Abstract
Only 3 medications are currently approved in the US for acute bipolar depression: 2 atypical antipsychotics and a combination atypical antipsychotic-selective serotonin reuptake inhibitor. Metabolic, neurologic, and hormonal adverse events are associated with all of the atypical antipsychotics approved for this indication. However, these agents differ in their propensity to cause weight gain or other side effects that significantly impact a patient’s physical health and ability to function, and the selection of medication—which may also include a mood stabilizer—as well as other forms of treatment, will affect the outcome. It is important to design treatment based on individual needs. Evidence suggests that the collaborative care model, which incorporates individualized systematic treatment, may be more appropriate for the management of bipolar depression than the acute care model.
J Clin Psychiatry 2015;76(3):e10
© Copyright 2015 Physicians Postgraduate Press, Inc.
From the Department of Psychiatry and the Depression Clinical and Research Program, Harvard Medical School and Massachusetts General Hospital, Boston (Dr Nierenberg); the Departments of Psychiatry and Pharmacology, University of Toronto, and the Mood Disorders Psychopharmacology Unit, University Health Network, Toronto, Ontario, Canada (Dr McIntyre); and the Department of Psychiatry, Harvard Medical School, and the Bipolar Clinic and Research Program, Massachusetts General Hospital, Boston (Dr Sachs).
Faculty
Andrew A. Nierenberg, MD, Chair
Medical Director, Bipolar Research Program, Clinical Psychopharmacology Unit, Massachusetts General Hospital, Boston, Massachusetts
Roger S. McIntyre, MD, FRCPC
Professor of Psychiatry and Pharmacology, University of Toronto and Head, Mood Disorders Psychopharmacology Unit, University Health Network, Toronto, Ontario, Canada
Gary S. Sachs, MD
Director, Bipolar Clinic, Massachusetts General Hospital, Partners Bipolar Treatment Center, Boston, Massachusetts
Faculty Disclosure
In the spirit of full disclosure, the faculty were asked to complete a statement regarding all relevant personal and financial relationships between themselves or their spouse/partner and any commercial interest. Faculty financial disclosures are as follows:
Dr Nierenberg has been a consultant for Abbott, American Psychiatric Association, Appliance Computing (Mindsite), Basliea, Brain Cells, Brandeis University, Bristol-Myers Squibb, Clintara, Corcept, Dainippon Sumitomo (now Sunovion), Dey, Eli Lilly, EpiQ LP/Mylan, Forest, Genaissance, Genentech, GlaxoSmithKline, Hoffman LaRoche, InfoMedic, Janssen, Jazz, Lundbeck, Medavante, Merck, Methylation Sciences, Naurex, Novartis, PamLabs, Pfizer, PGx Health, Ridge Diagnostics, Schering-Plough, Shire, Somerset, Sunovion, Takeda/Lundbeck, Targacept, and Teva; consulted through the MGH Clinical Trials Network and Institute (CTNI) for AstraZeneca, Brain Cells, Dainippon Sumitomo/Sepracor, Johnson & Johnson, Labopharm, Merck, Methylation Science, Novartis, PGx Health, Schering-Plough, Shire, Takeda/Lundbeck, and Targacept; has received grant/research support from AHRQ, American Foundation for Suicide Prevention, Brain and Behavior Research Foundation, Bristol-Myers Squibb, Cederroth, Cephalon, Cyberonics, Elan, Eli Lilly, Forest, GlaxoSmithKline, Janssen, Lichtwer, Marriott Foundation, Mylan, NIMH, PamLabs, PCORI, Pfizer, Shire, Stanley Foundation, Takeda, and Wyeth-Ayerst; has received honoraria from American Drug Utilization Review, American Society for Clinical Psychopharmacology, APSARD, Baystate Medical Center, Belvoir Publishing, Brandeis University, Bristol-Myers Squibb, Columbia University, CRICO, Dartmouth Medical School, Health New England, Harold Grinspoon Charitable Foundation, Hillside Hospital, Imedex, International Society for Bipolar Disorder, ISBD, Israel Society for Biological Psychiatry, Johns Hopkins University, MBL Publishing, Medscape, MGH Psychiatry Academy, MJ Consulting, National Association of Continuing Education, New York State, Physicians Postgraduate Press, SciMed, Slack Publishing, SUNY Buffalo, University of Miami, University of Michigan, University of Texas Southwestern Dallas, University of Pisa, University of Wisconsin, University of Wisconsin at Madison, and Wolters Klower Publishing; has stock ownership in Appliance Computing (MindSite), Brain Cells, and Medavante; and holds copyright to Clinical Positive Affect Scale and the MGH Structured Clinical Interview for the Montgomery-Asberg Depression Scale exclusively licensed to the MGH Clinical Trials Network and Institute (CTNI). Dr Nierenberg has not been on any speakers bureaus since 2003.
Dr McIntyre has been on advisory boards for Astra Zeneca, Bristol-Myers Squibb, Eli Lilly, France Foundation, GlaxoSmithKline, Janssen-Ortho, Lundbeck, Merck, Organon, Pfizer, and Shire; been on the speakers bureaus for Astra-Zeneca, Eli Lilly, Janssen-Ortho, Lundbeck, Merck, and Pfizer; and received research grants from Astra-Zeneca, Eli Lilly, Janssen-Ortho, Lundbeck, NARSAD, NIMH, Pfizer, Shire, and Stanley Medical Research Institute.
Dr Sachs has been consultant for AstraZeneca, Janssen, Lundbeck, Sunovion, and Takeda; has received grant/research support from NIMH; is on the speakers/advisory boards for Merck, Takeda, Sunovion and Otsuka; is a stock shareholder of Oracle, AthenaHealth, McKesson, Collaborative Care Initiative; and is an employee of Bracket and Massachusetts General Hospital.
Review Process
The faculty for this InfoPack discussed the content in a series of peer-review planning teleconferences, the chair and faculty reviewed the InfoPack for accuracy, and a member of the Journal Editorial Board who is without conflict of interest reviewed the InfoPack to determine whether the material is evidence-based and objective.
Acknowledgment
This InfoPack is based on a series of teleconferences by 3 experts on the treatment of Bipolar I disorder. These teleconferences were held in July and August 2014. This evidence-based, peer-reviewed InfoPack was prepared by Healthcare Global Village, Inc. Financial support for the preparation and dissemination of this InfoPack was provided by Sunovion Pharmaceuticals, Inc. The
authors acknowledge Nancy Groves, MS, Healthcare Global Village, Inc, for editorial assistance in development of the manuscripts. The opinions expressed herein are those of the authors and do not necessarily reflect the views of Healthcare Global Village, Inc; the publisher; the American Society of Clinical Psychopharmacology; or the commercial supporter.
