Abstract
Objective: To compare efficacy and safety of single daily dosing (Single-DD) vs multiple daily dosing (Multiple-DD) regimens of psychotropic drugs, the authors conducted a systematic review and meta-analysis.
Data Sources: A systematic literature search of MEDLINE and Embase was conducted with keywords related to dosing regimens and psychotropic drugs (last search: December 30, 2019)
Study Selection: Randomized controlled trials comparing clinical outcomes between Single-DD and Multiple-DD of the same formulation of the same psychotropic drugs in patients with psychiatric disorders were included.
Data Extraction: Data on study discontinuation, psychopathology, and treatment-emergent adverse events (TEAEs) were extracted.
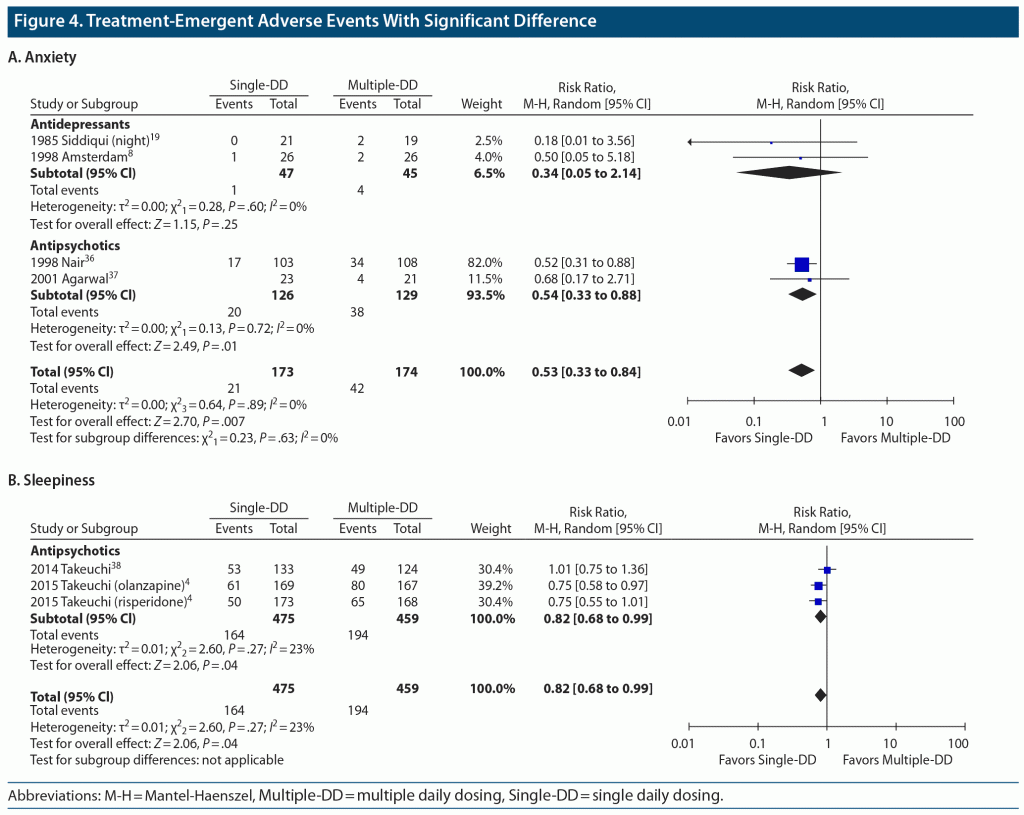
Results: A total of 32 studies with 34 paired comparisons involving 3,142 patients met the eligibility criteria and were included in the meta-analysis. Various types of psychotropic drugs were examined: antidepressants (22 comparisons), antipsychotics (7 comparisons), benzodiazepines (2 comparisons), mood stabilizers (2 comparisons), and antidepressant-benzodiazepine combination (1 comparison). There was no significant difference in study discontinuation due to all causes (30 comparisons, N = 2,883, risk ratio [RR] = 1.01, 95% CI = 0.94 to 1.09, P = .77), lack of efficacy (22 comparisons, N = 2,307, RR = 1.06, 95% CI = 0.84 to 1.33, P = .62), or adverse events (25 comparisons, N = 2,571, RR = 0.93, 95% CI = 0.75 to 1.14, P = .47) between the Single-DD and Multiple-DD groups. No significant difference was found in changes in psychopathology (8 comparisons, N = 1,337, standardized mean difference = 0.00, 95% CI = −0.11 to 0.11, P = .99) between the 2 groups. These results were also true for any type of psychotropic drugs. In terms of TEAEs, however, there were significant differences in anxiety (4 comparisons, N = 347, RR = 0.53, 95% CI = 0.33 to 0.84, P = .007) and sleepiness (3 comparisons, N = 934, RR = 0.82, 95% CI = 0.68 to 0.99, P = .04) in favor of the Single-DD group.
Conclusions: The findings suggest Single-DD can be clinically adopted regardless of type of psychotropic drugs in patients with psychiatric disorders in general.
J Clin Psychiatry 2021;82(2):20r13503
To cite: Kikuchi Y, Shimomura Y, Suzuki T, et al. Single versus multiple daily dosing regimens of psychotropic drugs for psychiatric disorders: a systematic review and meta-analysis. J Clin Psychiatry. 2021;82(2):20r13503.
