Developing Concepts in Negative Symptoms: Primary vs Secondary and Apathy vs Expression
Negative symptoms in schizophrenia, such as blunted affect, alogia, asociality, anhedonia, and avolition, remain challenging to treat in many patients, but new concepts may lead to a better understanding of the definition and treatment of these symptoms. The most widely used rating scales for negative symptoms (the Scale for the Assessment of Negative Symptoms and the Positive and Negative Syndrome Scale) were developed in the 1980s, but more recent findings, such as insight into aspects of anhedonia, have led to the creation of new rating scales (the Clinical Assessment Interview for Negative Symptoms and the Brief Negative Symptom Scale). Clinicians should differentiate between primary and secondary negative symptoms in order to select the best treatment option. Secondary negative symptoms may be caused by comorbid conditions, psychotic symptoms, medication side effects, and substance abuse. On most rating scales, negative symptoms have also been found to load onto 1 of 2 domains, apathy/anhedonia/asociality or diminished expression (blunted affect and alogia). This distinction may facilitate the development of new treatments.
(J Clin Psychiatry 2014;75[suppl 1]:3-7)
From the Department of Psychiatry and Behavioral Science, University of Nevada School of Medicine, Reno.
This article is derived from the planning teleconference series “Measurement-Based Strategies to Assess and Manage Schizophrenia,” which was held in August 2013 and supported by an educational grant from Genentech.
Dr Kirkpatrick is a consultant for Genentech/Roche and has received other financial or material support from ProPhase.
Corresponding author: Brian Kirkpatrick, MD, MSPH, Psychiatry & Behavioral Science, University of Nevada School of Medicine, 1664 N. Virginia St, Mail Stop 0354, Reno, NV 89557-0354 ([email protected]).
doi:10.4088/JCP.13049su1c.01
© Copyright 2014 Physicians Postgraduate Press, Inc.
Negative symptoms are an absence of or significant decrease in a behavior or psychological function, but this definition could include aphasia from a stroke or sensory neglect. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for schizophrenia include negative symptoms, which are defined as affective flattening, alogia, or avolition.1
In schizophrenia, negative symptoms are thought to be part of the illness, at least in some patients. Avolition in schizophrenia was noted as early as the mid-1800s, with Griesinger’s description of patients with psychosis and “a loss of will.”2 Later, Kraepelin spoke of “a weakening of . . . the mainsprings of volition.”3 Although many patients with schizophrenia do not have avolition, 1 or more negative symptoms were found in over 50% of outpatients with antipsychotic-treated schizophrenia spectrum disorders.4 Recognizing the need for improved assessment and treatment of negative symptoms in schizophrenia, researchers developed negative symptom rating scales.
Negative Symptom Scales
In treatment trials, negative symptoms are defined according to rating scales. Those used most commonly in treatment studies are the Scale for the Assessment of Negative Symptoms (SANS)5 and the Positive and Negative Syndrome Scale (PANSS).6 There is some disagreement across these scales as to the negative symptoms that have been included. In addition, because the SANS and the PANSS were developed in the 1980s, they do not reflect the latest research on negative symptoms.
The National Institute of Mental Health (NIMH) sponsored the consensus Development Conference on Negative Symptoms in 2005. Among the consensus points that came from the conference was agreement that negative symptoms include (1) blunted affect, (2) alogia, (3) asociality, (4) anhedonia, and (5) avolition.7 Other symptoms may be included in the future as more research is conducted.

- Differentiate between primary and secondary negative symptoms by ruling out factors that can cause secondary negative symptoms, such as anxiety/depression, severe positive psychotic symptoms, medication side effects, and substance abuse.
- Treat the causes of secondary negative symptoms whenever possible.
- Watch for new medication research using improved study designs and rating scales.
Another outcome from the NIMH Consensus Development Conference was a recommendation to develop new rating scales that included their agreed-upon domains and to clarify the current understanding of those domains. Eventually, 2 new rating scales were developed by conference participants: the Clinical Assessment Interview for Negative Symptoms (CAINS)8 and the Brief Negative Symptom Scale (BNSS).9 Both of these 13-item instruments incorporate the recommendations of the Consensus Development Conference and measure the 5 agreed-upon symptoms.9 Because the BNSS was intended to be used in treatment trials and can be administered in less time than the CAINS, it is likely to be used more widely for an institution’s clinical purposes, such as the documentation of efficacy.
