Objective: Stress exposures may have a differential impact on risk and resilience for depression depending on their timing across development. We sought to determine whether adverse childhood experiences (ACEs) and their onset with respect to puberty contribute to the increased risk observed in first-episode major depressive disorder (MDD) during the menopause transition.
Methods: Participants were from the Penn Ovarian Aging Study cohort, which is composed of women from Philadelphia County, Pennsylvania, who underwent behavioral, cognitive, and endocrine evaluations approximately yearly from 1996 to 2012 and completed the Adverse Childhood Experiences Questionnaire at study end point (n = 243). ACEs that first occurred 2 or more years before menarche were considered prepubertal. Incident menopause MDD was defined as first observed onset of the disorder in the perimenopause to postmenopause transition using the Structured Clinical Interview for DSM-III-R and the Primary Care Evaluation of Mental Disorders.
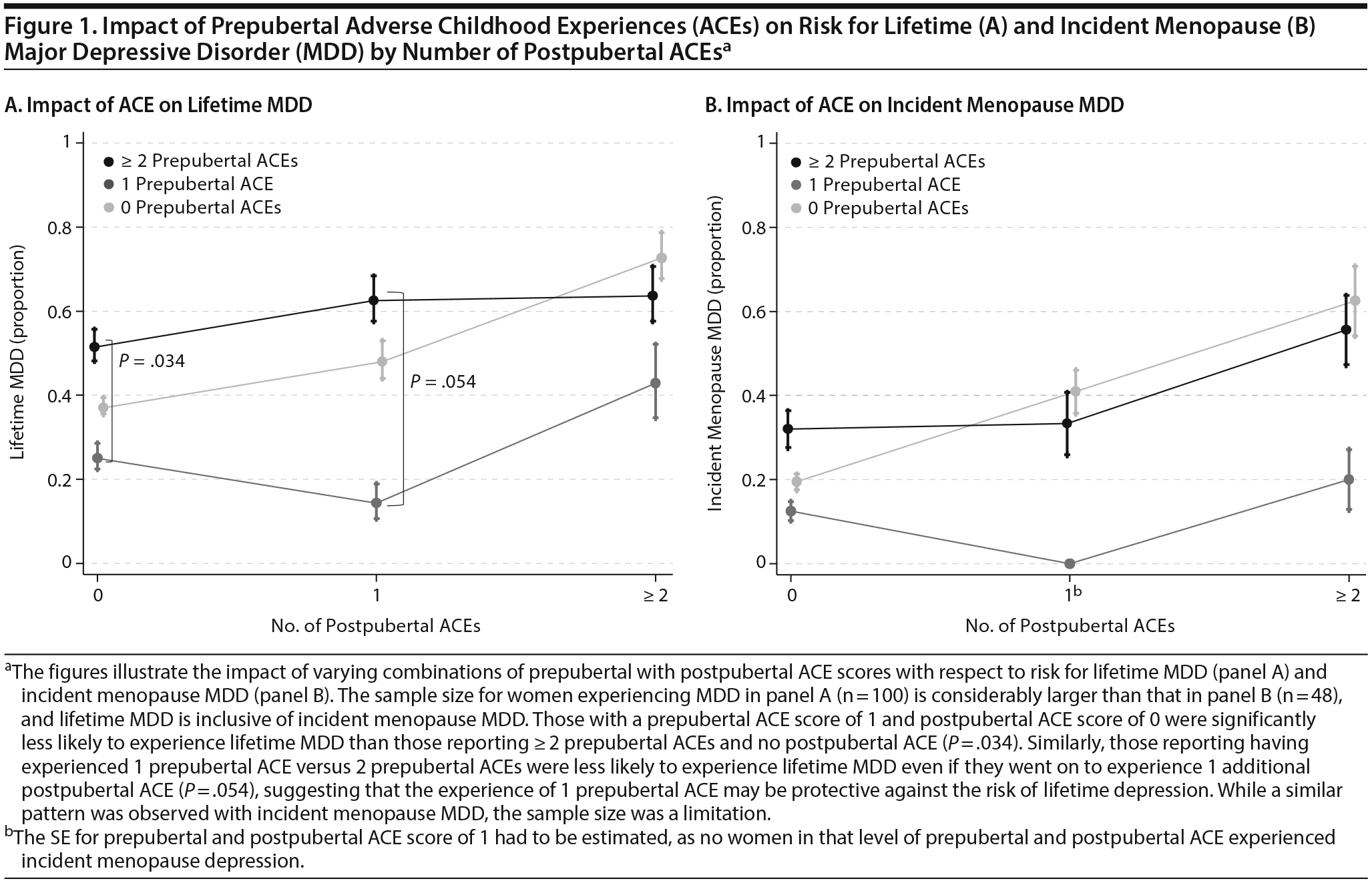
Results: Incident menopause MDD occurred in 48% of the 100 women who reported lifetime MDD. Women reporting ≥ 2 total ACEs were at significantly greater risk for lifetime MDD (adjusted odds ratio [aOR] = 2.05, P = .034) and incident menopause MDD (aOR = 2.58, P = .03) compared to those reporting 0 ACEs; women with ≥ 2 postpubertal ACEs were 2.3 times more likely to experience incidence menopause MDD (P = .024) after controlling for race, smoking, body mass index, and employment. Experiencing only 1 ACE in the prepubertal window, regardless of additional ACEs in postpuberty, was associated with reduced risk for lifetime and incident menopause MDD.
Conclusions: Timing and number of adverse experiences with respect to puberty differentially impacted risk and resilience for MDD across the female life span and during the menopause transition in this community cohort.

Adverse Childhood Experiences and Risk for First-Episode Major Depression During the Menopause Transition
ABSTRACT
Objective: Stress exposures may have a differential impact on risk and resilience for depression depending on their timing across development. We sought to determine whether adverse childhood experiences (ACEs) and their onset with respect to puberty contribute to the increased risk observed in first-episode major depressive disorder (MDD) during the menopause transition.
Methods: Participants were from the Penn Ovarian Aging Study cohort, which is composed of women from Philadelphia County, Pennsylvania, who underwent behavioral, cognitive, and endocrine evaluations approximately yearly from 1996 to 2012 and completed the Adverse Childhood Experiences Questionnaire at study end point (n = 243). ACEs that first occurred 2 or more years before menarche were considered prepubertal. Incident menopause MDD was defined as first observed onset of the disorder in the perimenopause to postmenopause transition using the Structured Clinical Interview for DSM-III-R and the Primary Care Evaluation of Mental Disorders.
Results: Incident menopause MDD occurred in 48% of the 100 women who reported lifetime MDD. Women reporting ≥ 2 total ACEs were at significantly greater risk for lifetime MDD (adjusted odds ratio [aOR] = 2.05, P = .034) and incident menopause MDD (aOR = 2.58, P = .03) compared to those reporting 0 ACEs; women with ≥ 2 postpubertal ACEs were 2.3 times more likely to experience incidence menopause MDD (P = .024) after controlling for race, smoking, body mass index, and employment. Experiencing only 1 ACE in the prepubertal window, regardless of additional ACEs in postpuberty, was associated with reduced risk for lifetime and incident menopause MDD.