To share: https://doi.org/10.4088/JCP.20r13503
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Neuropsychiatry, Keio University School of Medicine, Tokyo, Japan
bDepartment of Psychiatry, Komagino Hospital, Tokyo, Japan
cDepartment of Psychiatry, Yokohama Municipal Citizen’s Hospital, Kanagawa, Japan
dDepartment of Neuropsychiatry, University of Yamanashi Faculty of Medicine, Yamanashi, Japan
*Corresponding author: Hiroyoshi Takeuchi, MD, PhD, Department of Neuropsychiatry, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo, 160-8582, Japan ([email protected]).
How the dose of psychotropic drug is fragmented within a day (ie, daily dosing regimen of psychotropic drug) is traditionally determined based on its peripheral elimination half-life. In general, product monographs and package inserts recommend that psychotropic drugs with a < 24-hour half-life are to be administered in a divided dosing regimen (ie, multiple daily dosing [Multiple-DD] regimen) and those with a ≥ 24-hour half-life in a once-daily dosing regimen (ie, single daily dosing [Single-DD] regimen). However, real-world clinical practice indicates that this simple principle is not always followed. For instance, a cross-sectional survey showed that clozapine was prescribed in a once-daily dosing regimen in approximately 75% of patients in the United States and Canada, although the product monograph in both countries states that clozapine should be administered twice or 3 times a day.1
Maintaining good adherence to medications is critically important to maximize their therapeutic effects,2 and a simple drug regimen is advantageous from this viewpoint.3 The field of psychiatry is no exception; some past randomized controlled trials (RCTs) endeavored to compare efficacy and safety between Single-DD and Multiple-DD of various types of psychotropic drugs including antipsychotics,4–6 antidepressants,7–9 benzodiazepines,10–12 antiepileptics,13 and lithium.14 To our knowledge, there have been only 2 meta-analyses that focused on this topic,15,16 suggesting that Single-DD is not inferior to Multiple-DD in terms of efficacy and acceptability; however, these meta-analyses only included RCTs of antidepressants and were published more than 15 years ago. To address this clinically important question, we conducted a systematic review and meta-analysis of RCTs comparing Single-DD and Multiple-DD of all types of psychotropic drugs for psychiatric disorders.
METHODS
Literature Search and Study Selection
We conducted a systematic literature search in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement17 to identify RCTs comparing Single-DD and Multiple-DD regimens of all types of psychotropic drugs for psychiatric disorders (last search: December 30, 2019). To this end, MEDLINE and Embase were searched with the following keywords: (((once[ti] OR twice[ti] OR thrice[ti]) AND (daily[ti] OR day[ti])) OR ((dosing[ti] OR dose*[ti] OR dosage*[ti]) AND (regimen*[ti] OR schedule*[ti] OR single[ti] OR multiple[ti] OR divided[ti] OR split[ti] OR qd[ti] OR quaque die[ti] OR qhs[ti] OR quaque hora somni[ti] OR bid[ti] OR bis in die[ti] OR tid[ti] OR ter in die[ti]))) AND (psychotropic* OR antipsychotic* OR antidepressant* OR lithium OR divalproex OR valpro* OR lamotrigine OR carbamazepine OR mood stabilizer* OR benzodiazepine* OR antianxi* OR hypnotic*). We also searched CENTRAL using the same keywords to check if we had missed any other literature and conducted a hand search. Two authors (Y.K. and Y.S.) independently selected studies that met the following inclusion criteria: an RCT comparing clinical outcome(s) between Single-DD and Multiple-DD of the same formulation of the same psychotropic drugs(s) in patients with psychiatric disorder(s). Literature reported in languages other than English was excluded. Any disagreements about the study selection were resolved by consensus with 2 authors (Y.K. and Y.S.) under the supervision of the senior author (H.T.).
Two authors (Y.K. and Y.S.) independently assessed risk of bias for the selected studies according to the Cochrane Handbook for Systematic Reviews of Interventions (available at http://handbook.cochrane.org). Any disagreements about the assessment were resolved by consensus with 2 authors (Y.K. and Y.S.) under the supervision of the senior author (H.T.).
Data Extraction
Two authors (Y.K. and Y.S.) independently extracted the following clinical outcome data in both Single-DD and Multiple-DD groups from the selected studies: (1) the number of patients who discontinued the study due to all causes, lack of efficacy, and adverse events; (2) the mean ± SD of changes in scores on primary psychopathology scales from baseline to endpoint; and (3) the number of patients who experienced treatment-emergent adverse events (TEAEs) that were reported in ≥ 3 out of the identified comparisons. Because there were variants in expression of TEAEs, we combined those that were considered a synonymous term. Two studies included 2 Single-DD regimens (ie, at night and in the morning)18,19; we used the data in the night group. Also, 1 study included 2 Multiple-DD regimens (ie, twice and 3 times daily dosing)20; we used the data in the twice daily dosing group. We employed WebPlotDigitizer (available at https://automeris.io/WebPlotDigitizer/) if the included studies provided only graphs for the data. Any disagreements about the data extraction were resolved by consensus with 2 authors (Y.K. and Y.S.) under the supervision of the senior author (H.T.). If the selected studies provided insufficient data, we contacted the corresponding authors to obtain additional information necessary for the meta-analyses.
