Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
Some studies suggest that the use of selective serotonin reuptake inhibitors (SSRIs) during pregnancy increases the risk of preterm delivery. If so, to what extent is this a matter of clinical concern, and how should the subject be discussed with patients?
Some, but not all, studies suggest that the use of SSRIs during pregnancy increases the risk of preterm delivery; in the positive studies, the risk appears to be approximately doubled, on average, in SSRI-exposed relative to unexposed women.

- Some, but not all, studies suggest that selective serotonin reuptake inhibitor (SSRI) use during late pregnancy increases the risk of preterm delivery. Even if this is so, we cannot yet say whether the risk is related to SSRI treatment or to the depression (and its correlates) for which the SSRIs were prescribed.
- In studies that found significant differences, the duration of gestation was shorter by 2-6 days in women who received SSRIs during pregnancy relative to women who did not.
- A recent large randomized controlled trial found no differences in maternal and neonatal outcomes between deliveries conducted during week 38 and week 39 of gestation. This implies that even if SSRIs do anticipate delivery by a few days, it may not matter much.
Clinical Question
Some studies suggest that the use of selective serotonin reuptake inhibitors (SSRIs) during pregnancy increases the risk of preterm delivery. If so, to what extent is this a matter of clinical concern, and how should the subject be discussed with patients?
SSRIs and Preterm Delivery
Some,1-10 but not all,11-17 studies suggest that the use of SSRIs during pregnancy increases the risk of preterm delivery; in the positive studies, the risk appears to be approximately doubled, on average, in SSRI-exposed relative to unexposed women. An important limitation of these studies is that all were observational in design. In such studies, there is no way of knowing whether the adverse outcome is due to the drug that was used or to the indication for which the drug was prescribed; in other words, maternal depression and the severity thereof (and behaviors and experiences related thereto), rather than SSRI use, could have explained the increased risk of preterm delivery in SSRI-treated pregnant women.
In one study6 that compared SSRI-treated women with untreated depressed controls, there was no increase in risk. In the only study to date that described propensity-matching as a method to control for the presence and severity of maternal depression, Oberlander et al18 found that both the duration of gestation and the risk of preterm delivery did not differ significantly between depressed women who had used SSRIs during pregnancy and those who had not.
Most studies calculated odds ratios (ORs) to estimate the risk of preterm birth associated with SSRI use. This approach is useful for 2 reasons: preterm birth is an important categorical outcome, and calculating ORs allows for the adjustment for potential confounding variables such as maternal age, socioeconomic status, physical illness, smoking, and alcohol use. In the case of an actual patient, however, what could be more important is an estimate of the absolute degree of earlier birth, expressed in days, regardless of correction for confounds. This article will therefore focus on the implications of absolute estimates.
Absolute Estimate of Difference in the Duration of Gestation
Few papers that found an increased risk of SSRI-associated preterm delivery presented absolute estimates. When these data were available and the difference was statistically significant, the degree of earlier birth associated with SSRI exposure (relative to SSRI-unexposed women, regardless of presence of depression) was in the region of 2-6 days,3,5,7,8,16,18 with most studies finding smaller rather than greater differences. In a systematic review and meta-analysis of 15 studies, Ross et al19 found that gestational age was 3.2 (95% CI, 1.8-4.5) days shorter in women exposed to antidepressants relative to women in control groups; there was little difference in analyses restricted to different sets of controls.
So, the question that faces the reader is, “How serious a matter is it for a baby to be born 2-6 days earlier?” This article examines recent literature on the subject.