Conclusions: Timing and number of adverse experiences with respect to puberty differentially impacted risk and resilience for MDD across the female life span and during the menopause transition in this community cohort.
J Clin Psychiatry 2017;78(3):e298-e307
https://doi.org/10.4088/JCP.16m10662
© Copyright 2017 Physicians Postgraduate Press, Inc.
aPenn PROMOTES Research on Sex and Gender in Health, bDepartments of Psychiatry, cObstetrics and Gynecology, and dBiostatistics and Epidemiology, Center for Clinical Epidemiology and Biostatistics, Perelman School of Medicine, University of Pennsylvania, Philadelphia
eSchool of Veterinary Medicine, University of Pennsylvania, Philadelphia
fDepartment of Counseling Psychology, University of Florida, Gainesville
*Corresponding author: C. Neill Epperson, MD, Professor of Psychiatry and Obstetrics and Gynecology, Perelman School of Medicine, University of Pennsylvania, 3535 Market St, Rm 3001, Philadelphia, PA 19104 ([email protected])
Research confirms multiple sex differences in affective disorders, with women experiencing major depression, dysthymia, rapid-cycling bipolar, seasonal affective, posttraumatic stress, and panic disorders more often than men.1 Moreover, women experience sex-specific affective disorders such as premenstrual dysphoric disorder2 and are at increased risk for major depressive disorder (MDD) during certain reproductive events such as the menopause transition.3 Women with no previous history of depression are 2-3 times more likely to experience a first episode of depression during perimenopause and early postmenopause,4-8 although risk declines over the first few postmenopausal years,3,6 particularly among women who experienced their first episode of depression during perimenopause.3
Endocrine changes, associated physical symptoms, interpersonal conflicts, role changes, cognitive style, and early and recent stressful life events are purported to contribute to this sex bias in major affective disorders after puberty9,10 and to increased risk for depression during the menopause transition specifically.3-8 The relationship between early life stress reproductive hormones and risk for depression is complex, encompassing individual and aggregate effects on neurotransmitter systems, stress neural circuitry, and endocrine and immune function.11-16 Moreover, the impact of estradiol on the behavioral and endocrine sensitivity to psychosocial stress depends on reproductive status (premenopause vs postmenopause) of the individual.17,18
Several studies have suggested that current psychosocial stressors contribute to risk for new-onset depression in adults19 and that childhood trauma is associated with earlier onset mood episodes and greater years of illness among adults with bipolar disorder.20 Interestingly, childhood trauma is also associated with objective deficits in executive function domains in adults without a history of psychiatric disorders, suggesting that the negative sequelae of early life stress is enduring even among the relatively resilient.20 To our knowledge, no studies have focused on the role of childhood adversity in onset of major depressive disorder (MDD) during the menopause transition. Moreover, preclinical research suggests that timing of childhood adversity with respect to puberty is likely to contribute to depression risk among menopausal women. A nonhuman primate model (subordinance in social hierarchy) of prepubertal-onset stress provides support for an interactive effect of stress and estradiol with respect to enzymes critical for serotonin synthesis,21,22 hypothalamic-pituitary-adrenal axis function,23 and sexual behavior.24 Finally, rodent studies indicate that prepubertal and peripubertal exposures to stress adversely impact cognitive aging and affect regulation,25-27 in part through effects on the prefrontal cortex and hippocampus.28,29
We examined the impact of early life stress as assessed by the Adverse Childhood Experiences Questionnaire (ACE-Q)30,31 on the first episode of MDD during the menopause transition among participants in the 16-year, longitudinal Penn Ovarian Aging Study (POAS).3,4 We chose to examine childhood adversity instead of specific traumatic life events to more closely mimic the preclinical studies of chronic variable stress and social subordination, which reveal the importance of timing of adversity in relation to puberty with respect to aging outcomes.22,29 Furthermore, we explored whether adverse childhood experiences (ACEs) with onset prior to initiation of pubertal hormonal fluctuations more potently contributed to MDD risk over the life-course in addition to the menopause transition than ACEs that occurred later in childhood; we also examined whether any degree of early life adversity contributed to resilience with respect to risk for MDD in adulthood.
METHODS
Cohort Description
Between June 2012 and August 2012, 243 women (83.8%) of the 293 women remaining active in the POAS cohort were contacted by telephone to obtain history regarding early-life adversity using the ACE-Q.31 The POAS cohort is a population-based cohort (n = 436) identified by random-digit dialing to households in Philadelphia County, Pennsylvania, in 1996-1997 and stratified to obtain equal numbers of white and African American women. At enrollment, the participants were between the ages of 35 and 47 years, were premenopausal with regular menstrual cycles in normal range (22-35 days), and had an intact uterus and at least 1 ovary. Exclusion criteria included the use of psychotropic medication or hormonal contraception, presence of a serious health problem, or alcohol or drug abuse in the previous year. Attrition since POAS inception has been acceptable at 33%, and the cohort (n = 243) remaining who provided ACE-Q data is consistent with the original sample (see Supplementary eTable 1 at PSYCHIATRIST.COM).32,33 The POAS study was approved by the University of Pennsylvania institutional review board (IRB), and written informed consent was obtained from participants. In 2012, IRB approval was granted to contact the women who remained in the POAS to obtain ACE history. Participants gave assent by phone and were interviewed by members of the research team who were blind to the participant’s history of depression.
Assessment Periods for the POAS Cohort
Data were collected during 6 assessment periods at approximately 8-month intervals through year 5 and then yearly thereafter, with a 2-year gap between period 10 and period 11. Trained research interviewers administered a structured questionnaire and cognitive and mood assessments, measured height and weight to determine body mass index (BMI), and collected blood samples for hormone assays at each assessment period. Information regarding demographics, menstrual cycle dates, reproductive history, general health status and behaviors, and common menopausal symptoms was obtained at each assessment during in-home evaluations until 2010 (the 14th study year). Between years 14 and 16, women were interviewed by phone to obtain information regarding menopause status. History of ACE was obtained in year 16.
Study Variables
Menopausal status. Menopausal status was determined from the data on menstrual bleeding, recorded using daily diaries, the number of menstrual periods between assessments, and cycle length. The definitions for the menopausal status groups were based on the staging system for reproductive aging in women,34 with an added late-premenopause stage (Supplementary Methods).32
Adverse Childhood Experiences Questionnaire. The ACE-Q has been utilized to assess the relationship between childhood adversity and adult health outcomes in a number of studies, with the best known the Adverse Childhood Experiences (ACE) Study31 conducted in collaboration with the Centers for Disease Control and Prevention (CDC) and Kaiser Permanente. Numerous publications from this study emphasize the graded impact of ACEs on risk for a range of adverse adult health outcomes. The ACE-Q focuses on 3 general categories of childhood adversity (abuse, neglect, and household/family dysfunction), which are broken down into subcategories (ie, physical, sexual, and emotional abuse; emotional and physical neglect; parental separation; household violence; parental substance abuse or psychiatric disorders; and household member in prison). With 10 items, scores range from 0 to 10 depending on the number of different types of experiences reported. To examine the differential impact of prepubertal versus postpubertal ACEs in the present study, the ACE-Q was modified to ask all participants to recall the age at which a given ACE first occurred. Adverse childhood experiences that were reported to have occurred or started at least 2 years prior to menarche were considered prepubertal, even if they extended into the postpubertal window. All other ACEs with onset up to age 18 years were considered postpubertal. For female participants in the large ACE study,35 even having 2 ACEs was associated with a significant increased risk of lifetime MDD compared to having experienced no ACEs, but timing of the ACE with respect to puberty was not evaluated and neither was the impact of ACE on risk for new-onset menopause depression.