Data Analysis
We performed meta-analyses using Review Manager (RevMan) version 5.3 (The Nordic Cochrane Center, The Cochrane Collaboration; Copenhagen, Denmark; 2014). Outcome data were combined and compared between the Single-DD and Multiple-DD groups. For dichotomous and continuous outcomes, pooled estimates of risk ratios (RRs) and standardized mean differences (SMDs), respectively, were calculated with 2-sided 95% confidence intervals (CIs) using a random-effects model. Study heterogeneities were quantified using I2 statistic with I2 ≥ 50% indicating significant heterogeneity. All effect sizes with a P < .05 were considered significant. Two authors (Y.K. and Y.S.) independently performed the meta-analyses. Any disagreements about the meta-analyses were resolved by consensus with 2 authors (Y.K. and Y.S.) under the supervision of the senior author (H.T.).
As sensitivity analyses, we separately analyzed the following sets of studies: (1a) double-blind studies; (1b) non–double-blind studies; (2a) studies adopting intention-to-treat analysis; (2b) studies adopting modified intention-to-treat analysis; (2c) studies adopting completer analysis; (3a) studies examining psychotropic drugs with peripheral elimination half-life < 24 hours; (3b) studies examining psychotropic drugs with peripheral elimination half-life ≥ 24 hours; (4a) studies examining psychotropic drugs with the description in the product monograph that the drug should be administered once daily (ie, Single-DD); and (4b) studies examining psychotropic drugs without the description in the product monograph that the drug should be administered once daily (ie, Single-DD).
We further repeatedly performed these analyses in studies examining once vs twice or once vs 3 times daily dosing. One study included twice daily and 3 times daily dosing groups20; we used the data in the twice daily and 3 times daily dosing groups for the main analyses and the sensitivity analyses for studies examining once vs 3 times daily dosing, respectively.
RESULTS
Included Studies
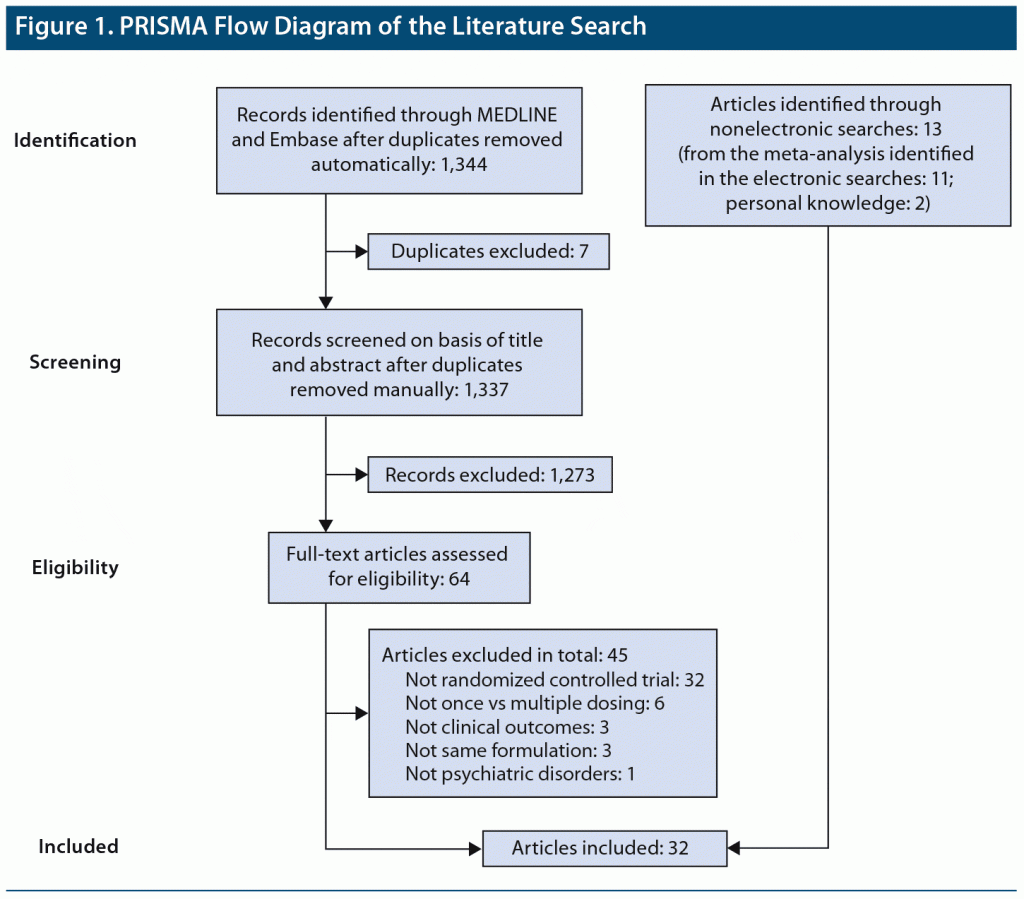
A total of 32 studies published from 1974 to 2015 involving 3,142 patients (N = 1,598 and N = 1,544 for the Single-DD and Multiple-DD groups, respectively) that met our inclusion criteria were identified (Figure 1).4–14,18–38 Only 7 studies were published after 2000. As 2 studies included 2 separate comparisons, a total of 34 comparisons were included in the meta-analysis. The characteristics of the eligible studies are summarized in Table 1. Among the 32 studies, 23, 1, and 5 were conducted in a double-blind, rater-blind, and open-label fashion, respectively. Various types of psychotropic drugs were examined: antidepressants (22 comparisons), antipsychotics (7 comparisons), benzodiazepines (2 comparisons), mood stabilizers (2 comparisons), and antidepressant-benzodiazepine combination (1 comparison). The Multiple-DD group included 3 types of regimens: twice daily dosing (17 comparisons), 3 times daily dosing (16 comparisons), and 4 times daily dosing (1 comparison). The authors of 5 studies provided additional data.4,6,8,14,38 The dose was fixed in 19 comparisons (ie, same doses for both groups), while the dose was flexible in 9 comparisons; the mean dose at the endpoint was lower and higher in the Single-DD group than the Multiple-DD group in 8 and 1 comparisons, respectively.