Bipolar Depression Update
Andrew A. Nierenberg, MD
The very term bipolar implies a disorder that swings between 2 opposite points. The reality for many patients, though, is a mixed state, one of experiencing both manic and depressive symptoms simultaneously—with anxiety potentially added to the mix—rather than drastic changes in mood and behavior from one extreme to the other.1
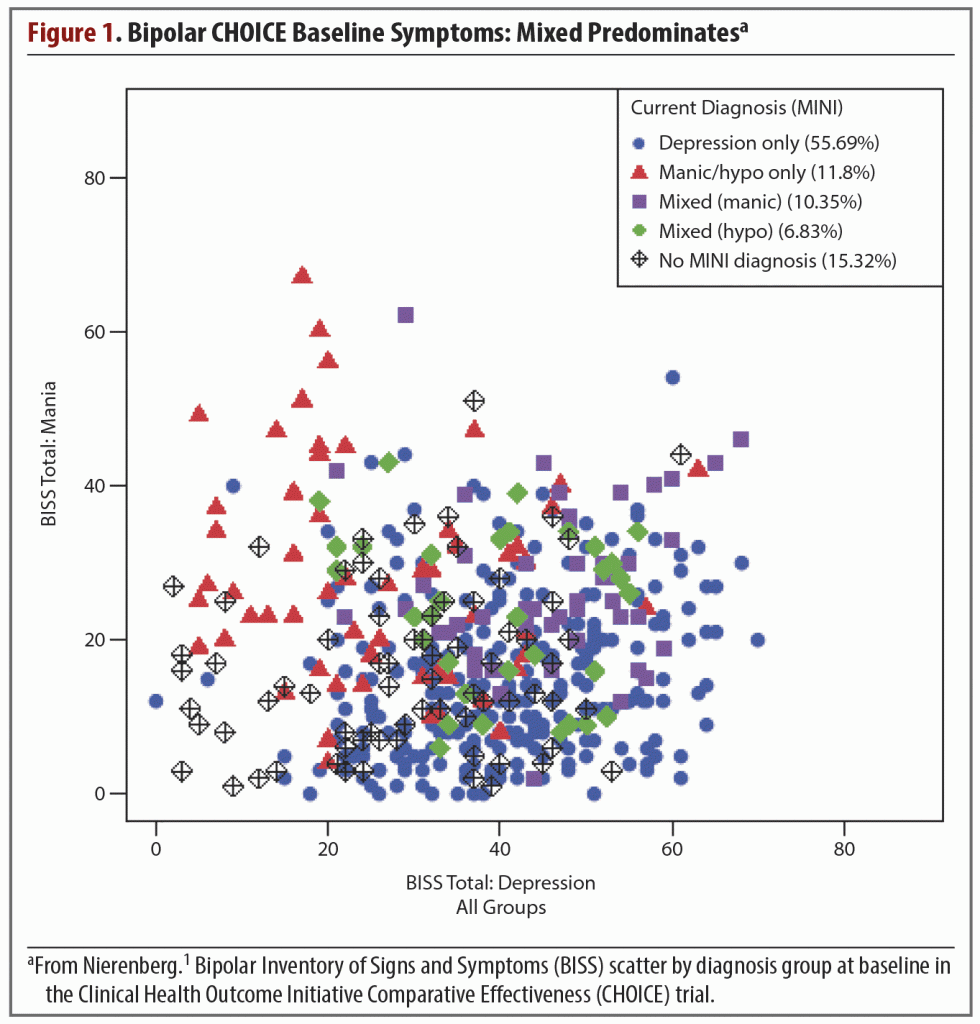
In some patients, the depressive symptoms are more disabling than the manic symptoms. However, mania and anxiety disorders such as posttraumatic stress disorder, social phobias, and attention-deficit/hyperactivity disorder are also common comorbidities, as is substance abuse.2 The coexistence of one or more of these illnesses will influence the course of the bipolar disorder or bipolar depression and complicate its management (Figure 1).
The duration and impact of depressive episodes are distinctive hallmarks leading to a diagnosis of bipolar depression. The chronicity of depressive episodes was evidenced in classic data from the Collaborative Depression Study,3 published in 2002 and including 146 patients with bipolar I disorder who had been followed 12.8 years. Depressive symptoms were present in 31.9% of total follow-up weeks versus 9.3% of weeks with pure mania/hypomania and 5.9% with cycling/mixed affective symptoms. The data also showed that subsyndromal and minor affective symptoms predominated in this group of patients.3
The same group of investigators also published a study of symptom status in patients with bipolar II disorder.4 Analysis of 86 patients with 13.4 years of follow-up showed that depressive symptoms were even more predominant than in bipolar I disorder. Patients experienced depressive symptoms approximately 39 times more than hypomanic symptoms (50.3% of all follow-up weeks vs 1.3%) and 22 times more frequently than cycling/mixed symptoms (2.3% of all follow-up weeks).4
FDA-APPROVED TREATMENTS FOR BIPOLAR DEPRESSION
Fewer treatment options are approved for treatment of acute bipolar depression than bipolar mania. While individual patients may be responsive or appear to be responsive to standard antidepressants, none of those agents approved for unipolar depression has received approval from the US Food and Drug Administration (FDA) for the treatment of bipolar disorder or bipolar depression. The International Society for Bipolar Disorders (ISBD) task force on antidepressant use in bipolar disorders concluded in a 2013 report that the evidence supporting efficacy and safety was weak.5 The task force also cited concerns over the risk for mood switch and recommended standard antidepressant use only as an adjunct to mood stabilizing medications in bipolar I disorder.5
Several medications initially approved as antipsychotics have gained FDA approval for the treatment of depressive episodes in bipolar disorder: quetiapine, olanzapine-fluoxetine, and lurasidone.
The antipsychotic-antidepressant combination olanzapine-fluoxetine received FDA approval for the treatment of depressive episodes of bipolar I disorder in 2003. Tohen et al6 reported in a 2004 study that both olanzapine monotherapy and the olanzapine-fluoxetine combination were statistically significantly superior to placebo (P < .001 for all). In comparison to the monotherapy group, however, the combination treatment group had statistically greater improvement at weeks 4 through 8 of the 8-week randomized controlled trial.6
Several aspects of the side effect profile of olanzapine-fluoxetine should be carefully considered in prescribing decisions. Clinical trial data show that clinically meaningful and sometimes very high elevations in triglyceride levels (> 500 mg/dL) as well as total cholesterol have been observed.7
Clinical trial evidence also raises concerns about the potential for significant weight gain during treatment with olanzapine in combination with fluoxetine as well as during olanzapine monotherapy treatment. In an analysis of 7 controlled short-term (median exposure to event of 6 weeks) clinical studies,7 22% of patients treated with the combination agent gained at least 7% of their baseline weight compared with 1.8% of the placebo-treated patients. The analysis also showed that approximately 3% of the patients in the active treatment group gained at least 15% of their baseline weight (median exposure to event of 8 weeks) versus 0% of those in the placebo group. Clinically significant weight gain was seen across all baseline body mass index (BMI) categories.7
Clinical trials of quetiapine have also demonstrated its efficacy in bipolar depression. In a study of over 500 patients diagnosed with bipolar disorder I or II,8 quetiapine 600 and 300 mg/d demonstrated significant improvement in Montgomery-Asberg Depression Rating Scale (MADRS)9 total scores compared with placebo. The analysis showed that 52.9% of patients in the quetiapine groups met MADRS remission criteria compared with 28.4% in the placebo group.8
However, both quetiapine and olanzapine-fluoxetine are associated with a substantial risk of sedation and somnolence. In an 8-week clinical trial of quetiapine XR 300 mg/d in patients with bipolar depression, the combined incidence of somnolence and sedation adverse reactions was 52% in the quetiapine group versus 13% in placebo.10 Somnolence/sedation was the most frequently cited adverse reaction for discontinuation (10.2% in the quetiapine XR group vs 0% in the placebo group).
In short-term controlled clinical studies in adults of olanzapine-fluoxetine, somnolence (somnolence, sedation, lethargy, and hypersomnia) was observed in 27% of patients receiving the combination agent versus 11% of the placebo group.7 These findings were recorded in studies including depressive episodes associated with bipolar I disorder and treatment-resistant depression.