The 13 items on the BNSS are categorized under 6 subscales, which measure the 5 NIMH conference domains7 plus a lack of normal distress. The first subscale, anhedonia, measures 3 aspects of the loss of experience of pleasure: (1) the intensity of pleasure during activities, (2) the frequency of pleasure during activities, and (3) the intensity of expected pleasure from future activities (how much a patient enjoys thinking about an upcoming activity). The asociality and avolition subscales measure behavior and the internal experience of these symptoms, which may be discrepant. For example, a patient may want to pursue a goal (in which case the patient does not have internal avolition) but may have trouble sustaining the effort required to reach the goal (the patient would have behavioral avolition). The fourth subscale, blunted affect, is divided into the following 3 components: (1) facial expression, (2) vocal expression, and (3) expressive gestures, which include body language. The alogia subscale captures both the quantity of speech (how many words are spoken) and spontaneous elaboration (when asked a question, the patient gives more information than is strictly required to answer). The sixth BNSS subscale rates the patient’s lack of normal distress; a lack of emotionality has long been recognized.9
The items on the anhedonia subscale of the BNSS capture the latest research on 2 distinctive types of anhedonia: appetitive anhedonia, which stems from a failure to enjoy thinking about a future activity, and consummatory anhedonia, which is failure to experience pleasure while participating in an activity.10 Some evidence suggests that patients with schizophrenia have decreased appetitive, or anticipatory, pleasure but intact consummatory pleasure.10,11 One theory is that their pessimistic expectations about activities may contribute to their avoidance of activities they might actually enjoy. However, another study12 found the opposite: that people with schizophrenia had normal anticipatory pleasure but decreased consummatory pleasure compared with healthy participants. The importance of the distinction between anticipatory and consummatory pleasure will require more research.
Should the appetitive/consummatory distinction be validated, there could be an important benefit to making the distinction with regard to the development of targeted treatments, especially psychosocial interventions. For patients with appetitive deficits, cognitive-behavioral therapy may help improve their negative expectations.11,13 Clinicians could discuss past positive experiences with patients to offset their negative thoughts or schedule activities or social skills training to improve their confidence in various settings.14
Primary Versus Secondary Negative Symptoms
A problem that is built into the use of rating scales is that simply recognizing the presence of negative symptoms does not reveal their cause. For instance, if a patient has a high score for asociality, the clinician should determine why. Is the patient’s lack of relationships the result of anxiety, depression, or suspiciousness, or is it because the patient is unemotional and uninterested? For patients with blunted affect, their lack of facial expression or expressive body language could be caused by depression, distraction from overwhelming hallucinations, or a true neurologic loss of expressive function. To determine the cause of negative symptoms, clinicians must distinguish between primary and secondary negative symptoms.
Differential Diagnosis
Primary negative symptoms, sometimes referred to as deficit symptoms, are enduring or trait-like.15 Secondary negative symptoms are caused by factors such as positive symptoms, treatment side effects, environmental deprivation, or depression.15,16
Depression. Depression is common in patients with schizophrenia and can cause symptoms that mimic primary negative symptoms. Patients with both comorbid depression and deficit symptoms of schizophrenia can exhibit fatigue, anhedonia, and social withdrawal.17 However, patients with primary negative symptoms typically deny depressive mood and do not exhibit the sleep disturbance that is common in patients with depression.17 A clearly depressed mood suggests depression.17 Primary negative symptoms also tend to be long-standing and not confined to a distinct episode of depression.
Psychotic symptoms. If psychotic symptoms are severe, negative symptoms are likely to be secondary to them, whether they are hallucinations, delusions, or disorganization. In this case, treating the positive symptoms can improve the related negative symptoms.16 If the negative symptoms do not improve with positive symptom treatment, they are more likely to be primary negative symptoms. Atypical antipsychotics, especially clozapine but also, to a lesser degree, olanzapine and risperidone, have some superiority over typical antipsychotics for improving psychotic symptoms in schizophrenia.18 Because each medication has different tolerability profiles, clinicians must individualize medication choice based on patients’ past response, tolerability issues, and preference.18
Side effects. Sedation can cause what appears to be an amotivational syndrome in some patients taking antipsychotics.19 Symptoms that were not present at baseline assessment and emerge during treatment may be secondary negative symptoms caused by medication.