The results of risk of bias assessment are described in Supplementary Figure 1. The risks of random sequence generation and of allocation concealment were unclear in all of the studies. The risk of incomplete outcome data was high in general, because older studies adopted completer analysis.
Study Discontinuation
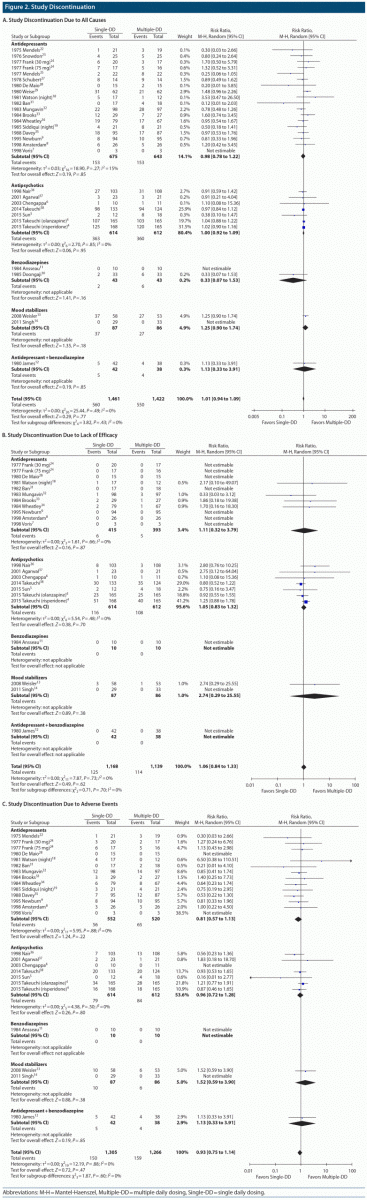
There was no significant difference in study discontinuation due to all causes (30 comparisons, N = 2,883, RR = 1.01, 95% CI = 0.94 to 1.09, P = .77), lack of efficacy (22 comparisons, N = 2,307, RR = 1.06, 95% CI = 0.84 to 1.33, P = .62), or adverse events (25 comparisons, N = 2,571, RR = 0.93, 95% CI = 0.75 to 1.14, P = .47) between the Single-DD and Multiple-DD groups of all psychotropic drugs (Figure 2). Moreover, no significant difference in any study discontinuation was found between the 2 groups in any subgroup of antidepressants, antipsychotics, benzodiazepines, mood stabilizers, and antidepressant-benzodiazepine combination.
Psychopathology
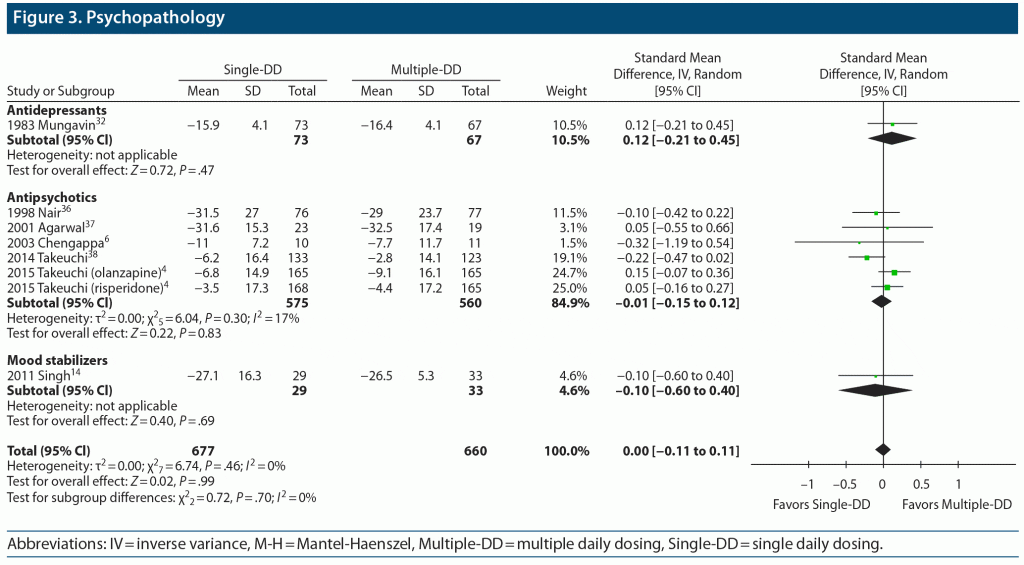
No significant difference was found in score changes on psychopathology scales (8 comparisons, N = 1,337, SMD = 0.00, 95% CI = −0.11 to 0.11, P = .99) between the Single-DD and Multiple-DD groups (Figure 3). There was no significant difference between the 2 groups in any subgroup of antidepressants, antipsychotics, and mood stabilizers, although caution is necessary as data were relatively scarce for psychopathology, because older studies frequently failed to provide standard deviation or standard error.
