between the psychiatric and the obstetric teams is critical. Complex psychiatric patients are often complex obstetric patients. At times, psychiatrists may need to educate and share the latest research evidence with the obstetric team surrounding the importance of psychiatric stability during pregnancy. Communication from the obstetric team about medication side effects as well as subtle signs of psychiatric decompensation can help the psychiatric team establish the appropriate dosage of medication.
In conclusion, given the rates of significant psychiatric illness and their associated risks of decompensation during the perinatal period, it is important for clinicians to become comfortable with antipsychotic use during pregnancy. Although we recognize the dearth of safety data on LAI formulations during pregnancy, we recommend overcoming the fear of prescribing an LAI to a pregnant woman when the lack of data is outweighed by substantial risks of psychiatric decompensation.
Published online: November 24, 2020.
REFERENCES
1.McNeil TF, Kaij L, Malmquist-Larsson A. Women with nonorganic psychosis: mental disturbance during pregnancy. Acta Psychiatr Scand. 1984;70(2):127-139. PubMed CrossRef
2.Di Florio A, Forty L, Gordon-Smith K, et al. Perinatal episodes across the mood disorder spectrum. JAMA Psychiatry. 2013;70(2):168-175. PubMed CrossRef
3.Leucht S, Tardy M, Komossa K, et al. Maintenance treatment with antipsychotic drugs for schizophrenia. Cochrane Database Syst Rev. 2012;(5):CD008016. PubMed
4.Lindström L, Lindström E, Nilsson M, et al. Maintenance therapy with second generation antipsychotics for bipolar disorder: a systematic review and meta-analysis. J Affect Disord. 2017;213:138-150. PubMed CrossRef
5.Petersen I, McCrea RL, Osborn DJ, et al. Discontinuation of antipsychotic medication in pregnancy: a cohort study. Schizophr Res. 2014;159(1):218-225. PubMed CrossRef
6.Cohen LS, Viguera AC, McInerney KA, et al. Reproductive safety of second-generation antipsychotics: current data from the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics. Am J Psychiatry. 2016;173(3):263-270. PubMed CrossRef
7.Brissos S, Veguilla MR, Taylor D, et al. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198-219. PubMed CrossRef
8.×–zdemir AK, Pak ÅžC, Canan F, et al. Paliperidone palmitate use in pregnancy in a woman with schizophrenia. Arch Women Ment Health. 2015;18(5):739-740. PubMed CrossRef
9.Ballester-Gracia I, Pérez-Almarcha M, Galvez-Llompart A, et al. Use of long acting injectable aripiprazole before and through pregnancy in bipolar disorder: a case report. BMC Pharmacol Toxicol. 2019;20(1):52. PubMed CrossRef
10.Teodorescu A, Ifteni P, Moga MA, et al. Dilemma of treating schizophrenia during pregnancy: a case series and a review of literature. BMC Psychiatry. 2017;17(1):311. PubMed CrossRef
11.Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29,823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686-693. PubMed CrossRef
12.Misawa F, Kishimoto T, Hagi K, et al. Safety and tolerability of long-acting injectable versus oral antipsychotics: a meta-analysis of randomized controlled studies comparing the same antipsychotics. Schizophr Res. 2016;176(2-3):220-230. PubMed CrossRef
13.McDonnell DP, Detke HC, Bergstrom RF, et al. Post-injection delirium/sedation syndrome in patients with schizophrenia treated with olanzapine long-acting injection, II: investigations of mechanism. BMC Psychiatry. 2010;10(1):45. PubMed CrossRef
14.Taylor CL, Broadbent M, Khondoker M, et al. Predictors of severe relapse in pregnant women with psychotic or bipolar disorders. J Psychiatr Res. 2018;104:100-107. PubMed CrossRef