NEW AGENT FOR BIPOLAR DEPRESSION
Lurasidone, approved by the FDA in 2013 as monotherapy and adjunctive therapy in adult patients with bipolar depression, is the latest medical treatment option to become available. It is a D2, 5-HT2A, and 5-HT7 receptor antagonist and 5-HT1A partial agonist. Its activity at 5-HT7 and other 5-HT receptors makes it an attractive candidate for the treatment of bipolar depression.11
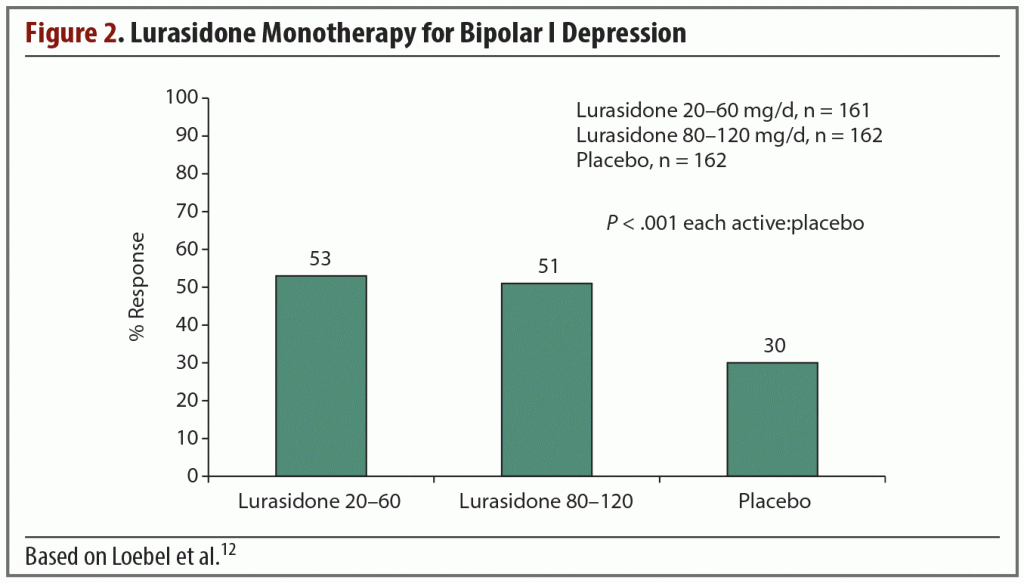
Efficacy and safety of lurasidone monotherapy were evaluated in a randomized, double-blind, placebo-controlled study by Loebel et al12; 166 patients were randomized to 20–60 mg/d, 169 patients to 80–120 mg/d, and 170 to placebo for 6 weeks. Treatment with both dosage ranges significantly reduced mean MADRS total scores at week 6 (−15.4; P < .001 [effect size = 0.51]) in both the lower and higher lurasidone dosage groups compared with −10.7 in placebo). Treatment with lurasidone also resulted in significantly greater endpoint reduction on the Clinical Global Impression Severity scale for use in bipolar illness (CGI-BP-S)13 compared with placebo. In the 20–60 mg/d group, the reduction was −1.8 versus −1.1 for placebo (P < .001 [effect size = 0.61]), while the reduction in the 80–120 mg/d group was −1.7 versus −1.1 in placebo (P < .001 [effect size = 0.50]).12
The most frequently reported adverse events for lurasidone were nausea, headache, akathisia, and somnolence. The incidence of somnolence was 4.3% in the lower dose group, 6.6% in the higher dose group, and 4.2% in placebo. The incidence of sedation, which is sometimes combined with somnolence in reports of adverse events, was reported separately; the incidence was 3.0% and 7.2% in the lower and higher lurasidone dosage groups, respectively, and 1.8% in the placebo group.12
The discontinuation rate due to adverse events was similar in all groups: 6.6%, lurasidone 20–60 mg/d; 5.9%, lurasidone 80–120 mg/d; and 6.5%, placebo.12 In this study, the mean change in weight was a gain of 0.56 kg for patients receiving the lower dosage range of lurasidone, +0.02 for those in the higher dosage range group, and −0.04 for the placebo group.14 There was no clinically meaningful change in fasting glucose or fasting lipids.12
The incidence of extrapyramidal events (EPS) was less than 10% in both lurasidone groups, although the increase appeared to be dose-related (4.9% in the lower dose group and 9.0% in the higher dose group). The incidence was 2.4% in the placebo group.12
A significantly greater percentage of subjects in the lurasidone groups met response criteria relative to the placebo group (Figure 2).12 This finding was both clinically and statistically significant. The comparable response rates from both lurasidone dosage groups suggest that a higher dose is not necessarily more effective in most patients, especially as the incidence of EPS was higher in the 80–120 mg/d treatment group.
Lurasidone is also approved as adjunctive therapy to lithium or valproate for bipolar depression. Its efficacy was evaluated in a double-blind, placebo-controlled study15 enrolling patients with bipolar I depression who were not responsive to at least 4 weeks of treatment with either lithium or valproate; following screening, 348 patients were randomly assigned to 6 weeks of treatment. Added to either lithium or valproate, flexibly-dosed lurasidone (20–120 mg/d) significantly reduced mean MADRS total score at week 6 compared with the placebo group (−17.1 vs −13.5; P = .005 [effect size = 0.34]). Treatment also resulted in significantly greater reduction in CGI-BP depression severity scores compared to placebo and significantly greater improvement in anxiety symptoms. The response rate for lurasidone adjunctive therapy was also highly statistically significant compared with placebo (57% vs 42%, P = .008). There was a minimal effect on weight, lipid parameters, and measures of glycemic control.15
Prior to this trial, no positive studies had shown that an atypical antipsychotic agent added to a mood stabilizer was effective for the treatment of bipolar depression.16 In 2013, Teva Pharmaceutical17 announced that a once promising compound in the pipeline, armodafinil, failed to meet the primary endpoint of demonstrating greater efficacy than placebo as adjunctive therapy to mood stabilizers and/or atypical antipsychotics in a series of Phase III studies. Armodafinil is approved as a treatment for improving wakefulness in adults who experience excessive sleepiness, but following the unsuccessful effort to prove its efficacy for the new indication of bipolar depression, Teva announced plans to discontinue the research.17
This leaves lurasidone as the only atypical antipsychotic specifically approved for adjunctive therapy for bipolar depression, although several agents are approved for maintenance treatment of bipolar I disorder in combination with lithium or valproate. Lurasidone is an option that may offer hope to patients whose symptoms have not been alleviated by monotherapy with mood stabilizers or who are judged to require combination treatment.
TREATMENT SELECTION
The management of bipolar depression is best guided by evidence of proven efficacy and tolerability in adequately controlled studies. There are now several options that meet this standard, but currently only 3 have FDA approval for treatment of bipolar I depression. As with all medications, safety and tolerability must be factored into the choice of treatment most appropriate for the individual patient. Weight gain, metabolic syndrome, and sedation are common side effects of particular concern.
The number needed to treat (NNT), number needed to harm (NNH), and likelihood of being helped or harmed (LHH) are additional measures that can be used to assess the relative benefits and harms of medications and translate research data into information useful in clinical practice. Citrome et al18 recently calculated the NNT, NNH, and LHH of lurasidone and also obtained data from studies of quetiapine immediate and extended release and olanzapine-fluoxetine combination to place lurasidone into clinical context in the treatment of bipolar I depression. Metrics for quetiapine and olanzapine-fluoxetine were derived from a series of published trials and product labeling.6,8,19–22 Using data from 2 prior studies of lurasidone,12,15 investigators found that it had single-digit NNT values versus placebo for response (5 for lurasidone monotherapy, both doses, and 7 for adjunctive therapy) and remission (6 for lurasidone monotherapy lower dose, and 7 for the higher dose; 7 for adjunctive lurasidone). These figures were comparable to those for quetiapine (6 for response, 6 for remission) and for olanzapine-fluoxetine (4 for response,
5 for remission).
The NNH values for lurasidone monotherapy and adjunctive therapy were also favorable. The NNH values for tolerability outcomes versus placebo were in the double or triple digits (14–130 for somnolence; 29–5,550 for ≥ 7% weight gain). In contrast, quetiapine’s NNH value for somnolence was 3, while the olanzapine-fluoxetine combination had an NNH value of 6 as calculated for ≥ 7% weight gain.
LHH, a tool for quantifying the benefit-risk ratio, was consistently more favorable for lurasidone versus the 2 earlier approved treatments for bipolar depression when used to analyze certain problematic side effects. The LHH for response versus somnolence was 2.8 for high-dose lurasidone, while the figure for quetiapine was 0.5. For response versus weight gain ≥ 7%, LHH values for lurasidone ranged from 5.8 to 1,110; the value for olanzapine-fluoxetine combination was ~1.18
The development of antipsychotic medications for the treatment of bipolar depression with dopamine D2 receptor blockers is intriguing, and the approved agents for the treatment of bipolar depression share some common pharmacologic mechanisms. However, not all of the atypical antipsychotics have demonstrated efficacy for this indication, and the difference may lie in other aspects of the receptor profile, perhaps including sedation. Data show that the incidence of sedation with lurasidone treatment is relatively low compared to quetiapine and olanzapine-fluoxetine,21 but there may be instances in which patients prefer some degree of sedation as a means of obtaining relief from their symptoms.23 Time and further studies will tell whether or to what extent the sedative component of dopamine blockers is advantageous.
Test Your Knowledge
Comorbidity and Pathophysiology of Bipolar Disorder
Roger S. McIntyre, MD, FRCPC
Antipsychotic agents, used alone or in combination with mood stabilizers, have helped control the psychotic symptoms of bipolar disorder and other mental health disorders in countless patients since they were introduced in the 1950s. Despite undeniable benefits, including the development of second-generation or atypical agents with a different side effect profile than their predecessors, antipsychotics are associated with a number of treatment-emergent adverse events and safety concerns. These must be balanced with the prospect of improvement in patients’ depressive symptoms.