Substance abuse. A connection has also been found between chronic cannabis abuse and an amotivational syndrome, which is characterized by apathy, but the causal relationship remains uncertain.20 Other substances of abuse may also create symptoms that mimic negative symptoms. Obtaining a history of substance use or its absence is important in the differential diagnosis of negative symptoms.
Deficit and Nondeficit Schizophrenia
One problem in the interpretation of research on negative symptoms is that patients with both primary and secondary negative symptoms are often included in studies, which creates some uncertainty about the conclusions reached. One solution is to define a subtype of schizophrenia, called deficit schizophrenia, which consists of patients who have primary negative symptoms. In addition to the DSM criteria for schizophrenia, criteria for deficit schizophrenia include the presence of at least 2 negative symptoms of clinically significant severity for 12 months, even during times of clinical stability, and these symptoms are not secondary to factors such as anxiety, medication side effects, psychotic symptoms, or depression.21
The deficit and nondeficit subtypes of schizophrenia differ on 5 dimensions that are used to distinguish diseases: (1) signs and symptoms, (2) course of illness, (3) pathophysiologic correlates, (4) risk and etiologic factors, and (5) treatment response.21 These differences cannot be attributed to group differences in demographics, antipsychotic treatment, duration of illness, or the severity of positive psychotic symptoms. Patients with deficit schizophrenia show worse psychosocial functioning than patients with nondeficit schizophrenia, and their impairment is not due to more severe positive or depressive symptoms or substance abuse.21 Risk factors differ for the deficit and nondeficit groups as well. For example, deficit schizophrenia is associated with summer birth while nondeficit schizophrenia is associated with winter birth.21
Negative Symptoms in Treatment Trials
There are few treatment trials focused on deficit patients, and to date, there is no established treatment for primary negative symptoms.15 Current medications for schizophrenia focus on action at dopamine receptors,22 but their overall effectiveness for negative symptoms has been disappointing.16 When psychotic, extrapyramidal, anxiety, or depression symptoms improve with medication, any change in negative symptoms may be due to improvement in these other symptoms.16 Therefore, most clinical trials that demonstrate an improvement in negative symptom ratings have an ambiguous interpretation: if other symptoms also improve, the improvement in negative symptoms is probably due to secondary negative symptoms.