We also examined impact of ACEs on participant characteristics at study enrollment. Those who reported 0 or 1 ACE were in the “low ACE” group; those who reported 2 or more ACEs were in the “high ACE” group given our interest in ACE impact on MDD risk. For all other analyses, comparisons were made between 1 ACE or ≥ 2 ACEs versus 0 ACEs. We parceled out those with only 1 ACE in an effort to examine the impact of relatively limited degrees of stress and to determine the possibility of a “stress inoculation” effect in humans that has been observed and reported in preclinical models.36,37
Major depressive disorder diagnosis and depressive symptoms. At initial screening, women were asked whether they had ever been diagnosed with depression by a doctor. During assessment period 1, MDD diagnosis was determined using the depression section from the Structured Clinical Interview for DSM-III-R (SCID).38 At each assessment from periods 2 to 6, trained researchers and interviewers administered the Primary Care Evaluation of Mental Disorders (PRIME-MD)39 to determine MDD diagnosis. From period 7 to study end, the self-administered version of the PRIME-MD, the 9-item Patient Health Questionnaire (PHQ-9),40,41 was administered to obtain a SCID diagnosis of MDD. Specifically, women had to endorse 1 of the 2 key symptoms of depression (ie, depressed mood or decreased interest or pleasure) and a total of 5 symptoms to meet criteria for MDD diagnosis. For each woman, the menopausal status at the first assessment in which she reported MDD was used to define the study outcome of interest. Lifetime MDD refers to individuals with any MDD diagnosis during the 14-year study period, as women who reported a history of depression at screening but were not observed to have ever met MDD criteria were not included in these analyses. Incident menopause MDD was defined as follows: no history of MDD during premenopause when menstrual cycles were regular and new-onset MDD after the first occurrence of a ≥ 7-day change in menstrual cycle length (stage 2). Women who had no history of MDD were considered as the reference group for analysis. The Center for Epidemiologic Studies Depression scale (CES-D),42 a 20-item self-report scale, was administered at each POAS assessment period to assess presence and severity of depressive symptoms. A score of ≥ 16 reflects a clinically meaningful level of depressive symptoms, while a score of ≥ 25 has greater specificity for a clinical diagnosis of MDD.42 We report here CES-D scores at study entry.
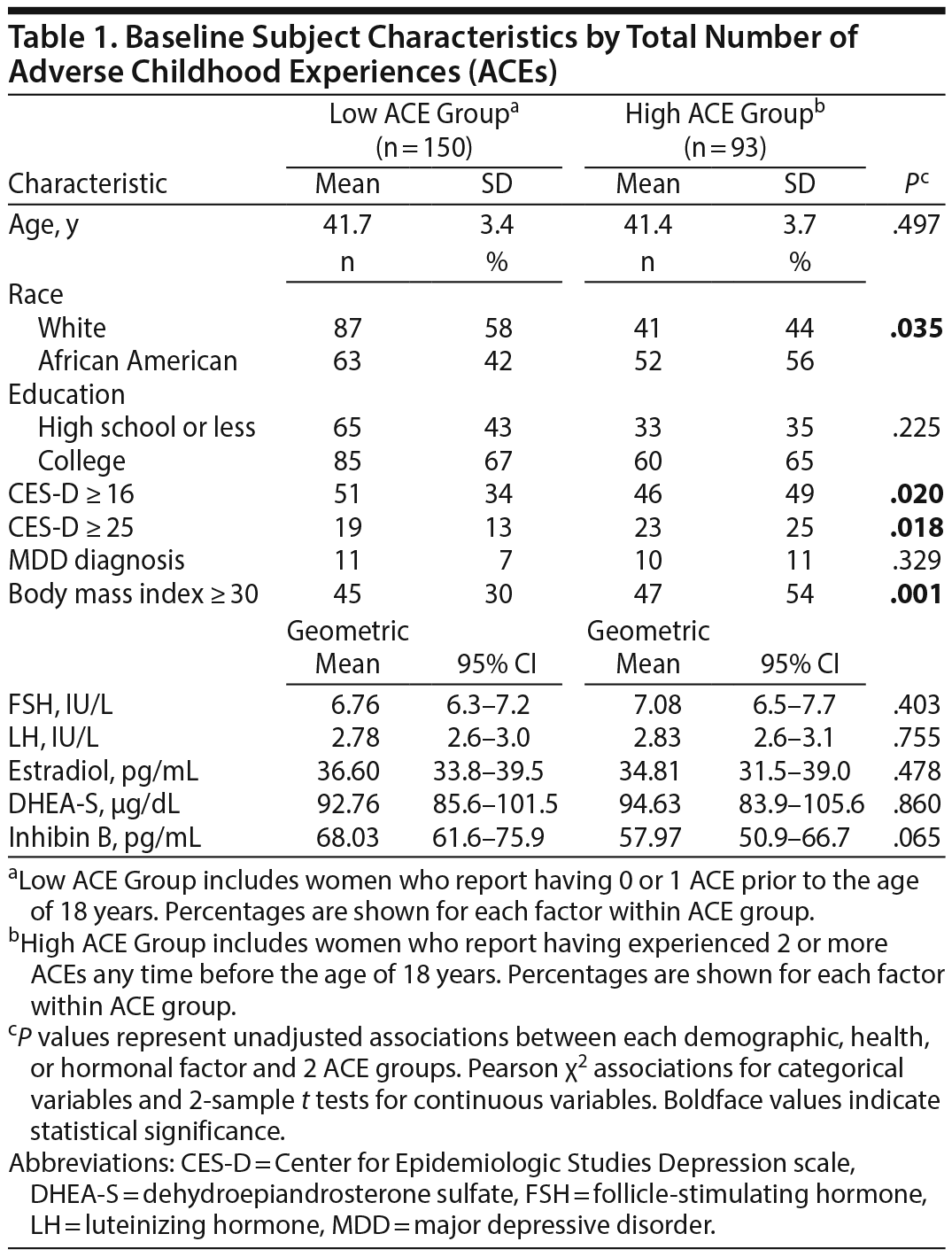
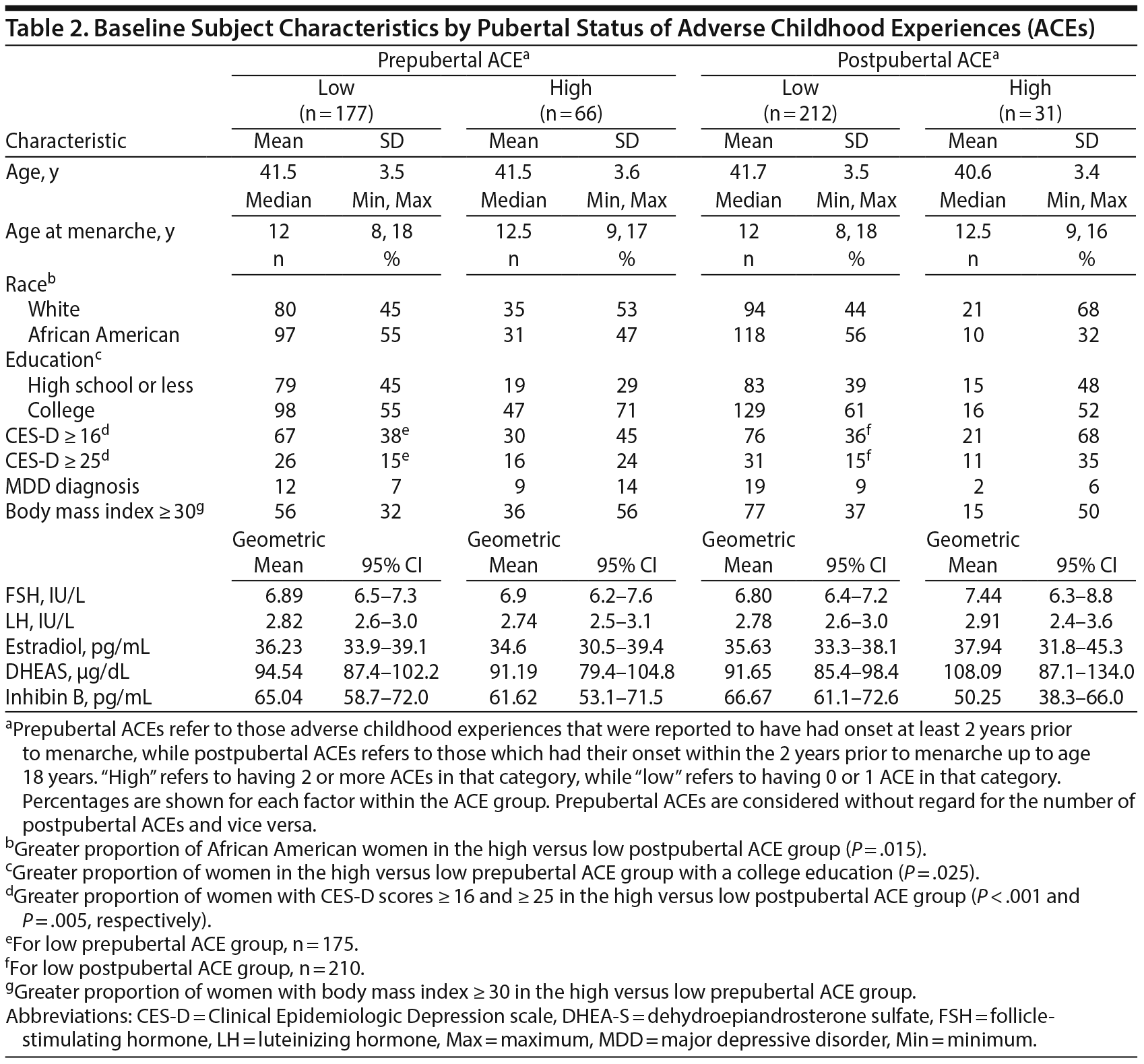
Hormone assessment. Blood samples for measurement of estradiol, follicle-stimulating hormone, and inhibin B were collected between days 2 and 6 of 2 consecutive menstrual cycles or 1 month apart in nonmenstruating women during each assessment period. The samples were prepared and stored at −80°C (-112°F). Methods for hormone quantification with interassay and intraassay coefficients of variation and lower limits of detection were conducted as described previously.4,32 We report baseline hormone levels in Tables 1 and 2 to demonstrate the sample’s premenopausal status.
Statistical Methods