The most commonly seen adverse events are those affecting the metabolic, neurologic, and hormonal systems. The metabolic concerns include weight gain (body weight, body mass, and body weight distribution), dyslipidemia, and glucose dysregulation, while somnolence and sedation, EPS, and tardive dyskinesia are the neurologic effects that most concern physicians. However, the risk of tardive dyskinesia appears to be higher with the first-generation agents, and sedation and somnolence are more often observed with agents having a strong antihistaminic effect.
The most often reported EPS events are acute dystonia and acute akathisia. Prolactin disorders are the primary effect of hormonal disruption due to antipsychotic medication. Prolactin elevation may manifest in forms such as loss of libido or galactorrhea.
Electrocardiographic changes triggered by use of antipsychotic medications are also a safety concern. QTc prolongation, associated with several antipsychotics as well as other drug categories, may cause the heart rhythm disorder torsades de pointes (TdP). If TdP persists, it may lead to ventricular fibrillation and sudden cardiac death.
Metabolic syndrome is generally considered the most significant of the adverse events due to both its impact and its prevalence. The prevalence of metabolic syndrome in the general population is an estimated 23.7% versus 20%–66% in the bipolar disorder population.24
As with other medications, the risk of any of the treatment-emergent adverse events varies with the antipsychotic prescribed for the treatment of bipolar disorder and the response of the individual patient; the severity of the event, should it develop, can also differ significantly. Metabolic disorders, for example, are a significant point of differentiation among antipsychotic agents. Among the drugs FDA-approved for treatment of bipolar disorder, aripiprazole, asenapine, lurasidone, and ziprasidone are least likely to cause clinically significant weight gain or changes in BMI and composition.
But neither metabolic syndrome nor other adverse effects of antipsychotic medication occur in a vacuum. To cite just one adverse event, the elevated risk of weight gain that is strongly associated with some of the agents has significant clinical implications for the likelihood that a patient will develop cardiovascular disease or type 2 diabetes or face premature mortality.
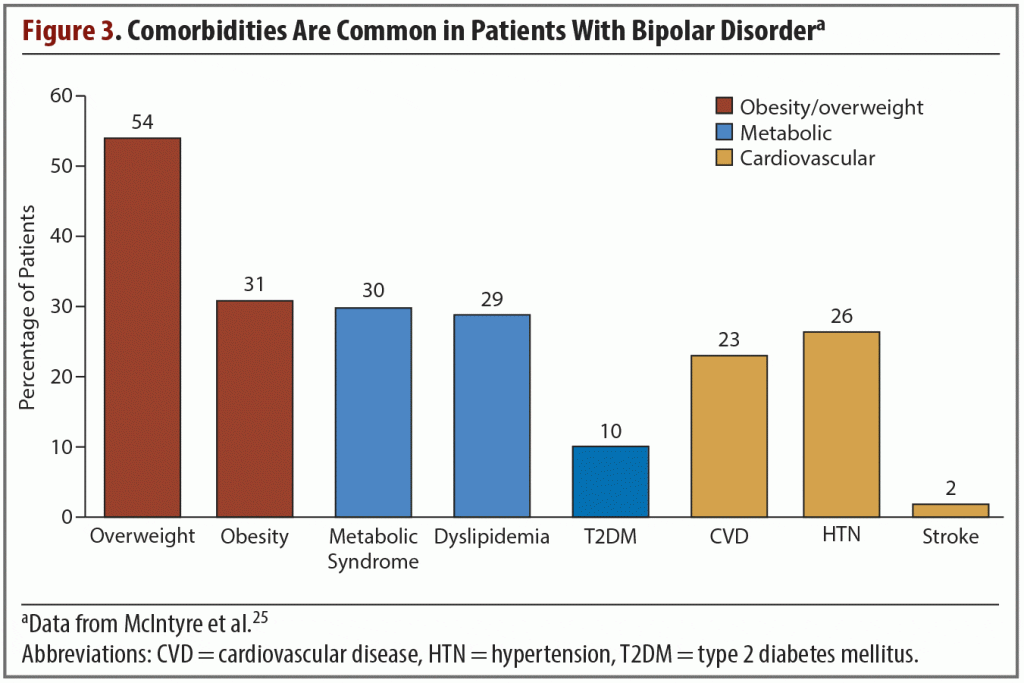
Evidence shows that these and other medical comorbidities are common in patients with bipolar disorder; one study, based on data collected worldwide, found a high prevalence of metabolic comorbidities. More than half of patients were overweight while a third were obese, and roughly one-quarter of them had been diagnosed with cardiovascular disease or hypertension (Figure 3).25
Medication used to treat bipolar disorder may contribute to the high rates of overweight and obesity, although the disease itself may also affect patient behavior, with poor diet and inadequate exercise eventually taking a toll.26
The consequences of comorbidities and risk factors associated with bipolar disorder cannot be underestimated. A recent Swedish cohort study27 found that all-cause mortality was increased 2.3-fold among women and 2.0-fold among men with bipolar disorder compared with the general population. Mortality was higher due to causes such as cardiovascular disease, diabetes, chronic obstructive pulmonary disease, influenza or pneumonia, unintentional injuries, and suicide in both men and women and stroke and cancer only in women.27
Even higher mortality rates have also been reported. In a 2013 study28 reviewing data from more than 40 psychopharmacology clinical trials, the mortality rate for patients with bipolar disorder was increased 3-fold compared with the general adult population. It was lower among patients assigned to atypical antipsychotic agents, mood stabilizers, or a combination of both than among individuals assigned to placebo.28
Clinicians face the challenge, then, of managing both the mood disorder and the possibility of multiple medical comorbidities that may have long-term or even life-threatening consequences.
OBESITY
Obesity is a particularly challenging comorbidity with the potential to contribute to a host of health-related issues in the bipolar population. One way to examine the clinical implications of obesity is through phenotypes. In these patients, the combination of bipolar disorder and obesity can be characterized by predominance of depressive symptoms, more severe symptoms (including risk of suicide), anxiety symptoms, and poor cognitive performance.
Looking more closely at the effect on cognitive function, one study29 showed that BMI was negatively correlated with attention and psychomotor processing speed as measured by the Digit Symbol Substitution Test in euthymic individuals with bipolar disorder. Further, overweight and obese patients with bipolar disorder had significantly lower scores on the Verbal Fluency Test when compared with normal-weight patients (P < .05). Except for measures of executive function and recollection memory, all other measures of cognitive function in this small study showed nonsignificant trends suggesting a negative association with BMI.29
Yet more evidence of the association between BMI and bipolar disorder comes from a study that found that overweight and obese patients with this condition had abnormal brain connectivity. Elevated BMI was associated with disruptions of brain white-matter integrity affecting the right parietal, temporal, and occipital regions of overweight patients compared with those maintaining a normal weight.30
Studies have also found that obesity is linked to both vascular dementia and Alzheimer’s dementia.31 Both of these conditions are overrepresented among individuals with bipolar disorder.32
In sum, obesity-related problems in the bipolar population have a direct adverse effect on the clinical presentation, the course, and the outcome of bipolar disorder. The substantial evidence supporting these findings underscores the relevance of selecting an antipsychotic agent with care.