Treatment-Emergent Adverse Events
A total of 35 types of TEAEs were included in the meta-analysis. There were significant differences between the Single-DD and Multiple-DD groups in anxiety (4 comparisons, N = 347, RR = 0.53, 95% CI = 0.33 to 0.84, P = .007) and sleepiness (3 comparisons, N = 934, RR = 0.82, 95% CI = 0.68 to 0.99, P = .04), both in favor of the Single-DD group (Figure 4). The same results were found in the subgroup of antipsychotics.
Sensitivity Analyses
While there were no significant differences in any study discontinuation or psychopathology between the Single-DD and Multiple-DD groups in any sensitivity analyses (Supplementary Table 1), some significant differences in TEAEs were found between the 2 groups in some sensitivity analyses (Supplementary Table 2). Overall, anxiety and sleepiness were favorable in the Single-DD group, while a couple of items including dizziness and drowsiness were in favor of the Multiple-DD group in some sensitivity analyses.
DISCUSSION
The current meta-analysis revealed no significant differences in all-cause study discontinuation, discontinuation due to lack of efficacy as well as adverse events, or changes in psychopathology between Single-DD and Multiple-DD regimens of both all and individual types of psychotropic drugs. In terms of TEAEs, however, there were significant differences in anxiety and sleepiness in favor of Single-DD regimen. The findings corroborate the previous meta-analyses focusing on antidepressants,15,16 but the advantage of our meta-analysis is that all types of psychotropic drugs were included.
Although dosing interval of a psychotropic drug is generally determined according to its peripheral elimination half-life, our meta-analysis found no superiority for Multiple-DD over Single-DD, regardless of the half-lives of the compounds. The dosing frequency is associated with medication adherence and is reasonably a topic of scrutiny; a systematic review showed that less frequent dosing is plausibly related to better medication adherence in chronic psychiatric diseases.41 In addition, a meta-analysis found that patients receiving Single-DD were more adherent than those receiving Multiple-DD in chronic diseases,42 and another meta-analysis of 4 RCTs indicated that Single-DD was associated with a lower risk of nonadherence than Multiple-DD in chronic cardiovascular disease.43 Although Single-DD may facilitate adherence to psychotropic drugs, thereby improving long-term outcomes in chronic conditions, the current meta-analysis was not able to address this clinically important question. In the current meta-analysis, only 6 included RCTs measured medication adherence as a clinical outcome4,6,13,29,35,38; 5 studies reported no significant difference between the Single-DD and Multiple-DD groups, and 1 study found superiority in the Single-DD group. Nevertheless, because these 6 studies did not provide sufficient data, we were not able to perform a meta-analysis. Moreover, medication adherence was variably assessed. Further RCTs are urgently needed to examine the effects of Multiple-DD vs Single-DD of psychotropic drugs on medication adherence as well as on long-term clinical consequences including psychopathology, functioning, and subjective well-being.
The finding of no difference in efficacy between Single-DD and Multiple-DD of psychotropic drugs may be supported by neuroimaging studies. In terms of antipsychotics, for instance, a recent systematic review of studies using positron emission tomography (PET) or single-photon emission computed tomography indicated that pharmacokinetic attenuation of antipsychotics was generally slower at the central level than the peripheral level.44 As for antidepressants, 2 studies using PET reported that duloxetine, escitalopram, and sertraline showed a sustained time-course of serotonin transporter occupancy compared to the plasma concentrations.45,46 Given that the PET studies have been consistent in showing that pharmacokinetics of at least some psychotropic drugs are substantially slower centrally than peripherally, drugs that affect the central nervous system may be administered once daily irrespective of the peripheral elimination half-life of the compound. Nonetheless, further investigations are necessary on this issue, and due caution is necessary because there are no neuroimaging studies examining other psychotropic drugs such as benzodiazepines on this topic, possibly due to a lack of good tracers.
Contrary to the findings on efficacy, the current meta-analysis indicated that Single-DD of psychotropic drugs was found to be superior to Multiple-DD in terms of anxiety and sleepiness. It is not clear why Single-DD was associated with less anxiety, although 1 included study speculated that higher peak plasma drug concentrations in Single-DD than Multiple-DD contributed to a greater degree of amelioration of anxiety.36 The superiority of Single-DD for sleepiness may be due to a more sedative effect during daytime in Multiple-DD. Indeed, 1 study reported that oral risperidone caused sedation more frequently a few hours after administration than at 24 hours,47 and another study reported that intramuscular olanzapine caused more sedation 2 hours after administration than at 24 hours.48 If a patient is taking psychotropic drug(s) in Multiple-DD and is suffering from sleepiness and/or anxiety, switching to Single-DD would be a reasonable treatment strategy.