Odds ratio estimates for the association between ACE groups and incident and lifetime MDD were estimated using logistic regression. Models for adjusted estimates included race, BMI (kg/m2) > 30, current smoking, and current employment (full time vs other). Two-sample t tests and Pearson χ2 tests were used to compare demographics between women with low (0 or 1) ACEs and those with high (2 or more) ACEs. The timing (prepuberty or postpuberty) of individual ACEs was compared using an exact binomial test of the hypothesis that the participants who report each individual ACE are equally likely to report the timing of the first occurrence to be prepuberty or postpuberty (see Table 4). Reproductive hormones were compared after natural log transformation. We report geometric mean hormone levels along with 95% confidence intervals in Tables 1 and 2.
We estimated, by using CDC reports, that 34.5% of the women would have no ACE history and that 41% would have ≥ 2 ACEs. Additional assumptions included a type I error with an α of 5% and 80% power. We computed that we would have sufficient statistical power to detect risk ratios on the order 2.4 or greater for incident MDD for women with ≥ 2 total ACES compared to women without history of ACE. The statistical analysis was conducted using STATA version 13 (College Station, Texas); 2-tailed P values of .05 were considered statistically significant.
RESULTS
Participants and Assessments
Of the 293 women active in the POAS cohort at study end, ACE-Q data were obtained from 243 (82.9%). Each woman contributed a mean of 12.3 assessments for a total of 2,986 assessments. Comparison between the entire POAS cohort and those for whom ACE-Q data were collected revealed no significant baseline differences with respect to race, age, BMI, employment, CES-D scores, or marital status (all P values > .05). Likewise, the proportion of women with lifetime or incident menopause MDD diagnoses at baseline did not differ (P > .05; Supplementary eTable 1). For the 243 women (128 white; 115 African American) who comprise the present group, mean (SD) age at enrollment in the POAS was 41.6 (3.5) years, and significant differences were found between the high and low ACE groups with respect to race (P = .035), with a greater proportion of the African American participants in the high versus low ACE groups. Similarly, the proportion of women with high levels of depressive symptoms and obesity as evidenced by CES-D scores ≥ 16 (P = .020) or ≥ 25 (P = .018) and BMI ≥ 30 (P = .001), respectively, was greater in the high versus low ACE groups (Table 1). Table 2 shows that baseline differences between high and low ACE groups are, in part, driven by whether the ACEs first occurred in the prepubertal or postpubertal period. Having ≥ 2 postpubertal ACEs is associated with having baseline CES-D scores ≥ 16 (P < .001) or ≥ 25 (P = .005), while having a prepubertal ACE score of ≥ 2 is associated with having a BMI ≥ 30 (P < .001). Interestingly, there were a greater proportion of college graduates among the group of women who reported ≥ 2 prepubertal ACEs (P = .025).
Lifetime and Incident Menopause Depression
At the time of enrollment, 95.5% of the cohort (232/243) was at risk of experiencing incident menopause MDD. Excluded from our analysis were 2 women who had no observed changes in cycle length and could therefore not be included because no menopause transition had yet transpired. An additional 9 women reported a history of depression at enrollment but were never observed to experience an episode of MDD over the 14 years that home visits were completed. Because their symptom severity and duration of their symptoms prior to study entry were not determined, they were also excluded. The cumulative incidence of lifetime MDD among the women who provided ACE-Q data was 43.1.% (100/232). Fifty-two women (22.4%) were diagnosed with MDD during premenopause, while 48 women (20.7%) were found to have their first diagnosis of MDD after onset of menstrual irregularity (incident menopause MDD).