METABOLIC SYNDROME
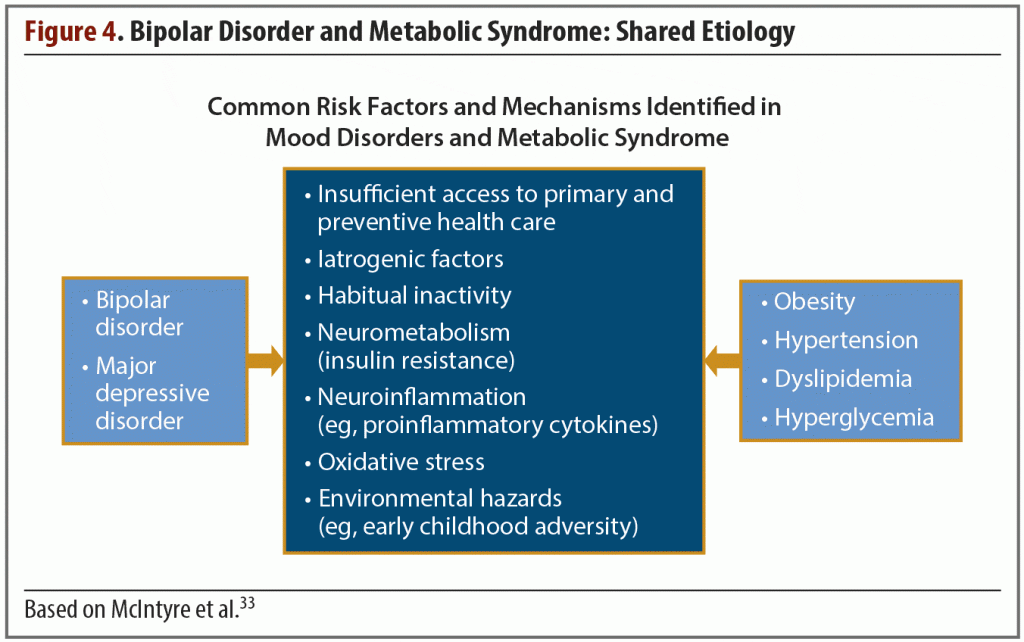
The widespread occurrence of metabolic disorders in patients with bipolar disease is well documented, but a simple yet comprehensive explanation of this association has yet to be established. What is known is that the 2 conditions have a shared etiology. Some are biological, such as neurometabolism (insulin resistance) or neuroinflammation (eg, proinflammatory cytokines), but other risk factors hint at the existence of a complex web of interconnected issues that may long predate the diagnosis of bipolar or other mood disorders.
For example, environmental stresses such as early childhood adversity or insufficient access to primary and preventive health care are 2 common components of bipolar disorder and metabolic syndrome.33
Viewed from yet another perspective, bipolar disorder can be considered a metabolic neuroendocrine disorder. Individuals diagnosed with this condition innately have endocrine disturbances such as abnormalities in the hypothalamic-pituitary-adrenal axis, increases in neuroinflammation, and oxidative stress (Figure 4).33
INFLAMMATION
Evidence is mounting that inflammation is highly relevant to bipolar disorder. Obesity, which we have already seen is strongly associated with bipolar disorder, is itself a proinflammatory state. Once obesity develops, regardless of the cause or causes, it produces inflammation, leading in turn to adverse effects on the brain neural substrates that are associated with bipolar disease and contribute as well to inflammatory comorbidities such as cardiovascular disease and diabetes.
One piece of evidence supporting a more extensive role for inflammation is a recent study suggesting that maternal influenza exposure may increase the risk for offspring to develop bipolar disorder with psychotic features. A 2014 study found that serologic evidence of influenza exposure during pregnancy was associated with a 5-fold higher risk of bipolar disorder, but only with psychotic features.34
The theory that inflammation is more than a consequence of bipolar disorder and, instead, etiologically relevant to the expression of bipolarity, is further bolstered by evidence that neuroinflammation is significantly increased in the hippocampus—a region relevant to memory—in patients with bipolar disorder relative to healthy controls.35
Additional findings from an evaluation of the tryptophan-kynurenine metabolism pathway showed that blood kynurenine concentrations and the kynurenine-to-tryptophan ratio were significantly higher in patients with bipolar disorder, and the increases were greater in a subsample of overweight patients. In addition, higher levels of neopterin concentrations were found in the overweight patients as well as in patients with later stage bipolar disorder.36 The abnormal levels of these biomarkers of inflammation in the individuals with bipolar disorder who were obese suggest that obesity creates a proinflammatory state that continually triggers adverse effects on the brain.
The combined impact of obesity and metabolic abnormalities in the bipolar population is, in effect, gravitationally pulling patients toward the depressive pole of this disorder, creating a more unstable illness.
ADVERSE EFFECTS OF ANTIPSYCHOTICS
A number of antipsychotic agents have gained FDA approval for the treatment of bipolar disorder. The efficacy findings from clinical trials are no guarantee, however, that every patient will be helped, and it is also understood that patients may be at increased risk of developing clinical toxicity after receiving certain medications. Some agents may, in fact, fail to prevent the return of a clinical syndrome phenotypically indistinguishable from depression or induce this syndrome.
Data from one small study37 showed that continued antipsychotic treatment following remission from an acute manic episode was associated with detrimental effects. In this study, 19 patients were randomized to receive placebo or continue treatment with perphenazine. Ongoing use of perphenazine was associated with a shorter time to depressive relapse, more depressive symptoms, higher rates of dysphoria and parkinsonism, and greater discontinuation rates.37 While this study was rigorous and well characterized, albeit small, and reinforces the need for more information on the effects of antipsychotics at all phases of treatment, the findings cannot be generalized to all agents. To date, evidence indicates that the FDA-approved treatments for bipolar depression—lurasidone, quetiapine, and olanzapine-fluoxetine—do not induce depression. However, these agents may have other adverse effects.
ADHERENCE
Given the comparatively high rates of sedation, somnolence, weight gain, and other adverse events observed with certain antipsychotics, it is not surprising that adherence is a challenge for many patients with bipolar disorder. In a large, prospective observational study of bipolar disorder,38 almost two-thirds of patients were prescribed combination therapy at their baseline visit; treatment changed for more than half of the patients during the trial’s 12-week acute phase. They either received additional antimanic medications or stopped or changed their initial medication.38
The literature also reveals that the reasons for discontinuation are often modifiable. A study in patients with schizophrenia and related disorders found that 53% of patients stopped treatment at an early stage; importantly, poor psychiatric response, paired with worsening symptoms, was the primary reason behind this decision.39 Poor response was cited by 36% of patients, while discontinuation due to poor tolerability was mentioned by just 12%.
TREATMENT DECISIONS
The FDA has approved 11 oral medications for the treatment of bipolar disorder as well as 3 antipsychotics specifically indicated for bipolar depression. Physicians have the opportunity and the obligation to choose wisely, to prescribe a medication that seems most likely to be effective for the individual patient’s symptoms as well as one with a low risk of significant sedation, somnolence, weight gain, or other side effects.
TEST YOUR KNOWLEDGE
A Pragmatic Approach: Managing Treatment for Bipolar Depression
Gary S. Sachs, MD
Managing a patient’s bipolar disorder is ultimately a matter of improving quality of life, and few things can blunt well-being more than recurrence. Often, a patient’s depressive episodes are both more frequent and more detrimental than the manic episodes. Controlling these depressive episodes through medication and psychotherapy with the goal of preventing recurrence is a pathway toward improving patients’ quality of life.
This improvement is easier said than done. One of the primary roadblocks is the prevailing acute care model of medical practice, which is a poor fit for bipolar disorder and other chronic conditions that can be managed but not “cured.” A disease such as bipolar disorder or bipolar depression requires an individualized care plan, something that may be difficult to prepare and implement in the current health care environment.
Bipolar disorder is considered one of the most difficult chronic conditions to treat and particularly ill suited to the current care model. Major obstacles to stability in these patients include:
- Chaos in the patient’s life
- Comorbidity, especially substance abuse
- Failure to accept the diagnosis
- Medication and treatment nonadherence
- Management of pregnancy, planned or unplanned40
Another barrier to managing patients with bipolar depression in a manner that effectively reduces their risk of recurrence is the difficulty of adapting best practice guidelines or the evidence base to the individual patient. Patients want the benefit of their physician’s knowledge and experience incorporated into their personal care plan, but when an attempt to apply population-based results to an individual case fails, the therapeutic alliance often suffers.
Lack of time, a perennial issue in all medical specialties, is also an obstacle to practicing personalized medicine for the treatment of patients with mood disorders. The practice environment works against the goal of personalized care since time constraints that may limit a doctor-patient encounter to 10 or 15 minutes are far from optimal for establishing rapport, conducting a formal assessment, maintaining clinically useful records of prior treatment, or providing meaningful patient education.