15.Tosato S, Albert U, Tomassi S, et al. A systematized review of atypical antipsychotics in pregnant women: balancing between risks of untreated illness and risks of drug-related adverse effects. J Clin Psychiatry. 2017;78(5):e477-e489. PubMed CrossRef
16.Jenkins A, Millar S, Robins J. Denial of pregnancy: a literature review and discussion of ethical and legal issues. J R Soc Med. 2011;104(7):286-291. PubMed CrossRef
17.Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24. PubMed CrossRef
18.Lפhteenvuo M, Tanskanen A, Taipale H, et al. Real-world effectiveness of pharmacologic treatments for the prevention of rehospitalization in a Finnish nationwide cohort of patients with bipolar disorder. JAMA Psychiatry. 2018;75(4):347-355. PubMed CrossRef
19.Ereshefsky L, Mascarenas CA. Comparison of the effects of different routes of antipsychotic administration on pharmacokinetics and pharmacodynamics. J Clin Psychiatry. 2003;64(suppl 16):18-23. PubMed
20.Westin AA, Brekke M, Molden E, et al. Treatment with antipsychotics in pregnancy: changes in drug disposition. Clin Pharmacol Ther. 2018;103(3):477-484. PubMed CrossRef
21.Freeman MP, Sosinsky AZ, Goez-Mogollon L, et al. Gestational weight gain and pre-pregnancy body mass index associated with second-generation antipsychotic drug use during pregnancy. Psychosomatics. 2018;59(2):125-134. PubMed CrossRef
22.Bryson CL, Ioannou GN, Rulyak SJ, et al. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol. 2003;158(12):1148-1153. PubMed CrossRef
23.Committee Opinion No. 666 Summary. Obstet Gynecol. 2016;127(6):1192-1193. PubMed
24.Jones I, Craddock N. Familiality of the puerperal trigger in bipolar disorder: results of a family study. Am J Psychiatry. 2001;158(6):913-917. PubMed CrossRef
25.Kronenfeld N, Berlin M, Shaniv D, et al. Use of psychotropic medications in breastfeeding women. Birth Defects Res. 2017;109(12):957-997. PubMed CrossRef
26.Marks JM, Spatz DL. Medications and lactation: what PNPs need to know. J Pediatr Health Care. 2003;17(6):311-317, quiz 318-319. PubMed
27.Hoffer ZS, Roth RL, Mathews M. Evidence for the partial dopamine-receptor agonist aripiprazole as a first-line treatment of psychosis in patients with iatrogenic or tumorogenic hyperprolactinemia. Psychosomatics. 2009;50(4):317-324. PubMed CrossRef
28.Nikolaev A, Blake CN, Carlson DL. Association between hyperprolactinemia and granulomatous mastitis. Breast J. 2016;22(2):224-231. PubMed CrossRef
aDepartment of Psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, New York
bDepartments of Psychiatry and Obstetrics & Gynecology, Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York
cFeinstein Institute for Medical Research, Manhasset, New York
*Corresponding author: Kristina M. Deligiannidis, MD, Department of Psychiatry, Division of Psychiatry Research, Zucker Hillside Hospital, Northwell Health, 75-59 263rd St, Glen Oaks, NY 11004 ([email protected]).
J Clin Psychiatry 2020;81(6):20ac13597
To cite: Reinstein SA, Cosgrove J, Malekshahi T, et al. Long-acting injectable antipsychotic use during pregnancy: a brief review and concise guide for clinicians. J Clin Psychiatry. 2020;81(6):20ac13597.
To share: https://doi.org/10.4088/JCP.20ac13597
© Copyright 2020 Physicians Postgraduate Press, Inc.
The ASCP Corner is a collection of brief peer-reviewed, evidence-based articles, authored by American Society of Clinical Psychopharmacology members, that examine the practice of psychopharmacology through the lens of clinical experience. The information contained herein only represents the opinion of the author(s).
See more ASCP Corner articles along with abstracts and updates from the last annual ASCP meeting at Psychiatrist.com/ASCP.
This PDF is free for all visitors!