This problem of an ambiguous interpretation of clinical trials that are focused on negative symptoms led to a recommendation by the Consensus Development Conference attendees of a specific study design for negative symptom trials.7 In this design, all patients enrolled in the study would be clinically stable (rather than suffering an exacerbation of their symptoms), and their negative symptoms would persist with adequate antipsychotic treatment. The study would be double-blind and have a placebo or active control condition, and the treatments would be administered to parallel groups. These patients would also have minimal psychotic symptoms, depression/anxiety, extrapyramidal side effects, or other significant causes of secondary negative symptoms.7 In these patients, changes in negative symptoms would probably reflect true improvement in primary negative symptoms due to the treatment under study.7
Apathy Versus Expression
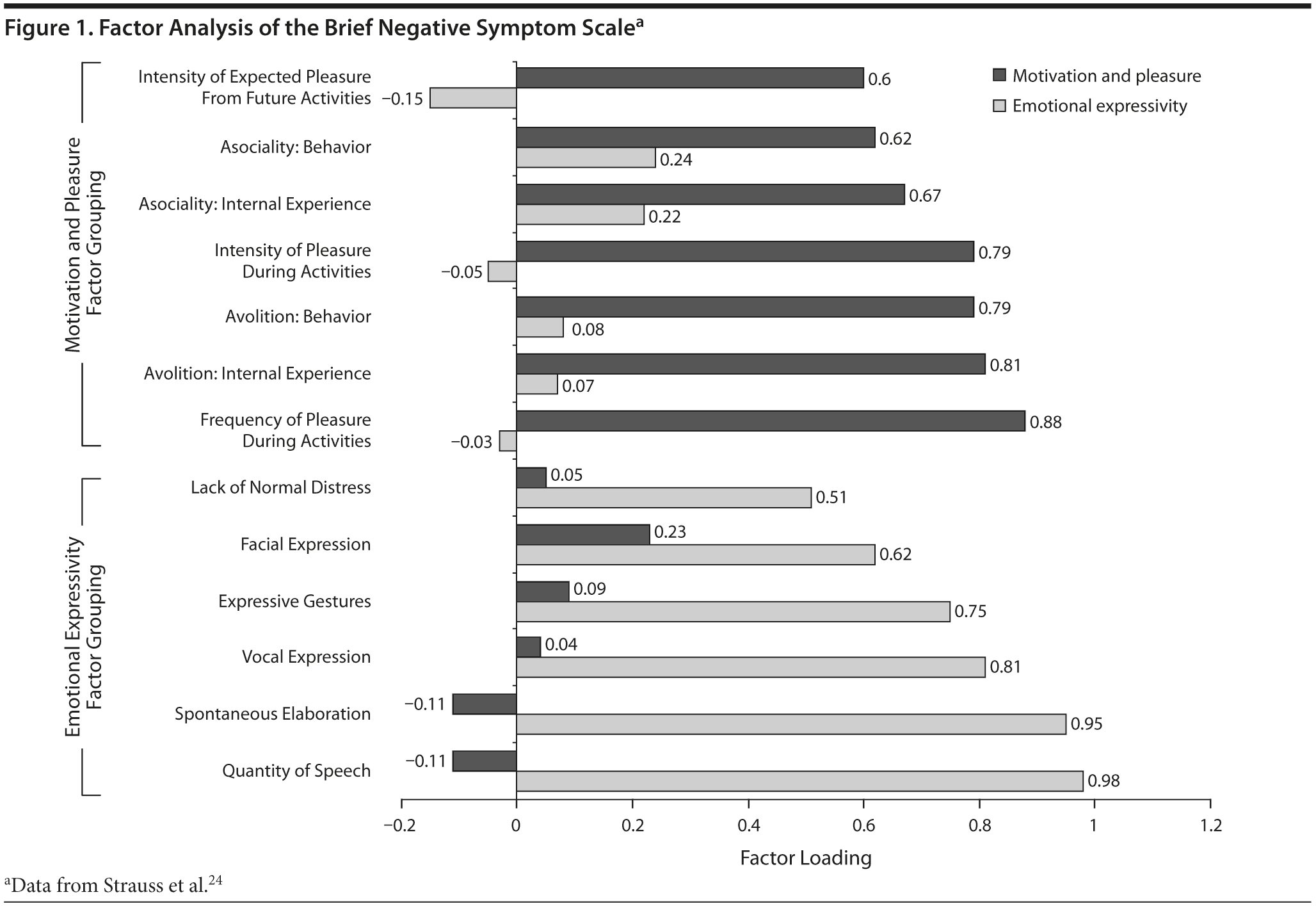
Another developing concept in the study of negative symptoms is that negative symptoms are not a single entity, but may consist of 2 groups of symptoms, or factors. A review23 of factor analytic studies of negative symptom scales examined which of the 5 negative symptoms generally increased or decreased together. The SANS studies identified 2 groups of symptoms that seem to have some independence from each other: (1) diminished expression, which consists of blunted affect and poverty of speech (alogia), and (2) apathy, which consists of anhedonia, avolition, and asociality.23 These 2 domains have been found in studies of 3 other instruments: the BNSS, CAINS, and the Schedule for the Deficit Syndrome (SDS).8,9,24-26 The 2 domains remained stable even after 5 years in one SDS study, with avolition related to social outcomes and diminished expression related to household functioning.27 The negative symptoms of the PANSS do not appear to have similar factors.28
On the BNSS, 6 of the 13 items loaded onto the diminished expression factor and 7 onto the apathy factor (Figure 1).24 If negative symptoms fall into 2 main groups, this clustering of symptoms could mean that the groups have different risk factors, course of illness, and pathophysiology, and perhaps should be considered separate treatment targets. Calculating the scores for each of these symptom groups separately, as a secondary outcome measure in clinical trials, should be easy in many clinical trials and could provide useful data for determining the importance of these factors.
