The use of prescription psychostimulants during pregnancy has been increasing in recent years. One large and 3 small studies have recently examined the risk of major congenital malformations following the use of methylphenidate and amphetamines during the first trimester of pregnancy. The broad findings of these studies are that first trimester gestational exposure to methylphenidate or amphetamines is associated with an increased risk of major congenital malformations but the associations are no longer statistically significant after adjusting analyses for confounding variables; that first trimester exposure to amphetamines is not associated with an increased risk of cardiovascular malformations; and that first trimester exposure to methylphenidate may increase the risk of cardiovascular malformations. A closer look at the data on the last-mentioned finding, however, suggests that the statistical significance of the finding is in doubt and that even if the finding is statistically significant, it is probably not clinically significant. Furthermore, all the findings emerged from observational studies that cannot exclude confounding by indication and other sources of confounding. A reasonable conclusion, therefore, is that there is no evidence, at present, to suggest that methylphenidate and amphetamines are teratogenic. Nevertheless, because absence of evidence of risk is not evidence of absence of risk, the benefits of continuing psychostimulant medication during pregnancy should be weighed against potential risks in an individualized and shared decision-making process.


ABSTRACT
The use of prescription psychostimulants during pregnancy has been increasing in recent years. One large and 3 small studies have recently examined the risk of major congenital malformations following the use of methylphenidate and amphetamines during the first trimester of pregnancy. The broad findings of these studies are that first trimester gestational exposure to methylphenidate or amphetamines is associated with an increased risk of major congenital malformations but the associations are no longer statistically significant after adjusting analyses for confounding variables; that first trimester exposure to amphetamines is not associated with an increased risk of cardiovascular malformations; and that first trimester exposure to methylphenidate may increase the risk of cardiovascular malformations. A closer look at the data on the last-mentioned finding, however, suggests that the statistical significance of the finding is in doubt and that even if the finding is statistically significant, it is probably not clinically significant. Furthermore, all the findings emerged from observational studies that cannot exclude confounding by indication and other sources of confounding. A reasonable conclusion, therefore, is that there is no evidence, at present, to suggest that methylphenidate and amphetamines are teratogenic. Nevertheless, because absence of evidence of risk is not evidence of absence of risk, the benefits of continuing psychostimulant medication during pregnancy should be weighed against potential risks in an individualized and shared decision-making process.
J Clin Psychiatry 2018;79(1):18f12108
To cite: Andrade C. Risk of major congenital malformations associated with the use of methylphenidate or amphetamines in pregnancy. J Clin Psychiatry. 2018;79(1):18f12108.
To share: https://doi.org/10.4088/JCP.18f12108
© Copyright 2018 Physicians Postgraduate Press, Inc.
Attention-deficit/
hyperactivity disorder (ADHD) develops in childhood and persists in adults in about 40%-50% of cases1; in one study, the pooled prevalence in adults was estimated to be 2.5%.2 Treatment of adult ADHD improves functioning3,4 and quality of life5 and reduces the risk of adverse outcomes such as accidents6 and problems related to substance abuse.7 Women on treatment for ADHD may therefore wish to continue their medications during pregnancy in order to continue to experience the benefits of treatment. In this context, a Danish study showed that, between 2003 and 2010, the use of ADHD medication during pregnancy increased from 5 per 100,000 person-years to 533 per 100,000 person-years.8 A North American study found that use in pregnancy increased from 0.2% during 1997-1998 to 1.3% during 2013.9
A question therefore arises: How safe is the use of ADHD medication during pregnancy? Four recent studies have examined the risk of major congenital malformations after first trimester gestational exposure to psychostimulants, specifically methylphenidate and amphetamines. These studies are briefly reviewed.