Number and Types of ACEs Reported
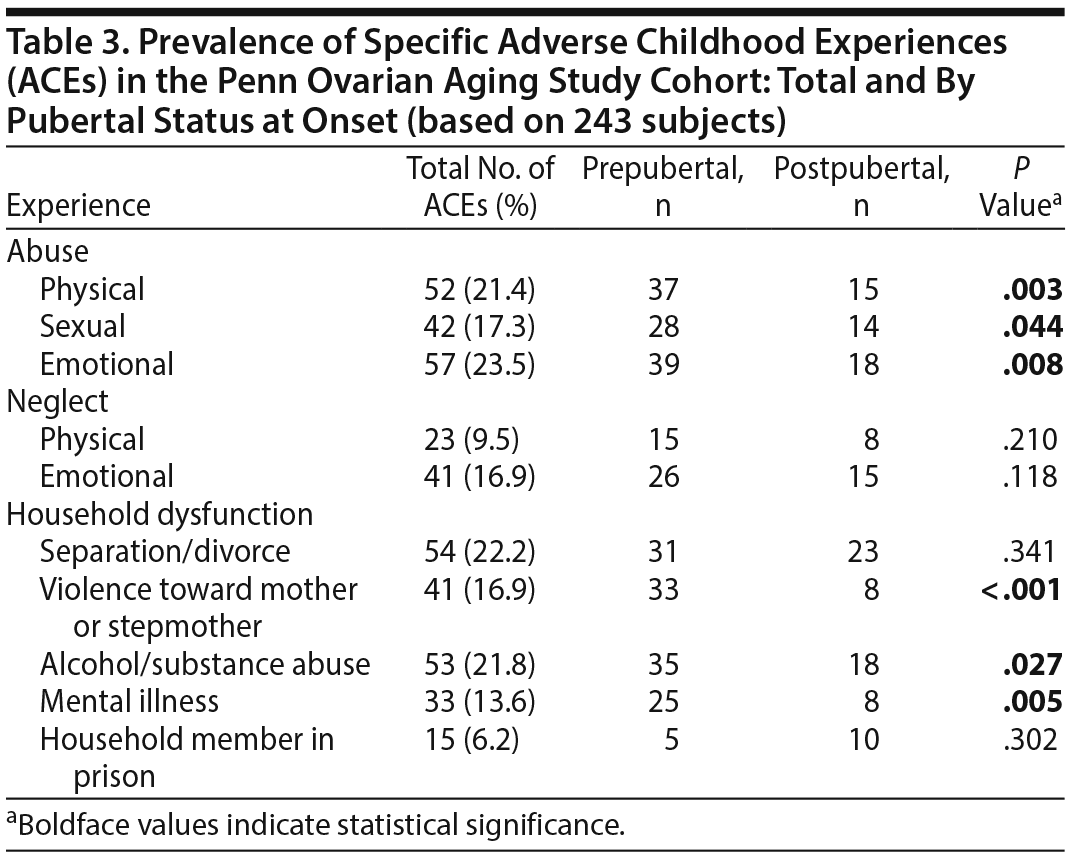
In this sample, 39.5%, 22.2%, and 38.3% of women reported having experienced 0, 1, or ≥ 2 ACEs, respectively. The number of individuals reporting each type of ACE is listed in Table 3 in total and by pubertal status. Overall, the most commonly reported adverse experiences were emotional abuse, parental separation or divorce, and living with someone with alcohol or substance abuse. Among the 54 women who reported a single ACE, the most commonly experienced event was familial drug or alcohol abuse, followed by separation/divorce. Most ACEs had an onset in the prepubertal window (P values < .05), suggesting that adversity exposures typically began quite early in development.
Relationship Between ACEs and MDD
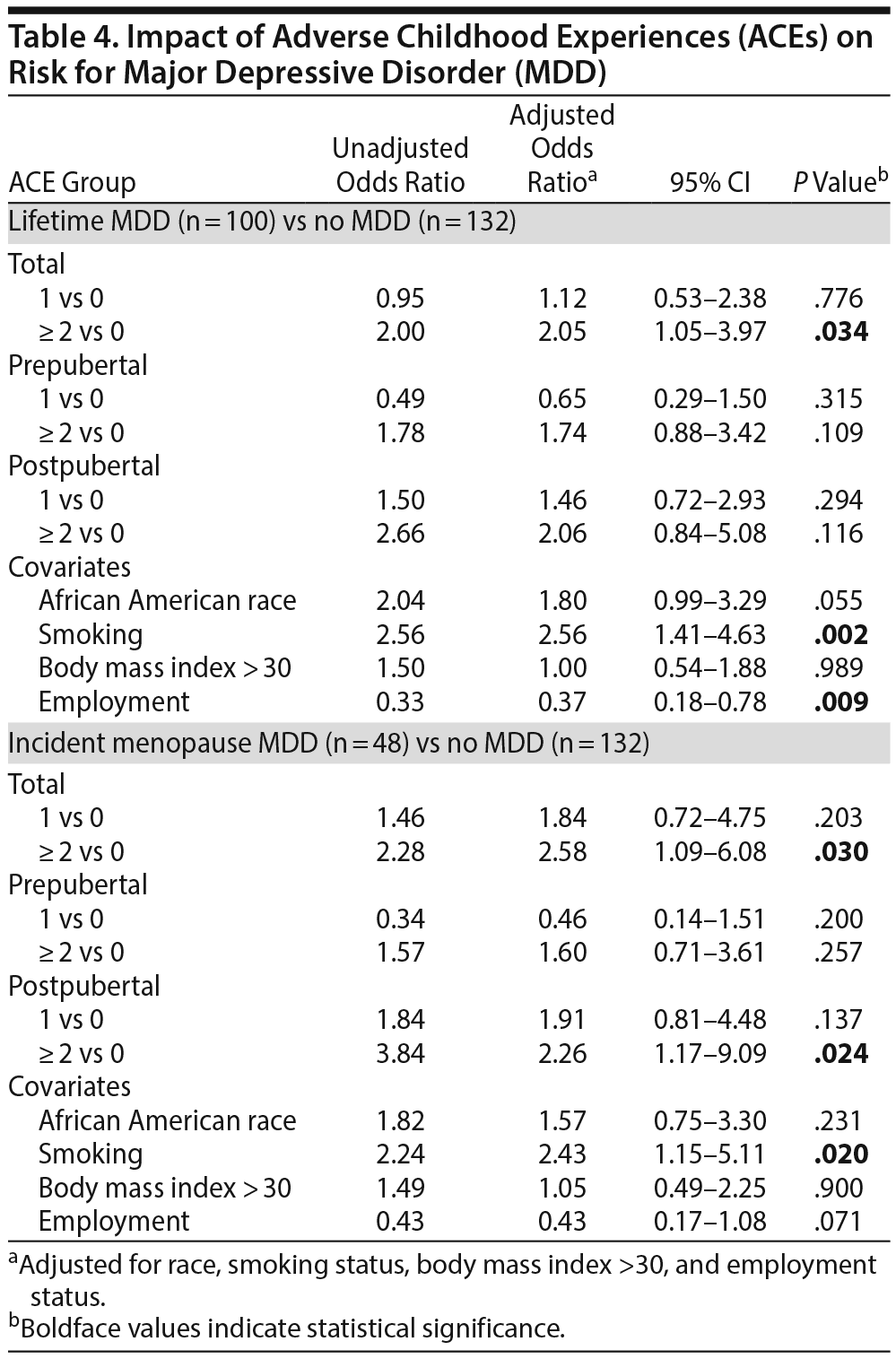
In models adjusted for race, smoking, BMI ≥ 30, and employment status (Table 4), those with a total ACE of ≥ 2 were significantly more likely to experience a lifetime MDD (adjusted odds ratio [aOR] = 2.05, P = .034) and incident menopause MDD (aOR = 2.58, P = .03) compared to those with 0 ACEs. Women who were smokers at study entry had an elevated risk for incident menopause MDD (aOR = 2.43, P = .02) and lifetime MDD (aOR = 2.56, P = .002). Employment was associated with reduced risk for lifetime MDD (aOR = 0.37, P = .009).