Some treatment trials have shown selective symptom improvement. A double-blind, placebo-controlled trial29 of adjunctive galantamine, an acetylcholinesterase inhibitor, examined the effects on cognitive impairments in patients with treated schizophrenia. Although no significant difference was found in the SANS total score between drug and placebo groups, the galantamine group showed significant symptom reduction in the SANS alogia subscale (P = .007).29 In contrast, a partial α7-nicotinic agonist, 3-(2,4-dimethoxybenzylidene) anabaseine (DMXB-A), improved negative symptoms on both the alogia and anhedonia SANS subscales in a phase 2 trial.30 A phase 2 study31 of adjunctive bitopertin, a selective glycine reuptake inhibitor, compared 3 doses (10 mg, 30 mg, 60 mg) of the agent with placebo in patients with schizophrenia. Investigators examined treatment response using 2 PANSS factors: avolition and expressive deficits. Avolition included emotional withdrawal, passive/apathetic social withdrawal, and active social avoidance, while expressive deficits included poor rapport, lack of spontaneity/conversation, and motor retardation. The 10-mg and 30-mg dose groups showed greater reduction in negative symptoms than the 60-mg dose and placebo. Bitopertin had a greater effect on apathy and social withdrawal than expressive deficits.31 The results of these trials, although interesting, do not yet provide enough information to conclude whether or not the 2 negative symptom factors will tend to have different responses to some treatments.
Conclusion
Several new concepts have emerged from the study of negative symptoms in schizophrenia. First, although widely used, the older rating scales (especially the SANS and PANSS) do not reflect the latest schizophrenia research, leading to the creation of 2 new scales, the BNSS and CAINS. Although rating scales are crucial in clinical trials, in the clinical setting it is important for clinicians to make a differential diagnosis of negative symptoms. Secondary negative symptoms can be caused by a number of factors, especially anxiety/depression, marked positive psychotic symptoms, medication side effects, and substance abuse. Clinicians should assess these possible causes to ascertain which patients have primary negative symptoms. Patients with enduring primary negative symptoms, or the deficit syndrome, have poor psychosocial functioning and treatment resistance. The pharmaceutical industry as well as academic investigators have come to focus on negative symptoms as a treatment target, and some promising treatments are now under study.
Finally, the 2 symptom domains that emerge in most factor analysis studies of negative symptom rating scales are apathy (which encompasses anhedonia, avolition, and asociality) and diminished expression (alogia and blunted affect). The symptoms in these 2 domains tend to vary over time as a group. This 2-factor model may help clarify new approaches to treatment, whether psychosocial or pharmacologic. However, the need remains for effective treatments of primary negative symptoms, either through psychosocial treatments or through different mechanisms of action than current antipsychotic agents.
Drug names: clozapine (Clozaril, FazaClo, and others), galantamine (Razadyne and others), olanzapine (Zyprexa), risperidone (Risperdal and others).
Disclosure of off-label usage: Dr Kirkpatrick has determined that, to the best of his knowledge, bitopertin and 3-(2,4-dimethoxybenzylidene) anabaseine (DMXB-A) are not approved by the US Food and Drug Administration for the treatment of negative symptoms in schizophrenia.
References
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
2. Griesinger W. Mental Pathology and Therapeutics (1867). Robertson CL, Rutherford J, trans. New York, NY: Hafner Publishing Co; 1965.
3. Kraepelin E. Dementia Praecox and Paraphrenia (1919). Barclay RM, trans. Robertson GM, ed. Huntington, NY: Robert E. Krieger Publishing Co Inc; 1971.
4. Bobes J, Arango C, Garcia-Garcia M, et al. CLAMORS Study Collaborative Group. Prevalence of negative symptoms in outpatients with schizophrenia spectrum disorders treated with antipsychotics in routine clinical practice: findings from the CLAMORS study. J Clin Psychiatry. 2010;71(3):280-286. PubMed doi:10.4088/JCP.08m04250yel
5. Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;39(7):784-788. PubMed doi:10.1001/archpsyc.1982.04290070020005
6. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed doi:10.1093/schbul/13.2.261
7. Kirkpatrick B, Fenton WS, Carpenter WT Jr, et al. The NIMH-MATRICS consensus statement on negative symptoms. Schizophr Bull. 2006;32(2):214-219. PubMed doi:10.1093/schbul/sbj053
8. Kring AM, Gur RE, Blanchard JJ, et al. The Clinical Assessment Interview for Negative Symptoms (CAINS): final development and validation. Am J Psychiatry. 2013;170(2):165-172. PubMed doi:10.1176/appi.ajp.2012.12010109
9. Kirkpatrick B, Strauss GP, Nguyen L, et al. The Brief Negative Symptom Scale: psychometric properties. Schizophr Bull. 2011;37(2):300-305. PubMed doi:10.1093/schbul/sbq059
10. Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr Bull. 2006;32(2):259-273. PubMed doi:10.1093/schbul/sbj009
11. Gard DE, Kring AM, Gard MG, et al. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93(1-3):253-260. PubMed doi:10.1016/j.schres.2007.03.008
12. Strauss GP, Wilbur RC, Warren KR, et al. Anticipatory vs consummatory pleasure: what is the nature of hedonic deficits in schizophrenia? Psychiatry Res. 2011;187(1-2):36-41. PubMed doi:10.1016/j.psychres.2011.01.012
13. Beck AT, Rector NA. Cognitive approaches to schizophrenia: theory and therapy. Annu Rev Clin Psychol. 2005;1(1):577-606. PubMed doi:10.1146/annurev.clinpsy.1.102803.144205
14. Morrison AK. Cognitive behavior therapy for people with schizophrenia. Psychiatry (Edgmont). 2009;6(12):32-39. PubMed
15. Buchanan RW. Persistent negative symptoms in schizophrenia: an overview. Schizophr Bull. 2007;33(4):1013-1022. PubMed doi:10.1093/schbul/sbl057
16. Tandon R, Jibson M. Negative symptoms of schizophrenia: how to treat them most effectively. Curr Psychiatry. 2002;1(9):36-42.