Methylphenidate, Amphetamines,
and Major Congenital Malformations
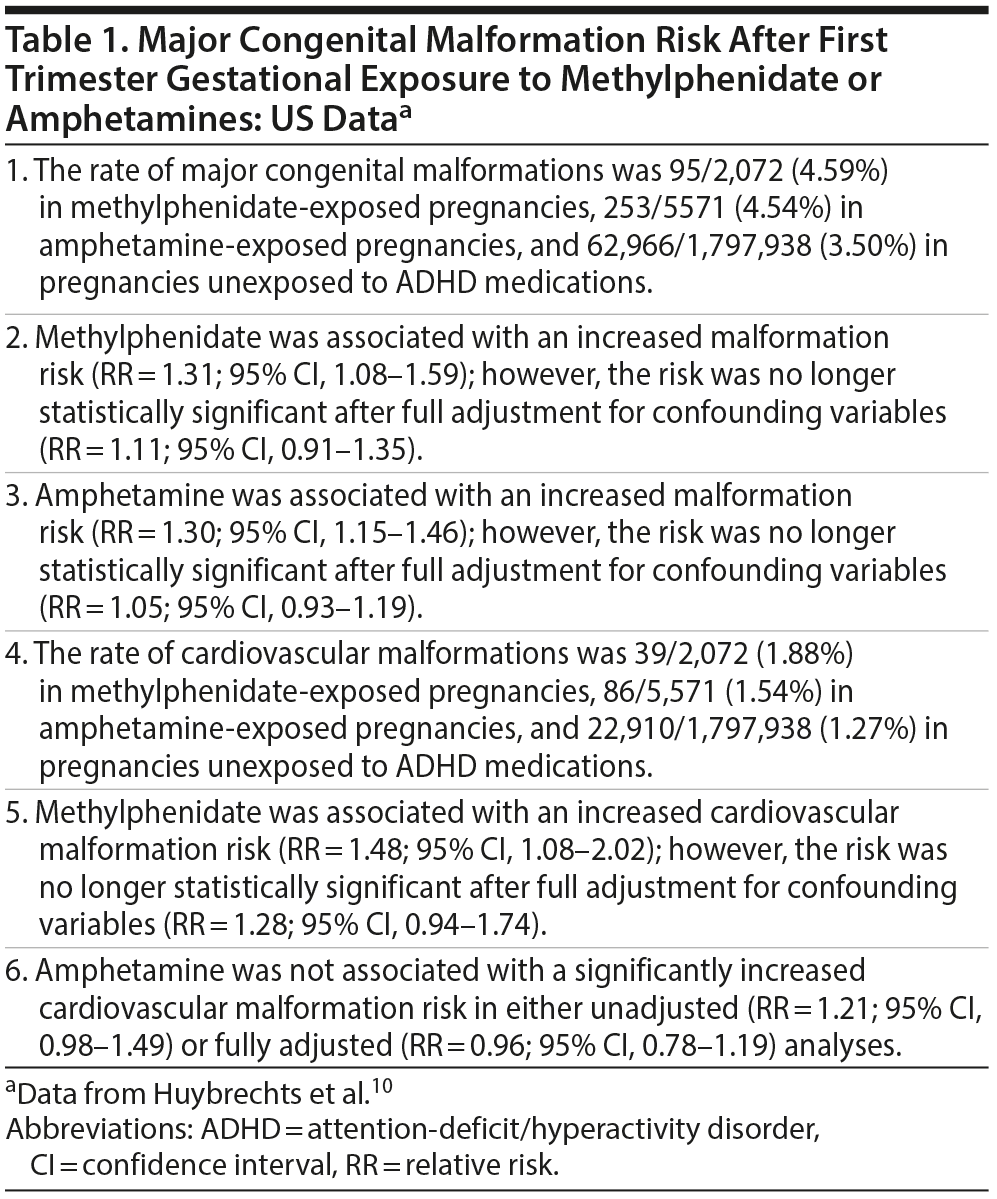
Huybrechts et al10 used 2000-2013 data from the Medicaid program in the United States to identify 2,072 women who had filled a prescription for methylphenidate and 5,571 women who had filled a prescription for an amphetamine (amphetamine or dextroamphetamine) during the first 90 days of pregnancy. A comparison group was identified comprising 1,797,938 women who had had no exposure to ADHD medications from 3 months before their last menstrual period to the end of the first trimester. Analyses were adjusted for a wide range of potential confounding variables, including sociodemographic variables, obstetric variables, medical and psychiatric illness variables, and medication variables. Sensitivity analyses and exploratory analyses reexamined the data in other ways, such as by increasing the adjustment for confounding, improving the likelihood of correct classification of medication exposure, and redefining outcomes.