Defining Term Pregnancy
Babies are ideally born at full term, that is, after 40 weeks of gestation. This is because many disadvantages related to health and development affect babies born preterm and because several problems are associated with postterm birth, as well.20
The onset of gestation is conventionally defined as the first day of the last menstrual period. With this date as the reference, preterm and postterm births are conventionally defined as birth before 37 weeks and birth after 42 weeks of gestation, respectively. In line with convention, term pregnancy in ICD-10 is defined as delivery between 37 weeks 0 days and 41 weeks 6 days of gestation.20
Babies born between 34 weeks 0 days and 36 weeks 6 days of gestation were formerly considered to be near term. In 2005, a US National Institute of Child Health and Human Development workshop suggested that “near term” be replaced by “late preterm” because the health disadvantages suffered by babies born during this period are more similar to those of preterm babies than those of term babies.20
Fetal maturation does not stop at week 37, and the period constituting term spans 5 weeks. It is therefore logical to expect that outcomes could vary depending on when delivery occurs during term pregnancies. In line with these expectations, observational data show that the best maternal and neonatal outcomes are associated with birth between 39 weeks 0 days and 40 weeks 6 days.21-24
To reduce heterogeneity of outcomes and to bring greater precision into the field, the National Institute of Child Health and Human Development, the American Congress of Obstetricians and Gynecologists, the American Academy of Pediatrics, the Society for Maternal-Fetal Medicine, the March of Dimes, and the World Health Organization participated in a workshop in December 2012.20 The recommendations of the workshop are summarized below:
- Births occurring between 37 weeks 0 days and 38 weeks 6 days should be designated as early term.
- Births occurring between 39 weeks 0 days and 40 weeks 6 days should be considered as full term.
- Births occurring between 41 weeks 0 days and 41 weeks 6 days should be designated as late term.
Spong20 observed that the new terminology has obvious implications for counseling, management, and research. What might be the implications for SSRI-exposed pregnancies?
Week 38 vs Week 39
The reclassification presented above describes splits that are 2 weeks in duration; what about splits that are narrower, such as a week in duration? After all, the average decrease in gestation duration after antenatal SSRI exposure was in the region of a few days, at the most, in the studies that did find differences between SSRI-exposed and unexposed pregnancies. A new study25 provides some interesting insights in this regard.
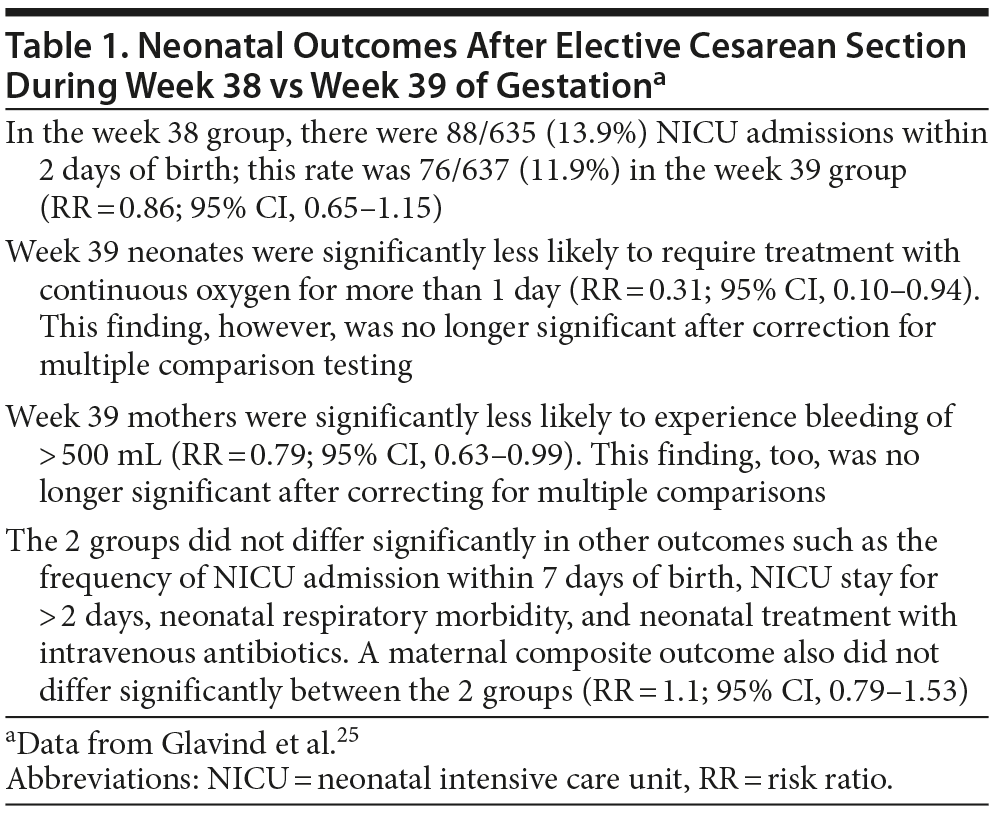
The authors25 describe a Danish, 7-center, open-label, randomized controlled trial (RCT) of perinatal outcomes after elective cesarean section that was scheduled at a gestational age of either 38 weeks 3 days or 39 weeks 3 days (with a window of 2 days on either side of the target day in each group). The sample included 1,274 women (mean age = 32 years) with uncomplicated, singleton pregnancies. The median difference in gestation duration between groups was 6 days.
