Circuits of Sexual Desire in Hypoactive Sexual Desire Disorder
Issue: Brain circuits of sexual desire overlap with the known reward pathways. Neurotransmitters, especially dopamine and serotonin, are key regulators of reward. Dysfunction of reward pathways is hypothetically linked to numerous conditions, from depression to substance abuse and, more recently, to sexual dysfunction, including hypoactive sexual desire disorder (HSDD).
What Is HSDD?
For both patients and health care providers, HSDD probably should mean “Having the Sexual Dysfunction Discussion” to diagnose and treat distressful conditions,1 but it really stands for Hypoactive Sexual Desire Disorder. The DSM-IV defines it as the persistent or recurring deficiency (or absence) of sexual fantasies or thoughts or desire for sexual activity associated with personal distress.2 Although surveys suggest that 31% of men and 43% of women experience some sort of sexual dysfunction1,3 (men usually erectile dysfunction and women usually lack of desire),4 both genders experience HSDD.
HSDD is the subject of much debate and even controversy, ranging from the gender equality angle that women’s sexual problems are finally receiving the same attention as men’s to the accusation that HSDD is really a form of corporate disease-mongering and a diabolical conspiracy by Pharma to invent a disorder that doesn’ t really exist in order to sell dangerous, expensive drugs that don’ t really work and thereby exploit unsuspecting women.5 The truth is probably somewhere in between. Certainly, women who have lost interest in their partner but want to have sex with the guy next door do not have HSDD, but a relationship issue. None of this debate should take away from the prospect of helping those women (and men) with distressful problems of reduced sex drive, decreased libido, and a reduced number of satisfying sexual events due to a dysfunctioning reward system. Treatments for HSDD are discussed in the next Brainstorms.6
What Circuits Are “Turned On” by Sexual Arousal?
Erotic videoclips that cause subjective sexual arousal in healthy men or women (during the mid-luteal vs menstrual phase) activate the prefrontal cortex (anterior cingulate and orbitofrontal cortex) as well as the insula, amygdala, and ventral striatum in both genders, giving a whole new meaning to being “turned on.”7-9 For men, there is a significant relationship between genital and subjective arousal, but for women, there is only a weak relationship between genital and subjective arousal or between genital arousal and brain activation.7-9
The ability of erotic visual stimuli to activate limbic and cortical areas is reduced in women after menopause but can be restored, as can sexual desire and number of sexually satisfying events, by hormone treatment with estrogens and androgens.7-9 Compared with young women without a history of sexual problems, when young women with HSDD view erotic videoclips, they have less subjective sexual arousal and greater activation of those brain areas that hypothetically suppress emotions.7-9 It’s as though HSDD patients are “mentalizing” erotic images but not enjoying them, a vantage point sometimes called “spectatoring.” Neuroimaging studies lead us to the question: Is HSDD due to hypofunctional sexual arousal, hyperfunctional sexual inhibition, or some combination of the two? So far, imaging studies8,9 in HSDD have revealed hyperfunctional inhibition of reward pathways. To understand this result, it is useful to review the neurotransmitters of sexual arousal and sexual inhibition.
Neurotransmitters of Sexual Arousal
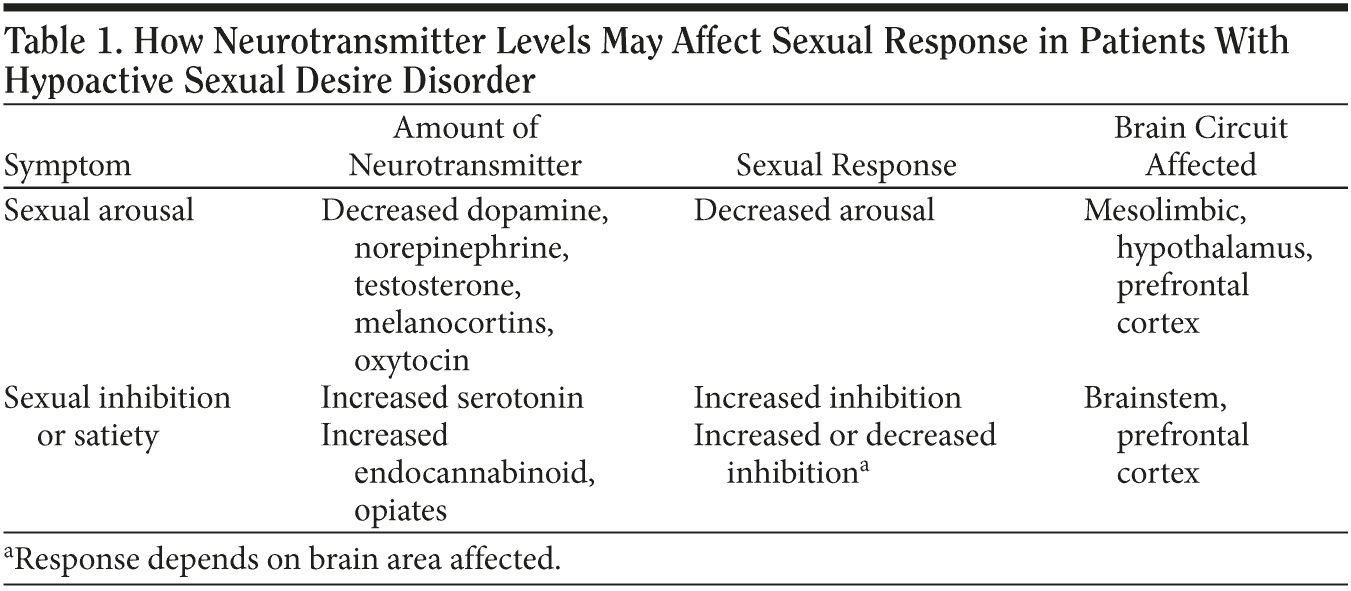
Dopamine (DA) is generally considered to be the major neurotransmitter of sexual arousal, due to its actions in mesolimbic and hypothalamic circuits.4,7 Low sexual desire in HSDD is theoretically due to hypoactivity of mesolimibic dopaminergic neurons (Table 1). This notion partially relies on anecdotal evidence suggesting that patients who take levodopa or DA agonists (eg, drugs given for Parkinson’s disease) experience an increase in sexual drive. Additionally, some patients who have been taking antidepressants that lead to increased DA release, such as the norepinephrine-dopamine reuptake inhibitor (NDRI) bupropion, can also experience an increase in sexual drive. Testosterone may actually enhance sexual interest via a dopaminergic mechanism, namely by interacting with neurons in the hypothalamus, and boosting the ability of DA to act in the hypothalamus. Animal models of sexual arousal also strongly support the role of DA in mesolimbic and hypothalamic circuits.7
Take-Home Points
- Sexual excitation is hypothetically mediated by the neurotransmitters dopamine, norepinephrine, oxytocin, and melanocortins.
