Suicide is the leading cause of death among young adults, with significant and independent associations with sleep alterations.1 Hypnotics are commonly prescribed in patients with major depressive disorder (MDD) and have been associated with a suicidal ideation reduction in patients with insomnia disorder.2 However, there remains a debate regarding the potential increased risk of suicidal behavior associated with the use of hypnotics.3 Dual orexin receptor antagonists (DORA), a new class of hypnotics, were recently marketed with precautionary measures for use in patients with MDD and suicidal ideation. Nevertheless, their safety data are very limited.
Our aim was to investigate the association between DORA and suicide ideation/behavior using real-world data.
Methods
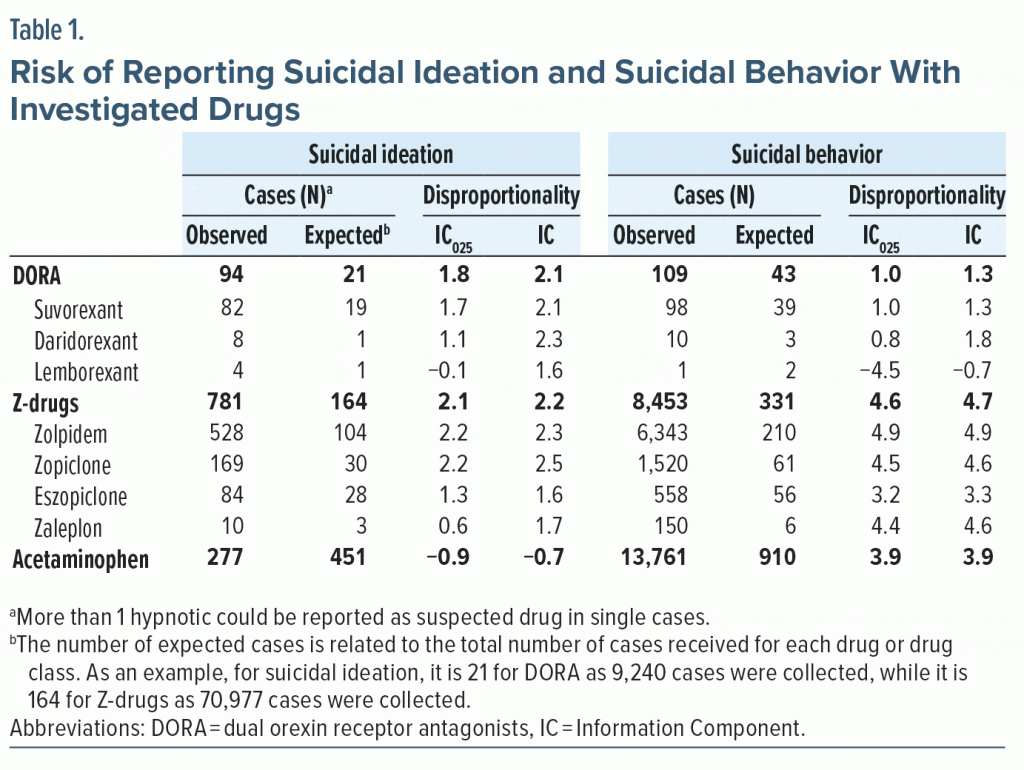
We queried the WHO pharmacovigilance database (VigiBase), which comprises more than 34 million cases of possible adverse reactions.4 We reviewed all terms related to “Suicide/self-injury” and characterized 2 phenotypes: ideation and behavior (completed or attempted suicide), to distinguish cases of possible adverse effects related to hypnotics intake from cases in which hypnotics were possibly used to commit suicide. We used Information Component (IC), the WHO Bayesian measure, to quantify the association between DORA and suicide reporting.5 Briefly, IC is a measure that, when significantly increased (IC025 ≥ 0), indicates that the observed number of cases is more frequent than expected, thus suggesting a possible increased risk. We conducted the same analysis for Z-drugs and acetaminophen (paracetamol). The latter was used as a positive control for suicidal behavior and as a negative control for suicidal ideation.
Results
Suicidal ideation. Among the 80,069 cases collected in VigiBase, we found 94 cases (1.2‰) related to DORA and 781 (9.8‰) to Z-drugs. DORA were the only suspected drugs in 85 cases (90.4%), while Z-drugs were in 396 cases (50.7%). Among cases reporting a drug withdrawal, a positive dechallenge (suicidal ideation improving) was recorded in 59.3% of DORA-related and 66.0% of Z-drugs–related cases.
Suicidal ideation was 2.1 times more frequent than expected with DORA and 2.2 times more frequent with Z-drugs, with no meaningful difference among drugs; data on daridorexant, lemborexant, and zaleplon were sparse. Acetaminophen was confirmed as the negative control (Table 1).
Suicidal behavior. Among the 161,574 cases collected in VigiBase, we found 43 cases (0.7‰) related to DORA and 8,453 (52.3‰) to Z-drugs. DORA were the only suspected drugs in 7 cases (16.3%), while Z-drugs were in 1,338 cases (15.8%). Among cases reporting a drug withdrawal, a positive dechallenge was recorded in 44.8% of DORA-related and 62.7% of Z-drugs–related cases.
Suicidal behavior was 1.3 times more frequent than expected with DORA and 4.7 times more frequent for Z-drugs; data on lemborexant were sparse. Acetaminophen was confirmed as the positive control (Table 1).