Pubertal Status and Risk for MDD
Again in adjusted models (Table 4), compared to those with no ACEs, women with ≥ 2 postpubertal ACEs were at greater risk of incident menopause MDD (aOR = 2.26, P = .02) but not lifetime MDD (P = .12). On the basis of preclinical evidence that some types and degrees of stress during development may induce resilience even in the face of future stressors,36,37 we examined the impact of varying number (0, 1, or ≥ 2) of prepubertal ACEs in the context of varying number (0, 1, or ≥ 2) of postpubertal ACEs on risk for lifetime MDD (Figure 1A) and incident menopause MDD (Figure 1B). Across the postpubertal ACE categories (x-axis), those reporting 1 (gray line) versus ≥ 2 (black line) prepubertal ACEs had significantly reduced MDD risk when there were no postpubertal ACEs (P = .034), with a trend toward significance (P = .054) observed for those with 1 prepubertal and 1 postpubertal ACE. The pattern was similar for incident MDD, although the relatively smaller sample size (n = 48 vs n = 100) was a limitation for statistical significance.
DISCUSSION
Early life adversity exerts a powerful influence on health outcomes across the life span. For women, the interplay between stress and reproductive hormonal shifts may be particularly relevant.11,43 Our findings add to the literature regarding the impact of childhood trauma and neglect15,16 and ACE history35 on lifetime risk for MDD. However, our novel finding that experiencing ≥ 2 total or postpubertal, but not prepubertal, ACEs is associated with increased risk for incident menopause MDD suggests that the hormonal milieu of the perimenopause may unmask risk for depression in women who experienced childhood adversity, particularly when the onset of adversity occurred in the context of postpubertal hormonal cyclicity. Our observation that postpubertal ACEs did not contribute significantly to risk for lifetime MDD may seem counter to the literature regarding history of childhood trauma and adversity and depression risk. It is important to note that a number of previous studies44-47 finding a relationship between childhood adversity and risk for lifetime MDD focused on maltreatment (abuse and/or neglect) or a specific type of trauma. Although age at onset of adversity has been considered, our focus on timing of events in relationship to puberty and impact of ACEs on MDD risk at menopause is unique.
Plausible explanations for this observation include the unique impact that early versus late childhood onset of early life stress imparts on the development and function of limbic and executive brain regions. Onset of early life stress in late childhood, defined as ages 8-17 years, is associated with greater reduction in anterior cingulate and insula cortex volumes than stress occurring earlier in life.15,16 Peak sensitivity to childhood maltreatment with respect to right amygdala volume has been previously observed in individuals whose experience of maltreatment occurred between ages 10 and 11 years.14 Postpubertal-onset early life stress occurs in the context of fluctuating estradiol, which itself alters neurochemistry and brain structure and function. Estradiol enhances enzymes critical to serotonin synthesis,22 monoamine oxidase inhibition,38 prefrontal dopaminergic tone,39 and neuronal dendrite density and morphology (reviewed by Shanmugan and Epperson11). Both preclinical and human studies17,43 suggest an interactive effect of estradiol and timing of stress onset across development with respect to neurochemistry and behavior.
Given the graded effect of ACEs on adverse health outcomes and preclinical studies36,37 demonstrating the capacity for exposure to early life stress to promote resilience, we were intrigued by the observation that the experience of 1 prepubertal ACE was associated with decreased risk for both lifetime and incident menopause MDD, even in the context of postpubertal ACEs (Table 4). Although they were significant only when considering the larger group who experienced lifetime MDD (Figure 1A; ie, inclusive of those with incident menopause MDD), these findings suggest that stress inoculation models should be explored in the human laboratory. While the findings are compelling, cautious interpretation is warranted given the relatively small sample size within specific strata defined by the prepubertal and postpubertal ACE categories.
In the CDC-sponsored ACE Study,31 the most commonly reported adverse experiences were household substance, physical, and sexual abuse. Emotional abuse posed the largest risk for lifetime or recent depression among women.35 Pubertal status was not reported and neither is it clear which ACE was the most common among those reporting only 1 type of adversity. In the present study, household substance abuse and separation/divorce were reported most frequently in those women who experienced only 1 prepubertal ACE. Whether our finding of relative resilience among the women with 1 prepubertal ACE would have been replicated had the 1 prepubertal ACE been physical, emotional, or sexual abuse could not be determined with this sample, but child abuse does not typically occur in isolation. Among women and men in the larger CDC study31 who experienced 1 ACE, 86.5% reported at least 1 other ACE and 38.5% reported 4 or more additional ACEs. For those individuals reporting childhood abuse or emotional neglect, the percentage reporting at least 1 additional ACE was even higher, at 98% and 93%, respectively.
The POAS is unique among the longitudinal studies of midlife women,6-8 as all participants were premenopausal, with regular menstrual cycles at the time of study enrollment, and standard instruments were administered yearly for the determination of MDD diagnosis. In addition, women underwent hormonal assessments and completed menstrual cycle calendars allowing for objective confirmation of menstrual cycle changes. Recruitment of all participants during the premenopause allowed us to examine the risk for incident MDD concurrent with the earliest changes in reproductive hormones leading to menstrual irregularity. While the prevalence of MDD in this study (43%) may appear high, it is important to remember that this proportion represents a cumulative incidence of MDD, as women were evaluated yearly. Epidemiologic studies1,6,8 reporting a 21% prevalence of lifetime MDD among women utilize a 1-time interview to determine past and current MDD. Moreover, a similar cumulative 13-year incidence of 39% was reported in the even larger longitudinal Study of Women’s Health Across the Nation.6 While we observed a greater percentage of African American women and obese women in the high ACE group, white women represented a greater proportion of those in the high postpubertal ACE group. These baseline differences, however, did not contribute significantly to risk for lifetime or incident MDD in analyses adjusted for race, BMI, smoking, and employment.