There are several limitations to be noted. First, the data on psychopathology and individual TEAEs were available for only 8 and up to 5 comparisons, respectively, which may have resulted in insufficient statistical power. Second, the current meta-analysis was associated with a high attrition bias, because the vast majority of the included studies were conducted in 1970s and 1980s, adopted the completer analysis method, and were associated with a high attrition rate; half of the studies were judged to have a high risk for incomplete outcome data. As 1970s and 1980s studies included in the meta-analysis examined relatively older antidepressants and benzodiazepines, the findings may not be generalizable to more recent psychotropic agents that are currently more widely utilized. Third, our meta-analysis did not cover all types of psychotropic drugs or psychiatric disorders; for example, no RCTs examining psychostimulants or antidementia drugs were found through our systematic literature search. Fourth, approximately two thirds of the studies did not provide such information, and we were unable to know if these studies examined efficacy of initiating a new drug in Single-DD vs Multiple-DD, switching Multiple-DD to Single-DD for an ongoing drug vs continuing Multiple-DD, or switching Single-DD to Multiple-DD for an ongoing drug vs continuing Single-DD. Fifth, the information on concomitant drugs that could have influenced the results was not sufficiently provided: no concomitant drugs (1 comparison), no psychotropic concomitant drugs (2 comparisons), benzodiazepines and antiparkinsonians allowed (13 comparisons), any drugs allowed (4 comparisons), and no information (14 comparisons). Sixth, no studies reported factors that could affect drug metabolism, such as smoking and metabolizer status. Finally, as previously stated, actual medication adherence has rarely been addressed, and long-term clinical consequences of Single-DD vs Multiple-DD are largely unknown. Further RCTs with various types of psychotropic drugs for diverse psychiatric disorders are needed to confirm the present findings.
In conclusion, the current meta-analysis showed no difference in effectiveness or efficacy between Single-DD and Multiple-DD regimens of psychotropic drugs, regardless of the type of drugs, in patients with psychiatric disorders. Nonetheless, given the superiority of Single-DD over Multiple-DD for tolerability and given its simplicity, Single-DD warrants serious clinical consideration, in particular for patients who suffer anxiety and/or sleepiness or are at risk of suboptimal medication adherence.
Submitted: June 13, 2020; accepted August 26, 2020.
Published online: February 23, 2021.
Potential conflicts of interest: Dr Suzuki has received manuscript or speaker’s fees from Astellas, Eisai, Eli Lilly, Elsevier Japan, Janssen, Kyowa, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, MSD, Novartis, Otsuka, Shionogi, Sumitomo Dainippon Pharma, Tsumura, Wiley Japan, and Yoshitomiyakuhin and research grants from Eisai, Mochida, Meiji Seika Pharma, and Shionogi. Dr Uchida has received grants from Eisai, Meiji Seika Pharma, Otsuka, and Sumitomo Dainippon Pharma; speaker’s fees from Eli Lilly, Meiji Seika Pharma, MSD, Otsuka, Pfizer, Sumitomo Dainippon, and Yoshitomiyakuhin; and advisory panel fees from Sumitomo Dainippon Pharma. Dr Mimura has received speaker’s fees from Daiichi Sankyo, Eisai, Eli Lilly, Fujifilm RI Pharma, Janssen, Mochida, MSD, Nippon Chemipher, Novartis, Ono, Otsuka, Pfizer, Sumitomo Dainippon Pharma, Takeda, Tsumura, and Yoshitomiyakuhin and research grants from Daiichi Sankyo, Eisai, Mitsubishi Tanabe Pharma, Pfizer, Shionogi, Takeda, and Tsumura. Dr Takeuchi has received speaker’s fees from Kyowa, Janssen, Meiji Seika Pharma, Mochida, Otsuka, Sumitomo Dainippon Pharma, and Yoshitomiyakuhin. Drs Kikuchi and Shimomura report no financial relationships with commercial interests.
Funding/support: This work was partially supported by Japan Society for the Promotion of Science (JSPS) KAKENHI grant number JP18K15492.
Role of the sponsor: The funding source had no role in study design, statistical analysis or interpretation of findings, or manuscript preparation or submission for publication.
Previous presentation: This work was presented in part at the 6th Asian College of Neuropsychopharmacology; October 13, 2019; Fukuoka, Japan.
Acknowledgments: The following individuals provided unpublished data: K. N. Roy Chengappa, MD (University of Pittsburgh School of Medicine); Jay D. Amsterdam, MD (University of Pennsylvania); and Lokesh Kumar Singh, MD (All India Institute of Medical Sciences).
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- This meta-analysis included 32 randomized controlled trials comparing clinical outcomes between single and multiple daily dosing regimens of the same formulation of the same psychotropic drugs in patients with psychiatric disorders.
- No significant differences were found in study discontinuation or psychopathology between single and multiple daily dosing regimens, while there were significant differences in anxiety and sleepiness in favor of a single daily dosing regimen.