THE COLLABORATIVE CARE MODEL
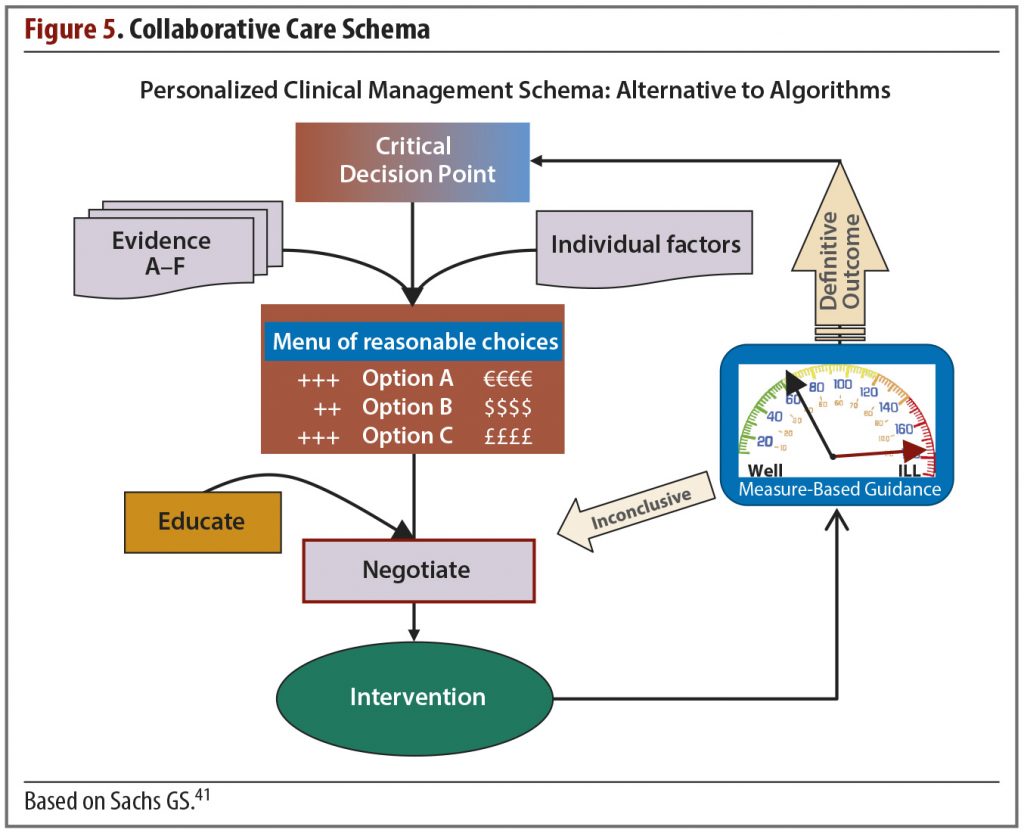
Still, for those willing to invest the time and effort, it may be possible to improve the management of bipolar depression through a model that helps patients, their caregivers, and the physician make decisions wisely and amicably. This is the collaborative care model (Figure 5).
The guiding principles of individualized systemic treatment in this model are the following:
- Aim for concordance
- Make a formal diagnosis
- Formulate a menu of reasonable choices appropriate for the current decision point
- Integrate measurement and management
- Utilize the collaborative chronic care model as much as possible
After establishing with the patient that the goal is agreement on a course of action, not a unilateral decision by the doctor, the next step is the diagnosis. The diagnosis includes both an assessment of the patient’s current clinical status and a lifetime assessment. For a patient with bipolar disorder, this means identifying the individual’s “highest high” as well as accounting for confounders.
The clinician then presents a menu of reasonable options; the choices are based on the factors relevant to the particular patient as well as the evidence base. Review of the available fund of knowledge from these 2 domains usually identifies several viable choices applicable to the current clinical decision point. As a rule, options that are proven treatments and those with lower risk should be offered first. Following an explanation of the risks and benefits of each and a period of discussion and negotiation, the patient can select a treatment based on his or her preferences.
In the collaborative care model, measurement and management are integrated in the intervention and used to help decide which options are presented to the patient and how the physician will explain the choices. This is done in part by incorporating key findings from the literature, which offers the best guidance for clinical management.
The quality of evidence can be ranked, with the highest ranking given to positive results from double-blind, placebo-controlled trials with an adequate sample size. In descending order, the other categories are double-blind comparison studies with an adequate sample; open comparison trials with an adequate sample; uncontrolled observational or controlled studies with ambiguous results; no published evidence; and available evidence with negative results.
Treatments that have category A evidence—double-blind comparison studies with an adequate sample size—should carry the most weight. When treatment options for acute bipolar depression that have a least one positive trial are stacked against those with only negative or failed trials, the framework for making the choice becomes clearer. Important patterns in the evidence base can be appreciated by considering the evidence across category A studies. For example, maintenance studies consistently demonstrate that early discontinuation of a treatment that has been effective for bipolar depression or mania acutely is associated with a high relapse rate.
Because the goal of the physician-patient collaboration is choosing a wise intervention amicably, numerous individual items should also be considered when formulating the reasonable choices. Important issues include the nature of the index episode, residual symptoms, patient gender, comorbid psychiatric illnesses, biomarkers, prior treatment response—both acute and prophylaxis—and adverse effect tolerance. Careful consideration is also given to the course of the patient’s illness—what is the predominant polarity, the number and frequency of prior episodes, is there a history of rapid cycling?
These merit serious review when patients have co-occurring general medical disorders or the treatments under consideration have a high risk of leading to or aggravating such disorders. Examples of these disorders and risk factors include cardiac conditions, which are associated with obesity and hypertension; endocrine disorders, contributing to thyroid problems or diabetes; and hemopoietic and immune function.
The therapeutic priority should also be incorporated in the doctor-patient discussion. Is an urgent care strategy required, or is it acceptable to follow a sequential care strategy?
A JANUS APPROACH
Another strategy to use when collaborating with the patient on treatment selection can be referred to as a Janus approach (named for the Roman deity known as the god of beginnings and transitions). The Janus approach to care recognizes that the doctor-patient collaboration is initially formed on the basis of looking backward at reports of the patient’s response to past treatments and the pertinent treatment research literature. Once the collaborators select an intervention, however, a shift is made to guide decisions based on the patient’s prospectively measured response to each new therapeutic regimen. This Janus approach is an example of incorporating measurement into the collaborative care model.
When evaluating the response to prior treatments, it is helpful to construct a table with these column headings:
- Medication prescribed
- Adequacy of dose and duration
- Efficacy (eg, no benefit, moderate antimanic benefit, much worse, slightly helpful)
- Tolerability (eg, no adverse effects, mild nausea, weight gain)
- Overall evaluation (eg, indeterminate, effective for depression, intolerable, ineffective, antimanic benefit)
This past information will help guide the next round of decisions; if a patient responded well to a certain treatment, it should at the least be one of the options and perhaps the preferred choice.
Whichever treatment is chosen, applying the Janus approach requires monitoring the patient’s response prospectively. If the chosen therapy seems to be effective with an acceptable adverse events profile, then it would be reasonable to continue.
Another pattern evident from analysis of the category A studies relates to the predictive value of response over the first 2 weeks of treatment. Substantial evidence points to the importance of the early absence of improvement. Based on a review of blinded data from 10 randomized controlled bipolar depression treatment trials, Kemp et al42 found that early improvement (improvement from baseline to day 14 was ≥ 20% reduction in depressive severity score) was a modest predictor of eventual response or remission (positive predictive value about 50%–60%), but the absence of early improvement was a more robust predictor of nonresponse (negative predictive value 80%–90%) at week 6–8.42
Predictive evidence such as this provides useful guidance for patients and clinicians based on early outcome. The data provide moderate encouragement to continue treatments that seem to be effective and strong indication to increase dosage or change treatment when early outcome indicates the treatment is ineffective or intolerable.
THE ADJACENT POSSIBLE
If quantitative measures are to play a central role in collaborative care, the question of how and when an already time-squeezed physician will obtain this information must be addressed. The concept of the “adjacent possible” may offer a solution. This concept encourages clinicians to consider what may become possible when a new product or new technology becomes available—for instance in medicine, the range of services that can be extended to patients in so-called “medical homes” though technology that allows the collection routine of vital signs and remote ECG monitoring in the home.
Health and fitness-related web-based tracking and mobile apps are proliferating and could help psychiatrists and other physicians monitor their patients’ progress and facilitate wise decision making. The value of apps is greatest, though, when the information they deliver has a clear relationship to the scientific evidence relevant to decision making.