Important findings from the US data are presented in Table 1. In summary, both methylphenidate and amphetamines were associated with an increased major malformation risk, and methylphenidate was also associated with an increased risk of cardiovascular malformations. However, none of these findings remained statistically significant in analyses that adjusted for confounding variables. The conclusions remained similar in sensitivity and exploratory analyses.
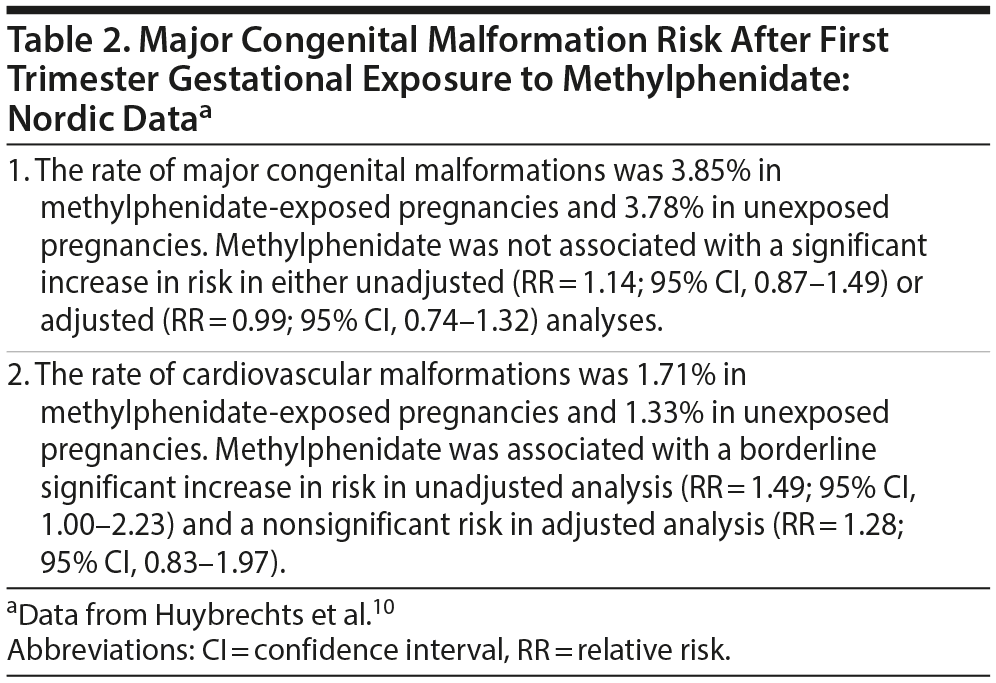
Huybrechts et al10 replicated their analyses using registry-derived data from Denmark (2005-2012), Finland (1996-2010), Iceland (2003-2012), Norway (2005-2012), and Sweden (2006-2013). The Nordic dataset included 1,402 pregnancies exposed to methylphenidate but only 99 to amphetamines; there were 2,557,001 unexposed controls. Statistical analysis was performed only for methylphenidate exposure. Risks for the Nordic countries were estimated and combined using meta-analysis.
Important findings from the Nordic data are presented in Table 2. In summary, methylphenidate exposure was not associated with an increased malformation risk in either unadjusted or adjusted analyses. Whereas it was associated with a borderline significant risk of cardiovascular malformations in unadjusted analysis, the risk was not significant in adjusted analysis.
When the US and Nordic estimates were combined using meta-analysis, first trimester gestational exposure to methylphenidate was not associated with a significant risk of major malformations (relative risk [RR] = 1.07; 95% confidence interval [CI], 0.91-1.26). However, there was a borderline risk of cardiovascular malformations (RR = 1.28; 95% CI, 1.00-1.64).