- A single daily dosing regimen can be a viable option regardless of psychotropic types in patients with psychiatric disorders in general.
References (48)

- Takeuchi H, Powell V, Geisler S, et al. Clozapine administration in clinical practice: once-daily versus divided dosing. Acta Psychiatr Scand. 2016;134(3):234–240. PubMed CrossRef NLM
- Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–226. PubMed CrossRef NLM
- Ryan R, Santesso N, Lowe D, et al. Interventions to improve safe and effective medicines use by consumers: an overview of systematic reviews. Cochrane Database Syst Rev. 2014;(4):CD007768. PubMed NLM
- Takeuchi H, Fervaha G, Lee J, et al. Effectiveness of different dosing regimens of risperidone and olanzapine in schizophrenia. Eur Neuropsychopharmacol. 2015;25(3):295–302. PubMed CrossRef NLM
- Sun X, Hamer R, McEvoy J. Asenapine once daily versus twice daily: impact on patient acceptance in a randomized, open-label, 14-day clinical trial. J Clin Psychiatry. 2015;76(7):992–993. PubMed CrossRef NLM
- Chengappa KN, Parepally H, Brar JS, et al. A random-assignment, double-blind, clinical trial of once- vs twice-daily administration of quetiapine fumarate in patients with schizophrenia or schizoaffective disorder: a pilot study. Can J Psychiatry. 2003;48(3):187–194. PubMed CrossRef NLM
- Voris JC, Shaurette GN, Sebastian PS, et al. Nefazodone: single versus twice daily dose. Pharmacotherapy. 1998;18(2):379–380. PubMed PubMed NLM
- Amsterdam JD, Hooper MB, Amchin J. Once- versus twice-daily venlafaxine therapy in major depression: a randomized, double-blind study. J Clin Psychiatry. 1998;59(5):236–240. PubMed CrossRef NLM
- Newburn G, Edwards R, Thomas H, et al. A comparison of the efficacy and tolerability of moclobemide given as a single daily dose or in three divided doses per day for the treatment of patients with a major depressive episode (DSM-III-R). J Clin Psychopharmacol. 1995;15(suppl 2):10S–15S. PubMed CrossRef NLM
- Doongaji DR, Sheth AS, Apte JS, et al. A comparative study of single and multiple doses of clobazam vs diazepam in anxiety neurosis. Current Therapeutic Research. 1985;37(3):398–405.
- Ansseau M, Doumont A, von Frenckell R, et al. Duration of benzodiazepine clinical activity: lack of direct relationship with plasma half-life: a comparison of single vs divided dosage schedules of prazepam. Psychopharmacology (Berl). 1984;84(3):293–298. PubMed CrossRef NLM
- James RT, Dean BC. A comparison of a single night-time and a divided daily dosage regimen of a chlordiazepoxide/amitriptyline combination. Curr Med Res Opin. 1980;6(8):573–575. PubMed CrossRef NLM
- Weisler RH, Kalali AH, Cutler AJ, et al. Efficacy and safety of once- versus twice-daily carbamazepine extended-release capsules for the treatment of manic symptoms in patients with bipolar I disorder. Psychiatry (Edgmont). 2008;5(3):35–48. PubMed NLM
- Singh LK, Nizamie SH, Akhtar S, et al. Improving tolerability of lithium with a once-daily dosing schedule. Am J Ther. 2011;18(4):288–291. PubMed CrossRef NLM
- Yildiz A, Pauler DK, Sachs GS. Rates of study completion with single versus split daily dosing of antidepressants: a meta-analysis. J Affect Disord. 2004;78(2):157–162. PubMed CrossRef NLM
- Yýldýz A, Sachs GS. Administration of antidepressants: single versus split dosing: a meta-analysis. J Affect Disord. 2001;66(2–3):199–206. PubMed NLM
- Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339(7716):b2535. PubMed NLM
- Watson JM, Tiplady B. Zimelidine: comparison of different dosage regimes in general practice. Acta Psychiatr Scand suppl. 1981;290:464–470. PubMed CrossRef NLM
- Siddiqui UA, Chakravarti SK, Jesinger DK. The tolerance and antidepressive activity of fluvoxamine as a single dose compared to a twice daily dose. Curr Med Res Opin. 1985;9(10):681–690. PubMed CrossRef NLM
- Wheatley D. Trazodone in depression. Int Pharmacopsychiatry. 1980;15(4):240–246. PubMed CrossRef NLM
- Pearce JB, Rees WL. A double-blind comparison of three times daily and single night dosage of the tricyclic anti-depressant dothiepin. J Int Med Res. 1974;2(1):12–19. CrossRef
- Mendels J, Schless A. A controlled comparison of doxepin h.s. and doxepin q.i.d. J Clin Pharmacol. 1975;15(7):534–539. PubMed CrossRef NLM
- Snowdon JA. Double-blind comparison of 3-times daily and single night dosage of amitriptyline, with special reference to side-effects. Curr Med Res Opin. 1976;4(6):381–387. PubMed CrossRef NLM
- Frank P. Comparisons of various clomipramine (Anafranil) dosage regimes. J Int Med Res. 1977;5(1 suppl):11–15. PubMed NLM