The study25 found no significant differences between the 2 groups in neonatal intensive care unit (NICU) admission within 48 hours of birth, NICU admission within 7 days, NICU length of stay, neonatal respiratory problems, neonatal treatment, or maternal outcomes. The results are summarized in Table 1.
Implications for Use of SSRIs During Pregnancy
The Danish study25 is important because it is the first RCT in the field; previous studies that examined the relationship between gestational age and perinatal outcomes were all observational in design. The findings of the Danish study25 suggest that, if a sample size in excess of 1,200 women was unable to find significant differences in neonatal or maternal outcomes between deliveries during week 38 and week 39 of gestation, then SSRI exposure-related anticipation of delivery by a few days is not likely to make a demonstrable difference. The Clinical Points listed at the beginning of this article summarize the evidence in a way that can be presented to patients. It must be kept in mind, though, that the Danish study25 examined only perinatal outcomes. We do not as yet know whether the difference of a week in gestational age could result in differences in neurodevelopment or in differences in health and health care utilization during childhood or later.
Parting Note
Precise definitions of preterm, term, and postterm have little meaning if the starting point (the date of the first day of the last menstrual period) is inaccurately remembered or if it inaccurately reflects fetal age. Spong therefore proposed an algorithm for the determination of gestational age based on the date of the last menstrual period, the results of a sonogram, and other data. Readers may wish to consult this algorithm.20
J Clin Psychiatry 2013;74(7):e633-e635 (doi:10.4088/JCP.13f08617)
© Copyright 2013 Physicians Postgraduate Press, Inc.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Chambers CD, Johnson KA, Dick LM, et al. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335(14):1010-1015. PubMed doi:10.1056/NEJM199610033351402
2. Ericson A, Källén B, Wiholm B. Delivery outcome after the use of antidepressants in early pregnancy. Eur J Clin Pharmacol. 1999;55(7):503-508. PubMed doi:10.1007/s002280050664
3. Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry. 2002;159(12):2055-2061. PubMed doi:10.1176/appi.ajp.159.12.2055
4. Wen SW, Yang Q, Garner P, et al. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol. 2006;194(4):961-966. PubMed doi:10.1016/j.ajog.2006.02.019
5. Suri R, Altshuler L, Hellemann G, et al. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry. 2007;164(8):1206-1213. PubMed doi:10.1176/appi.ajp.2007.06071172
6. Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557-566. PubMed doi:10.1176/appi.ajp.2008.08081170
7. Lund N, Pedersen LH, Henriksen TB. Selective serotonin reuptake inhibitor exposure in utero and pregnancy outcomes. Arch Pediatr Adolesc Med. 2009;163(10):949-954. PubMed doi:10.1001/archpediatrics.2009.164
8. Roca A, Garcia-Esteve L, Imaz ML, et al. Obstetrical and neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitors: the relevance of dose. J Affect Disord. 2011;135(1-3):208-215. PubMed doi:10.1016/j.jad.2011.07.022