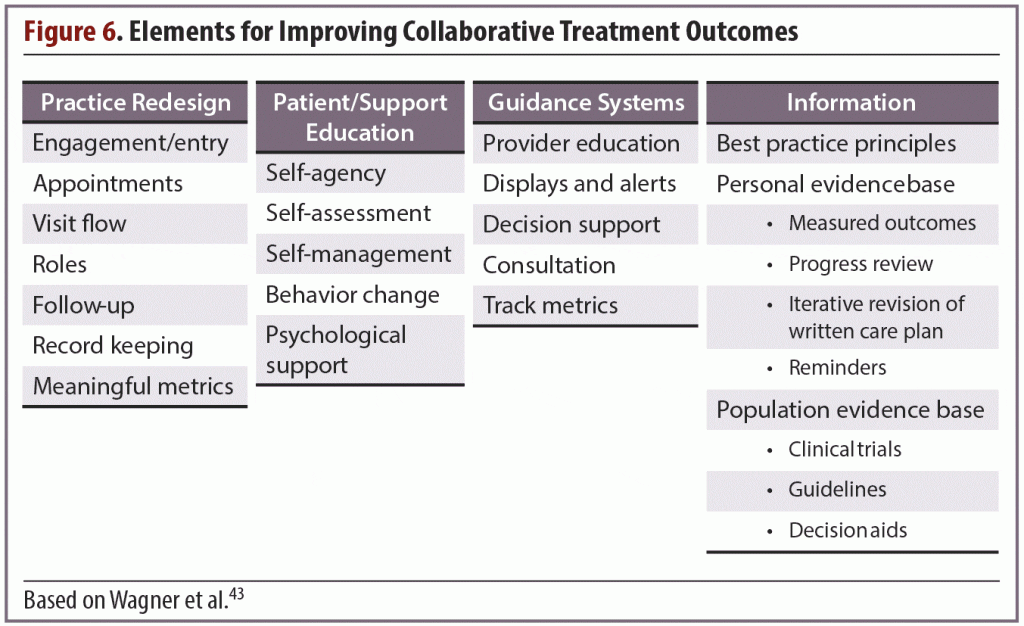
Technology has enabled many methods of gathering information, such as systems that allow patients to log into sites that collect information and send it to their physician’s office. Accumulated data can be reviewed and manipulated in various ways to measure different aspects of the patient’s progress, such as scores on rating scales and medication adherence (Figure 6). When this information is easily available as a “preassessment,” more time becomes available during the office visit for clinicians to use their highest skill set to assess the patient, interpret and discuss the findings, and make any necessary modifications to the treatment plan.
But this does not necessarily require high technology. How the information is compiled and delivered is less important than having these tasks completed outside of the office visit. A simple paper assessment completed in the waiting room before the visit can be quite serviceable.
SHARED DECISION MAKING
Shared decision making is one of the components of the collaborative care model. It has multiple steps, although not all of them need be taken at one time, nor do they necessarily need to be conducted in the presence of both the clinician and the patient. The steps are the following:
- Recognize that a decision needs to be made
- Identify collaborators as equals in the process
- State options as a “menu of reasonable choices”
- Explore understanding and expectations
- Identify preferences
- Negotiate options and aim for concordance (agreement between patient and clinician)
- Share the decision
- Arrange follow-up to evaluate outcomes
The shared decision-making process can have many benefits, but it can also have disadvantages. On the positive side, the collaborative process facilitates practitioners’ communication of relevant information on illnesses and intervention.44 Shared decision making may also promote consumer engagement in and responsibility for his or her care45 and empower individuals as active agents in their own care.44
The arguments against shared decision making include the proposition that too many choices can be overwhelming to the patient and may result in a sense of lost opportunities.46 It has also been suggested that anticipation of choice and control may lead to disappointment if high expectations run counter to clinical realities.47 Another point against shared decision making is the risk that those consumers who look to professionals, such as physicians, to tell them what to do may become frustrated when asked to exercise their preference in choosing a course of treatment.45
Patients respond differently to the idea of shared decision making and collaborative care, with some more receptive than others. Since the basis of collaborative care is personalized treatment, clinicians should modify their approach for patients who are less comfortable taking an assertive role in treatment decisions.
FINAL CARE MODEL CONSIDERATIONS
Bipolar disorder affects about 2.6% of the adult population in the United States (12-month prevalence), and 82.9% of these cases are classified as severe.48 The resulting impairment in psychosocial functioning and the economic burden of this disease affect individuals, families, and society in innumerable ways that may be obvious or unsuspected. Clinicians who treat patients with bipolar disorder and bipolar depression can recommend various medications and forms of psychotherapy, but a different way of managing the doctor-patient relationship may also be beneficial.
A collaborative chronic care model for iterative personalized medicine is more appropriate for individuals with bipolar depression than the acute care model. The fundamentals of the collaborative care schema are making wise, amicable decisions and treatment selection favoring agents with proven efficacy and patient preference. The care plan formulated in consultation with the patient is initially based on retrospective patient reports and population-based data but shifts to prospective outcomes once the intervention has begun. Going forward, the treatment plan is revised according to each individual patient’s prospectively reported efficacy and tolerability outcomes.
Although more research is needed, evidence suggests that the chronic collaborative care model is effective for mental health conditions. A 2012 meta-analysis49 of randomized controlled trials comparing this model with other forms of care demonstrated that the collaborative care model could improve mental and physical outcomes for individuals with mental disorders across a wide variety of care settings and was effective with no net increase in health care costs. The potential benefits make this model well worth pursuing.
TEST YOUR KNOWLEDGE
Drug names: aripiprazole (Abilify), armodafinil (Nuvigil and others), asenapine (Saphris), fluoxetine (Prozac and others), lurasidone (Latuda), olanzapine (Zyprexa and others), olanzapine and fluoxetine (Symbyax), quetiapine (Seroquel and others), ziprasidone (Geodon and others).
REFERENCES
1. Nierenberg AA. The spectrum of mixed states: a bipolar CHOICE study. Presented at the International Conference on Bipolar Disorders; June 14, 2013; Miami Beach, FL.
2. Krishnan KR. Psychiatric and medical comorbidities of bipolar disorder.PsychosomMed. 2005;67(1):1–8. doi:10.1097/01.psy.0000151489.36347.18 PubMed
3. Judd LL,AkiskalHS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530–537. doi:10.1001/archpsyc.59.6.530 PubMed
4. Judd LL,AkiskalHS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261–269. doi:10.1001/archpsyc.60.3.261 PubMed
5. PacchiarottiI, Bond DJ,Baldessarini RJ, et al. The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry. 2013;170(11):1249–1262. doi:10.1176/appi.ajp.2013.13020185 PubMed
6. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression.Arch Gen Psychiatry. 2003;60(11):1079–1088.doi:10.1001/archpsyc.60.11.1079 PubMed
7. Symbyax[package insert]. Indianapolis, IN: Eli Lilly and Company; 2013.http://pi.lilly.com/us/symbyax-pi.pdf. Updated January 7, 2015. Accessed October 21, 2014.
8. Calabrese JR, Keck PE Jr, MacfaddenW, et al. A randomized, double-blind, placebo-controlledtrial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162(7):1351–1360. doi:10.1176/appi.ajp.162.7.1351 PubMed
9. Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change.Br J Psychiatry. 1979;134(4):382–389.doi:10.1192/bjp.134.4.382 PubMed
10. Astra Zeneca. Seroquel: safety in bipolar disorhttp://www.seroquelxrtouchpoints.com/bipolar-disorder/seroquel-xr-side-effects. Updated June 2014. Accessed October 22, 2014.
11. Ishibashi T, Horisawa T, Tokuda K, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity.J Pharmacol Exp Ther. 2010;334(1):171–181.doi:10.1124/jpet.110.167346 PubMed
12. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study.Am J Psychiatry. 2014;171(2):160–168.doi:10.1176/appi.ajp.2013.13070984 PubMed
13. Spearing MK, Post RM, Leverich GS, et al. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP.Psychiatry Res. 1997;73(3):159–171.doi:10.1016/S0165-1781(97)00123-6 PubMed
14. Latuda [package insert]. Marlborough, MA: Sunovian; 2013.http://www.latuda.com/LatudaPrescribingInformation.pdf. Updated July 2013. Accessed Oct. 18, 2014.
15. Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study.Am J Psychiatry. 2014;171(2):169–177.doi:10.1176/appi.ajp.2013.13070985 PubMed
16. Sachs GS, Ice KS, Chappell PB, et al. Efficacy andsafety of adjunctive oral ziprasidone for acute treatment of depression in patients with bipolar I disorder: a randomized, double-blind, placebo-controlled trial.J Clin Psychiatry. 2011;72(10):1413–1422. doi:10.4088/JCP.09m05934 PubMed
17. Teva Pharmaceutical Industries Ltd. Investor Relations. News release: Teva reports top-line results from final phase III study of armodafinil (Nuvigil) in patients with major depression associated with bipolar I disorder, August 30, 2013.http://ir.tevapharm.com/phoenix.zhtml?c=73925&p=irol-newsarticle&id=1851019. Accessed January 1, 2015.