- Mendels J, Schless AP. Antidepressant effects of desipramine adminstered in two dosage schedules. Dis Nerv Syst. 1977;38(4):249–251. PubMed NLM
- Montgomery S, McAuley R, Montgomery DB. Relationship between mianserin plasma levels and antidepressant effect in a double-blind trial comparing a single night-time and divided daily dose regimens. Br J Clin Pharmacol. 1978;5(suppl 1):71S–76S. PubMed NLM
- Schubert DS, Miller SI. Are divided doses of tricyclic antidepressants necessary? J Nerv Ment Dis. 1978;166(12):875–877. PubMed CrossRef NLM
- De Maio D, Sesso M, Levi Minzi A, et al. Evaluation of the clinical efficacy of single daily doses of antidepressants. Prog Neuropsychopharmacol. 1980;4(6):607–612. PubMed CrossRef NLM
- Weise CC, Stein MK, Pereira-Ogan J, et al. Amitriptyline once daily vs three times daily in depressed outpatients. Arch Gen Psychiatry. 1980;37(5):555–560. PubMed CrossRef NLM
- Sharma SD. A comparison of a divided and a single dose regime of dothiepin and its therapeutic efficacy. Indian J Psychiatry. 1981;23(4):355–359. PubMed NLM
- Ban TA, Fujimori M, Petrie WM, et al. Systematic studies with amoxapine, a new antidepressant. Int Pharmacopsychiatry. 1982;17(1):18–27. PubMed CrossRef NLM
- Mungavin JM, Ankier SI. Comparison of two dosage regimens of trazodone in the treatment of depression. Clin Trials J. 1983;20(4):181–188.
- Brooks D, Prothero W, Bouras N, et al. Trazodone: a comparison of single night-time and divided daily dosage regimens. Psychopharmacology (Berl). 1984;84(1):1–4. PubMed CrossRef NLM
- Wheatley D. Trazodone: alternative dose regimens and sleep. Pharmatherapeutica. 1984;3(9):607–612 PubMed NLM
- Davey A. A comparison of two oral dosage regimens of 150 mg trazodone in the treatment of depression in general practice. Psychopharmacology (Berl). 1988;95(suppl):S25–S30. PubMed CrossRef NLM
- Nair NP; The Risperidone Study Group. Therapeutic equivalence of risperidone given once daily and twice daily in patients with schizophrenia. J Clin Psychopharmacol. 1998;18(2):103–110. PubMed CrossRef NLM
- Agarwal V, Chadda RK. Once daily risperidone in treatment of schizophrenia. Indian J Psychiatry. 2001;43(1):32–35. PubMed NLM
- Takeuchi H, Fervaha G, Uchida H, et al. Impact of once- versus twice-daily perphenazine dosing on clinical outcomes: an analysis of the CATIE data. J Clin Psychiatry. 2014;75(5):506–511. PubMed CrossRef NLM
- Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1–02):9–62. PubMed NLM
- Heel RC, Morley PA, Brogden RN, et al. Zimelidine: a review of its pharmacological properties and therapeutic efficacy in depressive illness. Drugs. 1982;24(3):169–206. PubMed NLM
- Medic G, Higashi K, Littlewood KJ, et al. Dosing frequency and adherence in chronic psychiatric disease: systematic review and meta-analysis. Neuropsychiatr Dis Treat. 2013;9:119–131. PubMed CrossRef NLM
- Coleman CI, Limone B, Sobieraj DM, et al. Dosing frequency and medication adherence in chronic disease. J Manag Care Pharm. 2012;18(7):527–539. PubMed NLM
- Caldeira D, Vaz-Carneiro A, Costa J. The impact of dosing frequency on medication adherence in chronic cardiovascular disease: systematic review and meta-analysis. Rev Port Cardiol. 2014;33(7–8):431–437. PubMed CrossRef NLM
- Kurose S, Mimura Y, Uchida H, et al. Dissociation in pharmacokinetic attenuation between central dopamine D2 receptor occupancy and peripheral blood concentration of antipsychotics: a systematic review. J Clin Psychiatry. 2020;81(5):19r13113. PubMed CrossRef NLM
- Arakawa R, Tateno A, Kim W, et al. Time-course of serotonin transporter occupancy by single dose of three SSRIs in human brain: a positron emission tomography study with [(11)C]DASB. Psychiatry Res Neuroimaging. 2016;251:1–6. PubMed CrossRef NLM
- Takano A, Suzuki K, Kosaka J, et al. A dose-finding study of duloxetine based on serotonin transporter occupancy. Psychopharmacology (Berl). 2006;185(3):395–399. PubMed CrossRef NLM
- Chung YC, Park TW, Yang JC, et al. Cognitive effects of a single dose of atypical antipsychotics in healthy volunteers compared with placebo or haloperidol. J Clin Psychopharmacol. 2012;32(6):778–786. PubMed CrossRef NLM
- Kittipeerachon M, Chaichan W. Intramuscular olanzapine versus intramuscular aripiprazole for the treatment of agitation in patients with schizophrenia: a pragmatic double-blind randomized trial. Schizophr Res. 2016;176(2–3):231–238. PubMed CrossRef NLM
Please sign in or purchase this PDF for $40.