Other Studies Examining Psychostimulant-Related Malformation Risk
Potteg×¥rd et al11 examined Danish registry data from 2005 to 2012; these data would probably have been substantially represented in the Nordic substudy of Huybrechts et al.10 There were 222 women who had redeemed a prescription for methylphenidate in a time window extending from 14 days before the start of the first trimester of pregnancy to the end of the first trimester. These women were propensity matched in a 1:10 ratio with 2,220 unexposed women, based on important variables such as maternal age, smoking status, body mass index, years of education, calendar year of completion of pregnancy, and concurrent use of antipsychotic, antidepressant, anxiolytic, and nonsteroidal antiinflammatory drugs. First trimester methylphenidate exposure was not associated with an increase in major malformations (point prevalence ratio = 0.8; 95% CI, 0.3-1.8) or cardiac malformations (point prevalence ratio = 0.9; 95% CI, 0.2-3.0). Similar findings were obtained in sensitivity analyses that employed other definitions of exposure and that considered previous users of methylphenidate as the unexposed comparison cohort.
In a study12 that examined 1996-2013 data from teratology information centers in Israel, Germany, Canada, and the United Kingdom, methylphenidate-exposed pregnancies (n = 382) were compared with pregnancies exposed to nonteratogenic substances (n = 382) after matching for maternal age, gestational age, and year at initial contact. In 89.5% of cases, exposure was recorded during the first trimester. There was no significant difference in the rate of major congenital malformations in methylphenidate-exposed cases vs unexposed controls (3.2% vs 3.6%, respectively); this held true even after exclusion of genetic or cytogenetic anomalies and after limiting the period of methylphenidate exposure to weeks 4-13 after the last menstrual period (malformation rate, 2.4% vs 3.4% in cases vs controls, respectively). The rate of cardiovascular malformations was 0.8% in each group.
One other small study of 208 exposures to methylphenidate identified 5 cases of major congenital malformations, all cardiovascular defects; however, the malformation risk was not statistically significant (RR = 1.81; 95% CI, 0.59-4.21).13 These data were drawn from Swedish registries (1996-2011) and would therefore have been partially represented in the study by Huybrechts et al.10
Other Gestational Outcomes Associated With Psychostimulant Exposure: Recent Data
In one small study,12 the miscarriage rate was significantly higher in methylphenidate-exposed pregnancies vs matched controls (14.1% vs 7.1%, respectively). There was no significant difference in the risk of other gestational outcomes, including gestational age at delivery and birth weight. This increased risk of miscarriage has been observed after gestational exposure to ADHD medications as a class.8,14
Another small study15 found an increased risk of hypertensive disorders of pregnancy following late gestational exposure to psychostimulant drugs. However, a large population-based cohort study16 found that pregnancies exposed to amphetamines or methylphenidate were not consistently associated with risks of preeclampsia, placental abruption, smallness for gestational age, and preterm birth in different analyses; the authors concluded that, on the basis of their findings, women with significant ADHD should not be counseled to stop their ADHD treatment.
Summary of the Findings
The broad findings of the reviewed studies may be summarized as follows:
- First trimester gestational exposure to methylphenidate or amphetamines is associated with an increased risk of major congenital malformations. However, such psychostimulant exposure is probably only a marker for the increased risk and not a driver of the increased risk because the association is no longer statistically significant after adjusting for potential confounding variables.
- First trimester gestational exposure to amphetamines is not associated with an increased risk of cardiovascular malformations; however, exposure to methylphenidate appears to be associated with a 28% increased risk of these malformations.
- Gestational exposure to ADHD medications may be associated with an increased risk of miscarriage and with an increased risk of other adverse gestational outcomes, as well.
