See reply by Andrade and article by Andrade
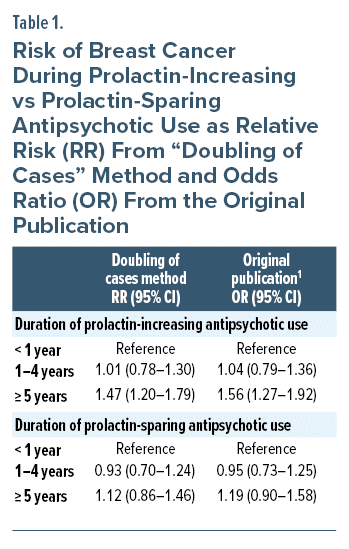
To the Editor: We thank Dr Andrade for reading and commenting on our article1 and calculation of the difference in breast cancer risk between prolactin-increasing and prolactin-sparing antipsychotic use.2 Following his suggestion, we have now calculated relative risk (RR) for the main results of our study, with a doubling of cases method as is recommended for the special case of nested case control studies.3 These RRs are well in line with odds ratios (ORs) reported in our original study as can be expected when the outcome is rare, such as incident breast cancer examined in our study.
With the doubling of cases method, we calculated an RR = 1.47 (95% confidence interval, 1.20–1.79) for ≥ 5 years of prolactin-increasing antipsychotic use, versus < 1 year use) and RR = 1.12 (0.86–1.46) for ≥ 5 years of prolactin-sparing antipsychotic use, versus < 1 year use). These RRs are very close to the ORs reported in the original study, namely OR = 1.56 (1.27–1.92) and OR = 1.19 (0.90–1.58), respectively (Table 1). When dividing the RR of prolactin-increasing antipsychotic use by the RR of prolactin-sparing antipsychotic use (RR 1.47/1.12), we get a value of 1.3125, ie, 31.3% increase in the risk of breast cancer. This is very similar to the value of 37% increase in odds in the discussion section of our original study.1
Of note, unfortunately, we cannot make direct comparisons between these exposure categories, as naturally the same persons have been prescribed both prolactin-sparing and prolactin-increasing antipsychotics during long follow-up times and, thus, exposure categories are not mutually exclusive, yet reciprocally controlled for each other in our analyses.
Article Information
Published Online: January 22, 2024. https://doi.org/10.4088/JCP.23lr15135
© 2024 Physicians Postgraduate Press, Inc.
J Clin Psychiatry 2024;85(1):23lr15135
To Cite: Taipale H, Solmi M, Correll CU, et al. Relative risk of breast cancer associated with prolactin-increasing antipsychotic use. J Clin Psychiatry. 2024;85(1):23lr15135
Author Affiliations: Department of Forensic Psychiatry, University of Eastern Finland, Niuvanniemi Hospital, Kuopio (Taipale, Tiihonen); Department of Clinical Neuroscience, Karolinska Institutet, Stockholm, Sweden (Taipale, Tiihonen); Department of Psychiatry, University of Ottawa, Ontario, Canada (Solmi); Regional Centre for the Treatment of Eating Disorders and On Track: The Champlain First Episode Psychosis Program, Department of Mental Health, The Ottawa Hospital, Ontario, Canada (Solmi); Ottawa Hospital Research Institute (OHRI), Clinical Epidemiology Program University of Ottawa, Ontario, Canada (Solmi); Department of Child and Adolescent Psychiatry, Charité Universitätsmedizin, Berlin, Germany (Solmi, Correll); The Zucker Hillside Hospital, Department of Psychiatry, Glen Oaks, New York (Correll); Department of Psychiatry and Molecular Medicine, Donald and Barbara School of Medicine at Hofstra/Northwell, Hempstead, New York (Correll); Center for Psychiatry Research, Stockholm City Council, Sweden (Tiihonen); Neuroscience Center, University of Helsinki, Finland (Tiihonen).
Corresponding Author: Heidi Taipale, PhD, University of Eastern Finland, Department of Forensic Psychiatry, Niuvankuja 65, Kuopio 70240 ([email protected]).
Relevant Financial Relationships: Drs Taipale and Tiihonen have participated in research projects funded by grants from Janssen-Cilag and Eli Lilly to their employing institution. Dr Taipale reports personal fees from Gedeon Richter, Janssen, Lundbeck, and Otsuka. Dr Tiihonen has been a consultant and/or advisor to and/or has received honoraria from HLS Therapeutics, Janssen-Cilag, Orion, Otsuka, and WebMed Global. Dr Solmi has received honoraria from/has been a consultant for AbbVie, Angelini, Lundbeck, and Otsuka. Dr Correll has been a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Adock Ingram, Alkermes, Allergan, Angelini, Aristo, Biogen, Boehringer-Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Denovo, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Jamjoom Pharma, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Neurelis, Newron, Noven, Novo Nordisk, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Sage, Seqirus, SK Life Science, Sumitomo Pharma America, Sunovion, Sun Pharma, Supernus, Takeda, Teva, Tolmar, Vertex, and Viatris; provided expert testimony for Janssen and Otsuka; served on data safety monitoring boards for Compass Pathways, Denovo, Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva; has received grant support from Janssen and Takeda; received royalties from UpToDate; and is a stock option holder of Cardio Diagnostics, Kuleon Biosciences, LB Pharma, Mindpax, and Quantic.
Funding/Support: None.
References (3)

- Taipale H, Solmi M, Lähteenvuo M, et al. Antipsychotic use and risk of breast cancer in women with schizophrenia: a nationwide nested case-control study in Finland. Lancet Psychiatry. 2021;8(10):883–891. PubMed CrossRef
- Andrade C. Understanding misunderstandings about the relative risk and odds ratio. J Clin Psychiatry. 2023;84(3):23f14943. PubMed CrossRef
- Ning Y, Lam A, Reilly M. Estimating risk ratio from any standard epidemiological design by doubling the cases. BMC Med Res Methodol. 2022;22(1):157. PubMed CrossRef
This PDF is free for all visitors!