18. Citrome L, Ketter TA, Cucchiaro J, et al. Clinical assessment of lurasidone benefit and risk in the treatment of bipolar I depression using number needed to treat, numberneeded to harm, and likelihood to be helped or harmed.J Affect Disord. 2014;155:20–27. doi:10.1016/j.jad.2013.10.040 PubMed
19. Thase ME, Macfadden W, Weisler RH, et al; BOLDER II Study Group. Efficacy of quetiapine monotherapy in bipolar I and IIdepression: a double-blind, placebo-controlled study (the BOLDER II study).J Clin Psychopharmacol. 2006;26(6):600–609. doi:10.1097/01.jcp.0000248603.76231.b7 PubMed
20. Suppes T, Datto C, Minkwitz M, et al. Effectiveness of the extended release formulation of quetiapine as monotherapy for the treatment of acute bipolar depJ Affect Disord. 2010;121(1-2):106–115.
21. Seroquel (quetiapine fumarate) [package insert]. Wilmington, DE: AstraZeneca; 2011.astrazeneca-us.com/pi/Seroquel.pdf. Updated December 2011. Accessed December 30, 2014.
22. Seroquel XR (quetiapine fumarate extended release) [package insert]. Wilmington, DE: AstraZeneca; 2011.astrazeneca-us.com/pi/Seroquelxr.pdf. Updated December 2011. Accessed December 30, 2014.
23. Belmaker RH. Lurasidone and bipolar disorder.
Am J Psychiatry. 2014;171(2):131–133. doi:10.1176/appi.ajp.2013.13091240 PubMed
24. McIntyre RS, Danilewitz M, Liauw SS, et Bipolar disorder and metabolic syndrome: an international perspective.J Affect Disord. 2010;126(3):366–387. doi:10.1016/j.jad.2010.04.012 PubMed
25. McIntyre RS, Soczynska JK, Beyer JL, et al. Medical comorbidity in bipolar disorder: re-prioritizing unmet needs.Curr Opin Psychiatry. 2007;20(4):406–416.doi:10.1097/YCO.0b013e3281938102 PubMed
26. Weiner M, Warren L, Fiedorowicz JG. Cardiovascular morbidity and mortality in bipolar disorder.Ann Clin Psychiatry. 2011;23(1):40–47.PubMed
27. Crump C, Sundquist K, Winkleby MA, et al. Comorbidities and mortality in bipolar disorder:a Swedish national cohort study.JAMA Psychiatry. 2013;70(9):931–939. doi:10.1001/jamapsychiatry.2013.1394 PubMed
28. Khan A, Faucett J, Morrison S, et al. Comparative mortality risk in adult patients with schizophrenia, depression, bipolar disorder, anxiety disorders, and attention-deficit/hyperactivity disorder participating in psychopharmacology clinical trials.JAMA Psychiatry. 2013;70(10):1091–1099.doi:10.1001/jamapsychiatry.2013.149 PubMed
29. Yim CY, Soczynska JK, Kennedy SH, et al. The effect of overweight/obesity on cognitive function in euthymic individuals with bipolar disorder.Eur Psychiatry. 2012;27(3):223–228.doi:10.1016/j.eurpsy.2011.02.004 PubMed
30. Kuswanto CN, Sum MY, Yang GL, et al. Increased body mass index makes an impact on brain white-matter integrity in adults with remitted first-episode mania.Psychol Med. 2014;44(3):533–541.doi:10.1017/S0033291713000858 PubMed
31. Kivipelto M, Ngandu T, Fratiglioni L, et Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease.Arch Neurol. 2005;62(10):1556–1560. doi:10.1001/archneur.62.10.1556 PubMed
32. Kessing LV, Nilsson FM. Increased risk of developing dementia in patients with major affective disorders compared to patients with other medical illnesses.J Affect Disord. 2003;73(3):261–269.doi:10.1016/S0165-0327(02)00004-6 PubMed
33. McIntyre RS, Alsuwaidan M, Goldstein BI, et al; Canadian Network for Mood and Anxiety Treatments (CANMAT) Task Force. The Canadian Network for Mood and Anxiety Treatments (CANMAT) task force recommendations for the management of patients with mooddisorders and comorbid metabolic disorders.Ann Clin Psychiatry. 2012;24(1):69–81. PubMed
34. Canetta SE, Bao Y, Co MDT, et al. Serological documentation of maternal influenza exposure and bipolar disorder in adult offspring.Am J Psychiatry. 2014;171(5):557–563.doi:10.1176/appi.ajp.2013.13070943 PubMed
35. Haarman BC, Riemersma-Van der Lek RF, de Groot JC, et al. Neuroinflammation in bipolar disorder: a [(11)C]-(R)-PK11195 positron emission tomography study.Brain Behav Immun. 2014;40:219–225.doi:10.1016/j.bbi.2014.03.016 PubMed
36. Reininghaus EZ, McIntyre RS, Reininghaus B, et al. Tryptophan breakdown is increased in euthymic overweight individuals with bipolar disorder:a preliminary report. Bipolar Disord. 2014;16(4):432–440. doi:1111/bdi.12166 PubMed
37. Zarate CA Jr, Tohen M. Double-blind comparison of the continued use of antipsychotic treatment versus its discontinuation in remitted manic patients.Am J Psychiatry. 2004;161(1):169–171.doi:10.1176/appi.ajp.161.1.169 PubMed
38. Reed C, Novick D, Gonzalez-Pinto A, et al. Observational study designs for bipolar disorder: what can they tell us about treatment in acute mania?ProgNeuropsychopharmacol Biol Psychiatry. 2009;33(4):715–721. doi:10.1016/j.pnpbp.2009.03.024 PubMed
39. Liu-Seifert H, Adams DH, Kinon BJ. Discontinuation of treatment of schizophrenic patients is driven by poor symptom response: a pooled post-hoc analysis of four atypical antipsychotic drugs.BMC Med. 2005;3(1):21.doi:10.1186/1741-7015-3-21 PubMed
40. Bishop S, Gaughran F, Scott J.The Prevalence and Needs of Individuals With Bipolar Disorders Using Mental Health Services in Lewisham, South London, and Maudsley. London, UK: National Health Service Trust; 2008.
41. Sachs GS. Strategies for improving treatment of bipolar disorder: integration of measurement and maActa Psychiatr Scand suppl. 2004;110(422):7–17.doi:10.1111/j.1600-0447.2004.00409.x PubMed
42. Kemp DE, Ganocy SJ, Brecher M, et al. Clinical value of early partial symptomatic improvement in the prediction of response and remission during short-term treatment trials in 3369 subjects with bipolar I or II depression.J Affect Disord. 2011;130(1–2):171–179.doi:10.1016/j.jad.2010.10.026 PubMed
43. Wagner EH, Austin BT, Von Korff M. Improving outcomes in chronic illness.Manag Care Q. 1996;4(2):12–25.PubMed
44. Charles C, DeMaio Lay participation in health care decision making: a conceptual framework.J Health Polit Policy Law. 1993;18(4):881–904. doi:10.1215/03616878-18-4-881 PubMed
45. Schauer C, Everett A, del Vecchio P, et al. Promoting the value and practice of shared decision-making in mental health care.Psychiatr Rehabil J. 2007;31(1):54–61.doi:10.2975/31.1.2007.54.61 PubMed
46. Kahneman D, Tversky A. Prospect theory: an analysis of decision under risk.Econometrica. 1979;47(2):263–292.doi:10.2307/1914185
47. Adams JR, Drake RE. Shared decision-making and evidence-based practice.Community Ment Health J. 2006;42(1):87–105.doi:10.1007/s10597-005-9005-8 PubMed
48. Kessler RC, Chiu WT, Demler O, et al.Prevalence, severity, and comorbidity of 12-monthDSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. doi:10.1001/archpsyc.62.6.617 PubMed
49. Woltmann E, Grogan-Kaylor A, Perron B, et al. Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis.Am J Psychiatry. 2012;169(8):790–804. doi:10.1176/appi.ajp.2012.11111616 PubMed
This PDF is free for all visitors!