Critical Appraisal: General Comments
All the reviewed studies were observational in nature, and, best attempts to adjust for confounding variables, notwithstanding, none could exclude the possibility of unknown, unmeasured, and insufficiently well-measured sources of confounding as an explanation for the significant associations identified. The problem of confounding in observational studies in pregnancy has been discussed in detail in earlier articles in this column and elsewhere17-19; whereas the use of preconception exposure controls, sibling controls, and paternal exposure controls may offer better insights, these research designs are also not infallible.20-22 The bottom line is that no matter how ingenious the research design is and no matter how careful the adjustment for confounding is, observational studies cannot establish that an exposure causes an outcome.
Critical Appraisal: Specific Comments
The finding that methylphenidate exposure increased the risk of cardiovascular malformations requires special consideration partly because the finding was described in a large and well-conducted study10 that was published in a leading journal and partly because the finding was endorsed in an accompanying commentary23 in the journal; therefore, the finding is likely to receive high visibility.
Assuming that the finding is valid, a 28% increase in cardiovascular malformations (by methylphenidate) will increase the risk from 1.30% (the approximate base rate in the US and the Nordic data; Tables 1 and 2) to 1.66%; that is, by 0.36%. This translates to a number needed to harm of 278,24 indicating that the clinical significance of the finding is very small.
However, is the finding valid? A large number of analyses were presented in the study,10 no primary outcome was stated, and no correction for a type I error was considered. Had the significance of this finding been corrected for multiple hypothesis testing, statistical significance would no longer have been declared. Most important of all, the finding was actually not statistically significant; the z value for the test for overall effect (Figure 2 in the published paper10) was 1.93; a threshold value of 1.96 is required for statistical significance with a P value of .05. Neither the authors of the study10 nor the commentator23 observed or acknowledged the lack of statistical significance when they discussed the finding.
Take-Home Message
The available data do not suggest that first trimester exposure to methylphenidate or amphetamines increases the risk of major congenital malformations in general or cardiovascular malformations in particular. Nevertheless, because absence of evidence of risk is not evidence of absence of risk, women who wish to continue to experience the benefits of psychostimulant medications during pregnancy should weigh the benefits of continued medication use against the theoretical risks of medication exposure. Decision-making should be individualized and shared.
Published online: January 23, 2018.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Sibley MH, Mitchell JT, Becker SP. Method of adult diagnosis influences estimated persistence of childhood ADHD: a systematic review of longitudinal studies. Lancet Psychiatry. 2016;3(12):1157-1165. PubMed doi:10.1016/S2215-0366(16)30190-0
2. Simon V, Czobor P, Bálint S, et al. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194(3):204-211. PubMed doi:10.1192/bjp.bp.107.048827
3. Sarkis E. Addressing attention-deficit/hyperactivity disorder in the workplace. Postgrad Med. 2014;126(5):25-30. PubMed doi:10.3810/pgm.2014.09.2797
4. Fredriksen M, Peleikis DE. Long-term pharmacotherapy of adults with attention deficit hyperactivity disorder: a literature review and clinical study. Basic Clin Pharmacol Toxicol. 2016;118(1):23-31. PubMed doi:10.1111/bcpt.12477
5. Agarwal R, Goldenberg M, Perry R, et al. The quality of life of adults with attention deficit hyperactivity disorder: a systematic review. Innov Clin Neurosci. 2012;9(5-6):10-21. PubMed