Although our findings are based on close to 3,000 observations among 243 women across 14 years, there are several important limitations to consider. A shortcoming of the ACE-Q is that it does not provide a granular assessment of the frequency and severity of given ACEs, such as sexual and physical abuse or the number of times an individual witnessed abuse against her mother. The benefit of the ACE-Q is that it provides a broader understanding of the impact of what the American Academy of Pediatrics refers to as “toxic stress” on adult health outcomes.48 We also chose a cutoff for low ACE of 0 or 1 and a cutoff for high ACE of ≥ 2 based on previous research35 examining ACE impact on MDD risk in women. In the current cohort, we conducted a sensitivity analysis for lifetime MDD (which includes incident menopause MDD) with respect to numbers of ACEs. Supporting our cutoff of ≥ 2 for high ACE (total, prepubertal, and postpubertal), we observed a significant increase in lifetime MDD risk between 1 and 2 ACEs for the prepubertal ACE score (P = .026) and a trend for the postpubertal ACE score (P = .06). The increase in MDD risk between 1 and 2 when considering total ACE score did not reach statistical significance (P > .05), perhaps due to the continued increase in risk between 2 and 6 total ACEs (Supplementary eFigure 1). As most of the ACEs endorsed by this cohort of women were reported to have started in the prepubertal window, we may have had insufficient power to fully determine the impact of postpubertal onset of ACEs alone on MDD risk. However, our observation that ≥ 2 postpubertal ACEs were associated with a significant increased risk for MDD in the smaller incident menopause MDD group than the larger lifetime MDD group would suggest that power is not the primary reason for the relative importance of postpubertal ACEs to incident MDD.
Other potential limitations include the reliance on recall of age at menarche and timing of adverse events occurring decades before. Some studies, but not all,49 suggest that recall for age at menarche is stable over time,50 and even 30 years later the correlation between recalled age at menarche and medical records is quite robust.51 The allowance of a 2-year window prior to the first menstrual period increases the likelihood that women were not actually postpubertal when they reported an ACE as occurring in the prepubertal window. A subset of 106 women from the current cohort have been interviewed twice 2 years apart to determine reliability of recall of age at menarche, age at which an ACE first began, and number of ACEs endorsed (data not shown). Recall of age at menarche was exact in 83% of women and within 1 year in 93%. With respect to total ACEs endorsed and timing of their onset, prepubertal ACE category switched from low to high in 4 individuals and from high to low in 1, a finding suggesting that, if anything, we have biased the outcome toward the null. We were also unable to examine the impact of hormone therapy by ACE group on risk for MDD, as relatively few women ( approximately 15%) in the POAS reported using hormone therapy, with the majority of these individuals reporting use at only 1 assessment. We did not examine the interaction between vasomotor symptoms and depression, as we previously reported that depression typically precedes onset of hot flashes.52
In summary, this study provides further evidence that childhood adversity exerts a powerful influence on MDD risk across the female life span and highlights the novel finding that risk is enhanced during the menopause transition, even among previously resilient women. Relatively low levels of adversity with onset prior to establishment of ovarian cyclicity may contribute to resilience in the face of postpubertal adversity, while postpubertal onset of adversity may enhance risk. Future research examining risk and resilience for psychopathology during adulthood after exposures to childhood adversity would benefit from considering the reproductive status of the child at the time exposures occur.
Submitted: January 12, 2016; accepted July 11, 2016.
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, no investigational information about pharmaceutical agents that is outside US Food and Drug Administration-approved labeling has been presented in this article.
Author contributions: All authors contributed to the collection and management of data, manuscript development, writing, and/or editing for clarity. The authors approve of the version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial disclosure: Dr Epperson serves as a consultant to Umecrine Mood, SAGE Therapeutics, and Asarina; acknowledges investments in Pfizer, Johnson & Johnson, Merck, Abbott, and AbbVie; and has received support for nonrelated research studies from SAGE and Shire. Dr Freeman has received research support from Forest. Drs Sammel, Bale, and Kim and Mss Conlin, Scalice, and Freeman have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: This research was supported by grants from the National Institute of Mental Health and Office of Research on Women’s Health (P50 MH099910), the National Institute on Drug Abuse (K24 DA030301 and R01 DA37289), and the National Institute on Aging (R01 AG048839 and R01 AG012745-15).
Role of the sponsor: The granting agencies had no role in the design, analysis, interpretation, or publication of this study.
Previous presentation: Poster presented at the 54th Annual Meeting of the American College of Neuropsychopharmacology; December 6-10, 2015; Hollywood, Florida.
Supplementary material: See accompanying pages.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry. 1994;51(1):8-19. PubMed doi:10.1001/archpsyc.1994.03950010008002
2. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475. PubMed doi:10.1176/appi.ajp.2012.11081302
3. Freeman EW, Sammel MD, Boorman DW, et al. Longitudinal pattern of depressive symptoms around natural menopause. JAMA Psychiatry. 2014;71(1):36-43. PubMed doi:10.1001/jamapsychiatry.2013.2819
4. Freeman EW, Sammel MD, Lin H, et al. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375-382. PubMed doi:10.1001/archpsyc.63.4.375
5. Freeman EW, Sammel MD, Liu L, et al. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61(1):62-70. PubMed doi:10.1001/archpsyc.61.1.62
6. Bromberger JT, Kravitz HM, Chang YF, et al. Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med. 2011;41(9):1879-1888. PubMed doi:10.1017/S003329171100016X
7. Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry. 2004;161(12):2238-2244. PubMed doi:10.1176/appi.ajp.161.12.2238
8. Cohen LS, Soares CN, Vitonis AF, et al. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385-390. PubMed doi:10.1001/archpsyc.63.4.385
9. Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35(3):320-330. PubMed doi:10.1016/j.yfrne.2014.05.004
10. Accortt EE, Freeman MP, Allen JJ. Women and major depressive disorder: clinical perspectives on causal pathways. J Womens Health (Larchmt). 2008;17(10):1583-1590. PubMed doi:10.1089/jwh.2007.0592
11. Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35(3):847-865. PubMed doi:10.1002/hbm.22218
12. Cisler JM, James GA, Tripathi S, et al. Differential functional connectivity within an emotion regulation neural network among individuals resilient and susceptible to the depressogenic effects of early life stress. Psychol Med. 2013;43(3):507-518. PubMed doi:10.1017/S0033291712001390
13. Hoeijmakers L, Lucassen PJ, Korosi A. The interplay of early-life stress, nutrition, and immune activation programs adult hippocampal structure and function. Front Mol Neurosci. 2015;7:103. PubMed doi:10.3389/fnmol.2014.00103