17. Mulholland C, Cooper S. The symptom of depression in schizophrenia and its management. Adv Psychiatr Treat. 2000;6(3):169-177. doi:10.1192/apt.6.3.169
18. Citrome L. A systematic review of meta-analyses of the efficacy of oral atypical antipsychotics for the treatment of adult patients with schizophrenia. Expert Opin Pharmacother. 2012;13(11):1545-1573. PubMed doi:10.1517/14656566.2011.626769
19. Miller DD. Atypical antipsychotics: sleep, sedation, and efficacy. Prim Care Companion J Clin Psychiatry. 2004:6(suppl 2);3-7. PubMed
20. Schmits E, Quertemont E. So called “soft” drugs: cannabis and the amotivational syndrome. Rev Med Liege. 2013;68(5-6):281-286. PubMed
21. Kirkpatrick B, Galderisi S. Deficit schizophrenia: an update. World Psychiatry. 2008;7(3):143-147. PubMed
22. Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch Gen Psychiatry. 2012;69(8):776-786. PubMed doi:10.1001/archgenpsychiatry.2012.169
23. Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32(2):238-245. PubMed doi:10.1093/schbul/sbj013
24. Strauss GP, Hong LE, Gold JM, et al. Factor structure of the Brief Negative Symptom Scale. Schizophr Res. 2012;142(1-3):96-98. PubMed doi:10.1016/j.schres.2012.09.007
25. Kimhy D, Yale S, Goetz RR, et al. The factorial structure of the Schedule for the Deficit Syndrome in schizophrenia. Schizophr Bull. 2006;32(2):274-278. PubMed doi:10.1093/schbul/sbi064
26. Nakaya M, Ohmori K. A two-factor structure for the Schedule for the Deficit Syndrome in schizophrenia. Psychiatry Res. 2008;158(2):256-259. PubMed doi:10.1016/j.psychres.2007.10.008
27. Galderisi S, Bucci P, Mucci A, et al. Categorical and dimensional approaches to negative symptoms of schizophrenia: focus on long-term stability and functional outcome. Schizophr Res. 2013;147(1):157-162. PubMed doi:10.1016/j.schres.2013.03.020
28. Liemburg E, Castelein S, Stewart R, et al, for the Genetic Risk and Outcome of Psychosis (GROUP) Investigators. Two subdomains of negative symptoms in psychotic disorders: established and confirmed in two large cohorts. J Psychiatr Res. 2013;47(6):718-725. PubMed doi:10.1016/j.jpsychires.2013.01.024
29. Buchanan RW, Conley RR, Dickinson D, et al. Galantamine for the treatment of cognitive impairments in people with schizophrenia. Am J Psychiatry. 2007;165(1):82-89. PubMed doi:10.1176/appi.ajp.2007.07050724
30. Freedman R, Olincy A, Buchanan RW, et al. Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry. 2008;165(8):1040-1047. PubMed doi:10.1176/appi.ajp.2008.07071135
31. Umbricht D, Lentz E, Santarelli L, et al. A post-hoc analysis of the negative symptom factor score in a proof-of-concept study of glycine reuptake inhibitor bitopertin in schizophrenia. Papers of the 25th ECNP Congress. October 13-17, 2012; Vienna, Austria. Eur Neuropsychopharmacol. 2012;22(suppl 2):SB11.