6. Chang Z, Quinn PD, Hur K, et al. Association between medication use for attention-deficit/hyperactivity disorder and risk of motor vehicle crashes. JAMA Psychiatry. 2017;74(6):597-603. PubMed doi:10.1001/jamapsychiatry.2017.0659
7. Quinn PD, Chang Z, Hur K, et al. ADHD medication and substance-related problems. Am J Psychiatry. 2017;174(9):877-885. PubMed doi:10.1176/appi.ajp.2017.16060686
8. Haervig KB, Mortensen LH, Hansen AV, et al. Use of ADHD medication during pregnancy from 1999 to 2010: a Danish register-based study. Pharmacoepidemiol Drug Saf. 2014;23(5):526-533. PubMed doi:10.1002/pds.3600
9. Louik C, Kerr S, Kelley KE, et al. Increasing use of ADHD medications in pregnancy. Pharmacoepidemiol Drug Saf. 2015;24(2):218-220. PubMed doi:10.1002/pds.3742
10. Huybrechts KF, Bröms G, Christensen LB, et al. Association between methylphenidate and amphetamine use in pregnancy and risk of congenital malformations: a cohort study from the International Pregnancy Safety Study Consortium [published online ahead of print December 13, 2017]. JAMA Psychiatry. PubMed doi:10.1001/jamapsychiatry.2017.3644
11. .Potteg×¥rd A, Hallas J, Andersen JT, et al. First-trimester exposure to methylphenidate: a population-based cohort study. J Clin Psychiatry. 2014;75(1):e88-e93. PubMed doi:10.4088/JCP.13m08708
12. Diav-Citrin O, Shechtman S, Arnon J, et al. Methylphenidate in pregnancy: a multicenter, prospective, comparative, observational study. J Clin Psychiatry. 2016;77(9):1176-1181. PubMed doi:10.4088/JCP.15m10083
13. Kפllén B, Borg N, Reis M. The use of central nervous system active drugs during pregnancy. Pharmaceuticals (Basel). 2013;6(10):1221-1286. PubMed doi:10.3390/ph6101221
14. Bro SP, Kjaersgaard MI, Parner ET, et al. Adverse pregnancy outcomes after exposure to methylphenidate or atomoxetine during pregnancy. Clin Epidemiol. 2015;7:139-147. PubMed doi:10.2147/CLEP.S72906
15. Newport DJ, Hostetter AL, Juul SH, et al. Prenatal psychostimulant and antidepressant exposure and risk of hypertensive disorders of pregnancy. J Clin Psychiatry. 2016;77(11):1538-1545. PubMed doi:10.4088/JCP.15m10506
16. Cohen JM, Hernández-D×az S, Bateman BT, et al. Placental complications associated with psychostimulant use in pregnancy. Obstet Gynecol. 2017;130(6):1192-1201. PubMed doi:10.1097/AOG.0000000000002362
17. Andrade C. Antidepressant use in pregnancy and risk of autism spectrum disorders: a critical examination of the evidence. J Clin Psychiatry. 2013;74(9):940-941. PubMed doi:10.4088/JCP.13ac08607
18. Andrade C. Adverse outcomes following serotonin reuptake inhibitor exposure during pregnancy. J Clin Psychiatry. 2016;77(2):e199-e200. PubMed doi:10.4088/JCP.15com10041
19. Andrade C. Propensity score matching in nonrandomized studies: a concept simply explained using antidepressant treatment during pregnancy as an example. J Clin Psychiatry. 2017;78(2):e162-e165. PubMed doi:10.4088/JCP.17f11446
20. Andrade C. Offspring outcomes in studies of antidepressant-treated pregnancies depend on the choice of control group. J Clin Psychiatry. 2017;78(3):e294-e297. PubMed doi:10.4088/JCP.17f11509
21. Andrade C. Antidepressant exposure during pregnancy and risk of autism in the offspring, 1: meta-review of meta-analyses. J Clin Psychiatry. 2017;78(8):e1047-e1051. PubMed doi:10.4088/JCP.17f11903
22. Andrade C. Antidepressant exposure during pregnancy and risk of autism in the offspring, 2: do the new studies add anything new? J Clin Psychiatry. 2017;78(8):e1052-e1056. PubMed doi:10.4088/JCP.17f11916
23. Cooper WO. Shedding light on the risks of methylphenidate and amphetamine in pregnancy [published online ahead of print December 13, 2017]. JAMA Psychiatry. PubMed doi:10.1001/jamapsychiatry.2017.3882
24. Andrade C. The numbers needed to treat and harm (NNT, NNH) statistics: what they tell us and what they do not. J Clin Psychiatry. 2015;76(3):e330-e333. PubMed doi:10.4088/JCP.15f09870
This PDF is free for all visitors!