14. Pechtel P, Lyons-Ruth K, Anderson CM, et al. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 2014;97(97):236-244. PubMed doi:10.1016/j.neuroimage.2014.04.025
15. Teicher MH, Andersen SL, Polcari A, et al. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1-2):33-44. PubMed doi:10.1016/S0149-7634(03)00007-1
16. Baker LM, Williams LM, Korgaonkar MS, et al. Impact of early vs late childhood early life stress on brain morphometrics. Brain Imaging Behav. 2013;7(2):196-203. PubMed doi:10.1007/s11682-012-9215-y
17. Albert K, Pruessner J, Newhouse P. Estradiol levels modulate brain activity and negative responses to psychosocial stress across the menstrual cycle. Psychoneuroendocrinology. 2015;59:14-24. PubMed doi:10.1016/j.psyneuen.2015.04.022
18. Dumas JA, Albert KM, Naylor MR, et al. The effects of age and estrogen on stress responsivity in older women. Am J Geriatr Psychiatry. 2012;20(9):734-743. PubMed doi:10.1097/JGP.0b013e31825c0a14
19. Chou KL, Mackenzie CS, Liang K, et al. Three-year incidence and predictors of first-onset of DSM-IV mood, anxiety, and substance use disorders in older adults: results from Wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2011;72(2):144-155. PubMed doi:10.4088/JCP.09m05618gry
20. Marshall DF, Passarotti AM, Ryan KA, et al. Deficient inhibitory control as an outcome of childhood trauma. Psychiatry Res. 2016;235:7-12. PubMed doi:10.1016/j.psychres.2015.12.013
21. Asher J, Michopoulos V, Reding KM, et al. Social stress and the polymorphic region of the serotonin reuptake transporter gene modify oestradiol-induced changes on central monoamine concentrations in female rhesus monkeys. J Neuroendocrinol. 2013;25(4):321-328. PubMed doi:10.1111/jne.12009
22. Shively CA, Mirkes SJ, Lu NZ, et al. Soy and social stress affect serotonin neurotransmission in primates. Pharmacogenomics J. 2003;3(2):114-121. PubMed doi:10.1038/sj.tpj.6500166
23. Michopoulos V, Reding KM, Wilson ME, et al. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Horm Behav. 2012;62(4):389-399. PubMed doi:10.1016/j.yhbeh.2012.07.014
24. Reding K, Michopoulos V, Wallen K, et al. Social status modifies estradiol activation of sociosexual behavior in female rhesus monkeys. Horm Behav. 2012;62(5):612-620. PubMed doi:10.1016/j.yhbeh.2012.09.010
25. Howell BR, Sanchez MM. Understanding behavioral effects of early life stress using the reactive scope and allostatic load models. Dev Psychopathol. 2011;23(4):1001-1016. PubMed doi:10.1017/S0954579411000460
26. Kim DR, Bale TL, Epperson CN. Prenatal programming of mental illness: current understanding of relationship and mechanisms. Curr Psychiatry Rep. 2015;17(2):5. PubMed doi:10.1007/s11920-014-0546-9
27. Goel N, Bale TL. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J Neuroendocrinol. 2009;21(4):415-420. PubMed doi:10.1111/j.1365-2826.2009.01843.x
28. Hara Y, Waters EM, McEwen BS, et al. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol Rev. 2015;95(3):785-807. PubMed doi:10.1152/physrev.00036.2014
29. Morrison KE, Narasimhan S, Fein E, et al. Peripubertal stress with social support promotes resilience in the face of aging. Endocrinology. 2016;157(5):2002-2014. PubMed doi:10.1210/en.2015-1876
30. Dong M, Anda RF, Felitti VJ, et al. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse Negl. 2004;28(7):771-784. PubMed doi:10.1016/j.chiabu.2004.01.008
31. Felitti VJ, Anda RF, Nordenberg D, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245-258. PubMed doi:10.1016/S0749-3797(98)00017-8
32. Gracia CR, Sammel MD, Freeman EW, et al. Defining menopause status: creation of a new definition to identify the early changes of the menopausal transition. Menopause. 2005;12(2):128-135. PubMed doi:10.1097/00042192-200512020-00005
33. Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab. 2013;98(9):3829-3838. PubMed doi:10.1210/jc.2013-1808
34. Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW). Fertil Steril. 2001;76(5):874-878. PubMed doi:10.1016/S0015-0282(01)02909-0
35. Chapman DP, Whitfield CL, Felitti VJ, et al. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord. 2004;82(2):217-225. PubMed doi:10.1016/j.jad.2003.12.013
36. Lyons DM, Parker KJ, Katz M, et al. Developmental cascades linking stress inoculation, arousal regulation, and resilience. Front Behav Neurosci. 2009;3:32. PubMed doi:10.3389/neuro.08.032.2009
37. Russo SJ, Murrough JW, Han MH, et al. Neurobiology of resilience. Nat Neurosci. 2012;15(11):1475-1484. PubMed doi:10.1038/nn.3234
38. Spitzer RL, Williams JBW, Gibbon M, et al. Instruction Manual for the Structured Clinical Interview for DMS-III-R (SCID). New York, NY: Biometrics Research; 1988.
39. Spitzer RL, Williams JBW, Kroenke K, et al. Validity and utility of the PRIME-MD patient health questionnaire in assessment of 3000 obstetric-gynecologic patients: the PRIME-MD Patient Health Questionnaire Obstetrics-Gynecology Study. Am J Obstet Gynecol. 2000;183(3):759-769. PubMed doi:10.1067/mob.2000.106580
40. Spitzer RL, Kroenke K, Williams JBW; Patient Health Questionnaire Primary Care Study Group. Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study. JAMA. 1999;282(18):1737-1744. PubMed doi:10.1001/jama.282.18.1737
41. Moriarty AS, Gilbody S, McMillan D, et al. Screening and case finding for major depressive disorder using the Patient Health Questionnaire (PHQ-9): a meta-analysis. Gen Hosp Psychiatry. 2015;37(6):567-576. PubMed doi:10.1016/j.genhosppsych.2015.06.012
42. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385-401. doi:10.1177/014662167700100306
43. Shansky RM, Lipps J. Stress-induced cognitive dysfunction: hormone-neurotransmitter interactions in the prefrontal cortex. Front Hum Neurosci. 2013;7:123. PubMed doi:10.3389/fnhum.2013.00123
44. Schalinski I, Teicher MH, Nischk D, et al. Type and timing of adverse childhood experiences differentially affect severity of PTSD, dissociative and depressive symptoms in adult inpatients. BMC Psychiatry. 2016;16:295. PubMed doi:10.1186/s12888-016-1004-5
45. Klein DN, Glenn CR, Kosty DB, et al. Predictors of first lifetime onset of major depressive disorder in young adulthood. J Abnorm Psychol. 2013;122(1):1-6. PubMed doi:10.1037/a0029567
46. Gillespie CF, Bradley B, Mercer K, et al. Trauma exposure and stress-related disorders in inner city primary care patients. Gen Hosp Psychiatry. 2009;31(6):505-514. PubMed doi:10.1016/j.genhosppsych.2009.05.003
47. Arnow BA, Blasey CM, Hunkeler EM, et al. Does gender moderate the relationship between childhood maltreatment and adult depression? Child Maltreat. 2011;16(3):175-183. PubMed doi:10.1177/1077559511412067
48. Garner AS, Shonkoff JP; Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood, Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics. Early childhood adversity, toxic stress, and the role of the pediatrician: translating developmental science into lifelong health. Pediatrics. 2012;129(1):e224-e231. PubMed doi:10.1542/peds.2011-2662
49. Soliman A, Allen K, Lo AC, et al. Differences in reliability and reproductive history recall among women in North Africa. Int Electron J Health Educ. 2009;12(1):150-161. PubMed
50. Bosetti C, Tavani A, Negri E, et al. Reliability of data on medical conditions, menstrual and reproductive history provided by hospital controls. J Clin Epidemiol. 2001;54(9):902-906. PubMed doi:10.1016/S0895-4356(01)00362-6
51. Must A, Phillips SM, Naumova EN, et al. Recall of early menstrual history and menarcheal body size: after 30 years, how well do women remember? Am J Epidemiol. 2002;155(7):672-679. PubMed doi:10.1093/aje/155.7.672
52. Freeman EW, Sammel MD, Lin H, et al. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117(5):1095-1104. PubMed doi:10.1097/AOG.0b013e318214f0de
This PDF is free for all visitors!