9. Yonkers KA, Norwitz ER, Smith MV, et al. Depression and serotonin reuptake inhibitor treatment as risk factors for preterm birth. Epidemiology. 2012;23(5):677-685. PubMed doi:10.1097/EDE.0b013e31825838e9
10. El Marroun H, Jaddoe VW, Hudziak JJ, et al. Maternal use of selective serotonin reuptake inhibitors, fetal growth, and risk of adverse birth outcomes. Arch Gen Psychiatry. 2012;69(7):706-714. PubMed doi:10.1001/archgenpsychiatry.2011.2333
11. Pastuszak A, Schick-Boschetto B, Zuber C, et al. Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac). JAMA. 1993;269(17):2246-2248. PubMed doi:10.1001/jama.1993.03500170076037
12. Nulman I, Rovet J, Stewart DE, et al. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336(4):258-262. PubMed doi:10.1056/NEJM199701233360404
13. Kulin NA, Pastuszak A, Sage SR, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study. JAMA. 1998;279(8):609-610. PubMed doi:10.1001/jama.279.8.609
14. Malm H, Klaukka T, Neuvonen PJ. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol. 2005;106(6):1289-1296. PubMed doi:10.1097/01.AOG.0000187302.61812.53
15. Pearson KH, Nonacs RM, Viguera AC, et al. Birth outcomes following prenatal exposure to antidepressants. J Clin Psychiatry. 2007;68(8):1284-1289. PubMed doi:10.4088/JCP.v68n0817
16. Toh S, Mitchell AA, Louik C, et al. Antidepressant use during pregnancy and the risk of preterm delivery and fetal growth restriction. J Clin Psychopharmacol. 2009;29(6):555-560. PubMed doi:10.1097/JCP.0b013e3181bf344c
17. Nordeng H, van Gelder MM, Spigset O, et al. Pregnancy outcome after exposure to antidepressants and the role of maternal depression: results from the Norwegian Mother and Child Cohort Study. J Clin Psychopharmacol. 2012;32(2):186-194. PubMed doi:10.1097/JCP.0b013e3182490eaf
18. Oberlander TF, Warburton W, Misri S, et al. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63(8):898-906. PubMed doi:10.1001/archpsyc.63.8.898
19. Ross LE, Grigoriadis S, Mamisashvili L, et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry. 2013;70(4):436-443. PubMed
20. Spong CY. Defining “term” pregnancy: recommendations from the Defining “Term” Pregnancy Workgroup [published online ahead of print May 3, 2013]. JAMA. 2013;1-2. PubMed doi:10.1001/jama.2013.6235
21. Oshiro BT, Henry E, Wilson J, et al; Women and Newborn Clinical Integration Program. Decreasing elective deliveries before 39 weeks of gestation in an integrated health care system. Obstet Gynecol. 2009;113(4):804-811. PubMed
22. Tita AT, Landon MB, Spong CY, et al. Eunice Kennedy Shriver NICHD Maternal-Fetal Medicine Units Network. Timing of elective repeat cesarean delivery at term and neonatal outcomes. N Engl J Med. 2009;360(2):111-120. PubMed doi:10.1056/NEJMoa0803267
23. Tita AT, Lai Y, Landon MB, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units Network (MFMU). Timing of elective repeat cesarean delivery at term and maternal perioperative outcomes. Obstet Gynecol. 2011;117(2 pt 1):280-286. PubMed doi:10.1097/AOG.0b013e3182078115
24. MacDorman MF, Kirmeyer SE, Wilson EC. Fetal and perinatal mortality, United States, 2006. Natl Vital Stat Rep. 2012;61(1).
25. Glavind J, Kindberg S, Uldbjerg N, et al. Elective caesarean section at 38 weeks versus 39 weeks: neonatal and maternal outcomes in a randomised controlled trial [published online ahead of print May 20, 2013]. BJOG. PubMed doi:10.1111/1471-0528.12278
This PDF is free for all visitors!