Discussion
DORA were related to a warning concerning suicidal ideation,3 but, to date, we found no specific pharmacovigilance signal or unexpected increase in the number of cases, in particular when compared with Z-drugs. Nevertheless, the limitations of spontaneous reporting studies (such as underreporting) and the specific pharmacologic profile of DORA, in particular the link between orexin and serotonin pathways,6 require further pharmacovigilance attention and neurobehavioral studies.
Our findings regarding suicidal ideation were coherent between DORA and Z-drugs, with a positive dechallenge occurring in one-third of the reported cases, and that was associated with an improvement in around two-thirds of cases related to both hypnotics. These results could be at least in part related to an indication bias, as insomnia disorder is closely associated with MDD and suicidal ideation.1 However, it is important to note that the available data on suicidal ideation and DORA are still preliminary, and further studies using real-world data are necessary to provide more comprehensive and updated information.
Concerning suicidal behavior, in comparison with Z-drugs, we found a relative paucity of cases of voluntary intoxication with DORA.
In perspective, comparative data on validated efficacy and safety outcomes are essential to clarify the role of hypnotics in suicidal risk in patients with insomnia disorder. Elucidating this role is important for thoughtful and evidence-based prescription of hypnotics to improve sleep alteration in patients with MDD and suicidal ideation.
Article Information
Published Online: September 27, 2023. https://doi.org/10.4088/JCP.23br14923
© 2023 Physicians Postgraduate Press, Inc.
Submitted: April 23, 2023; accepted June 23, 2023.
J Clin Psychiatry 2023;84(6):23br14923
To Cite: Salvo F, Micoulaud-Franchi J-A, Palagini L, et al. Dual orexin receptor antagonists and suicide risk: findings from the WHO spontaneous reporting database. J Clin Psychiatry. 2023;84(6):23br14923.
Corresponding Author: Prof Pierre Alexis Geoffroy, MD, PhD, University Hospital Bichat-Claude Bernard, Department of Psychiatry and Addictive Medicine, 46 rue Henri Huchard, 75018, Paris, France ([email protected]).
Author Affiliations: Department of Medical Pharmacology, Regional Pharmacovigilance Center of Bordeaux, University Hospital of Bordeaux, Inserm U1219, Université de Bordeaux, F-33000, Bordeaux, France (Salvo); University Sleep Clinic Service, University Hospital of Bordeaux, F-33076, Bordeaux, France; UMR CNRS 6033 SANPSY, University Hospital of Bordeaux, F-33076, Bordeaux, France (Micoulaud-Franchi); Psychiatric Clinic Department of Experimental Medicine, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy (Palagini); Département de Psychiatrie et d’Addictologie, AP-HP, GHU Paris Nord, DMU Neurosciences, Hopital Bichat—Claude Bernard, F-75018, Paris, France; Centre ChronoS, GHU Paris—Psychiatry & Neurosciences, Paris, France; Université Paris Cité, NeuroDiderot, Inserm, FHU I2-D2, F-75019, Paris, France (Geoffroy).
Relevant Financial Relationships: Dr Micoulaud-Franchi has received speaker honoraria from Bioprojet, EISAI, and Resmed and fees for consulting from AirLiquid, Bayer, and Gilead. Dr Palagini has received consultant honoraria from Idorsia, Pfizer, Bruno SpA, and Fidia. Dr Geoffroy has received speaker honoraria from Biocodex, Bioprojet, Idorsia, Isis Medical, Janssen-Cilag, Jazz Pharmaceuticals, Lundbeck, MySommeil, and Withings; has received fees for consulting from Apneal, Biocodex, Dayvia, Idorsia, Janssen-Cilag, Jazz Pharmaceuticals, Myndblue, Posos, ResilEyes, and Withings; and is a member of the advisory boards of Apneal, Idorsia, Mindblue, and Mysommeil. Dr Salvo has no conflicts of interest to disclose.
Funding/Support: None.
Disclaimer: The information presented in this study does not represent the opinion of the World Health Organization.
ORCID: Pierre Alexis Geoffroy: https://orcid.org/0000-0001-9121-209X
References (6)

- Geoffroy PA, Oquendo MA, Courtet P, et al. Sleep complaints are associated with increased suicide risk independently of psychiatric disorders: results from a national 3-year prospective study. Mol Psychiatry. 2021;26(6):2126–2136. PubMed CrossRef
- McCall WV, Benca RM, Rosenquist PB, et al. Reducing suicidal ideation through insomnia treatment (REST-IT): a randomized clinical trial. Am J Psychiatry. 2019;176(11):957–965. PubMed CrossRef
- Nobile B, Olié E, Courtet P. Possible suicidal risk with daridorexant, a new treatment for insomnia. J Clin Psychiatry. 2022;84(1):22l14628. PubMed CrossRef
- Lindquist M. VigiBase, the WHO Global ICSR database system: basic facts. Drug Inf J. 2008;42(5):409–419. CrossRef
- Bate A, Lindquist M, Edwards IR, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998;54(4):315–321. PubMed CrossRef
- Wang C, Wang Q, Ji B, et al. The orexin/receptor system: molecular mechanism and therapeutic potential for neurological diseases. Front Mol Neurosci. 2018;11:220. PubMed CrossRef
This PDF is free for all visitors!