- Sexual inhibition is hypothetically mediated by other neurotransmitters: serotonin, opioids, and endocannabinoids.
- Reward pathways, classically dopaminergic input to the nucleus accumbens, also include important circuits interconnecting other key brain areas, especially the amygdala, hypothalamus, and prefrontal cortex.
- Hypoactive sexual desire disorder (HSDD) may result from hypofunctional excitation, hyperfunctional inhibition, or some combination of the two, due to dysregulation of neurotransmitters in reward circuits.
Norepinephrine (NE) is also strongly linked to sexual arousal in that it is the principle neurotransmitter regulating arousal of all types, including control of the autonomic nervous system.7 Melanocortins are neuropeptides linked to sexual arousal, especially their actions at 2 different melanocortin receptors (MC3 and MC4) in hypothalamus and limbic areas.7 Oxytocin, well known as the “affinity” neurotransmitter or the “bonding” hormone, is another neuropeptide that can promote sexual arousal.7
Neurotransmitters of Sexual Inhibition
Although it is clear for reproductive reasons and for survival of the species why we should have sexual arousal circuits and neurotransmitters, why do we need sexual inhibition circuits and neurotransmitters? One notion is that to allow sexual activity to be sufficiently rewarding, it is a good idea to have a restorative phase of sexual satiety that also serves as a “refractory phase” when sexual activity is inhibited.7 This phase occurs naturally following orgasm.
Serotonin (5-HT) is the best known “satiety” neurotransmitter and mediates a sense of satisfaction in behaviors ranging from eating to sexual arousal.4,7 Increasing brain 5-HT levels with the widely prescribed selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs) is infamous for causing sexual dysfunction, from inhibiting orgasm to reducing sexual desire,4 which occurs, in part, by inhibiting DA in both brainstem circuits and prefrontal cortex circuits (Table 1).4,7 Serotonin regulates the output of pyramidal neurons from prefrontal cortex that project to brainstem and inhibit DA release.4,7 The problem in HSDD may come when serotonergic actions are too high, since this could theoretically lead to an overactive sexual inhibition system and, thus, inhibition of the dopaminergic sexual arousal system.4,7 Just this situation is now the widely hypothesized mechanism of disease action in HSDD, namely overactive 5-HT causing underactive DA.4,7
Other neurotransmitters are also inhibitory to sexual functioning, but in complex ways. Opiates and endocannabinoids can both promote and inhibit sexual actions, depending upon brain area and receptor involved.4 Perhaps the best example of opiate action on sexual arousal is the rush of euphoria that they produce, followed by a prolonged period of relaxation that some call a “pharmacogenic orgasm.”4
Summary
The brain has separate but interacting neural systems for sexual arousal and for sexual inhibition. HSDD is currently conceptualized to be a disorder of reward circuitry, caused by either hypofunctional excitation or hyperfunctional inhibition or both.
References
1. Neuroscience Education Institute. Having the Sexual Dysfunction Discussion. Carlsbad, CA: NEI Press; 2009.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychiatric Association; 2004.
3. Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281(6):537-544. doi:10.1001/jama.281.6.537 PubMed
4. Stahl SM. Stahl’s Essential Psychopharmacology. 3rd ed. New York, NY: Cambridge University Press; 2008.
5. Moynihan R. The marketing of a disease: female sexual dysfunction. BMJ. 2005;330(7484):192-194. doi:10.1136/bmj.330.7484.192 PubMed
6. Stahl SM. Targeting circuits of sexual desire as a treatment strategy for hypoactive sexual desire disorder. J Clin Psychiatry. In press
7. Pfaus JG. Pathways of sexual desire. J Sex Med. 2009;6(6):1506-1533. doi:10.1111/j.1743-6109.2009.01309.x PubMed
8. Arnow BA, Millheiser L, Garrett A, et al. Women with hypoactive sexual desire disorder compared to normal females: a functional magnetic resonance imaging study. Neuroscience. 2009;158(2):484-502. doi:10.1016/j.neuroscience.2008.09.044 PubMed
9. Holstege G, Willemsen A, Beers C, et al. Differences in brain activity in premenopausal women with hypoactive sexual desire disorder (HSDD) compared to women without sexual dysfunction. In: Abstracts of the 12th Congress of the European Society for Sexual Medicine (ESSM), November 15-18, 2009; Lyon, France.