Improvement in Refractory Psychosis With Dronabinol: Four Case Reports
To the Editor: We recently reported that 4 out of 6 patients with refractory schizophrenia improved with the cannabinoid agonist dronabinol (synthetic Δ9-tetrahydrocannabinol).1 We have subsequently tried dronabinol in a purely clinical manner for 8 other inpatients with refractory psychosis in our tertiary care hospital. Inclusion criteria were severe psychosis refractory to standard medication, a history of symptomatic improvement when using marijuana, and good physical health. We now report on the 4 patients who improved with dronabinol. The trials reported here occurred between November 2009 and April 2010. Dronabinol use was approved by the joint institutional review board of the Rockland Psychiatric Center and Nathan S. Kline Institute for Psychiatric Research (Orangeburg, New York) and given according to our hospital’s formal protocol for administering US Food and Drug Administration-approved medication for off-label use.
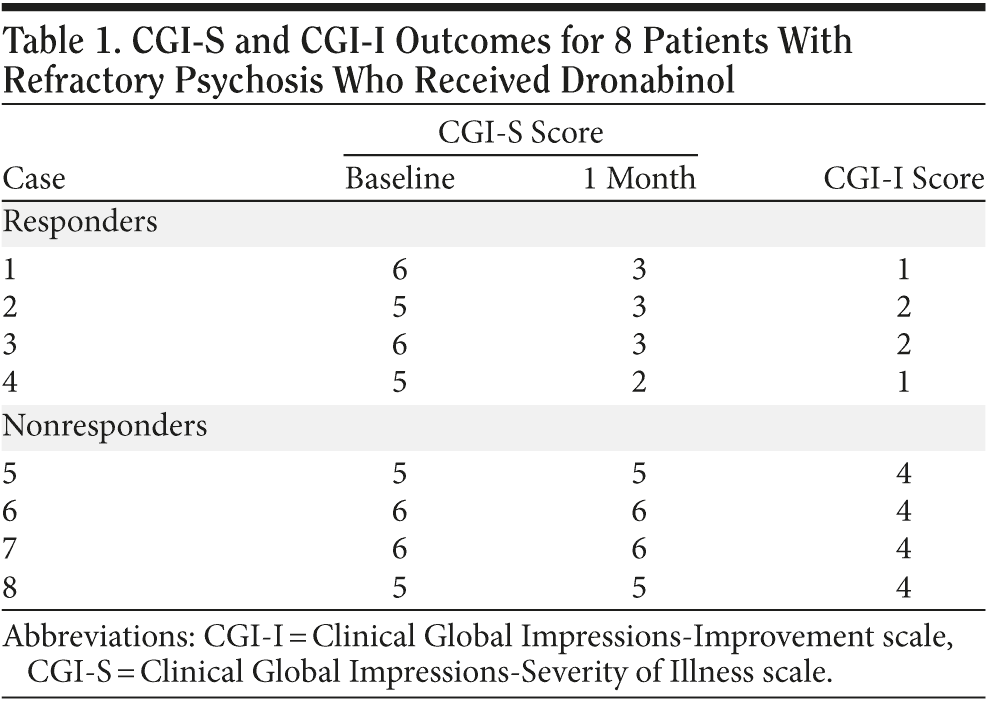
Case 1. Mr A, a 54-year-old man with DSM-IV schizoaffective disorder, bipolar type, had been continuously aggressive, intrusive, and disorganized for years in spite of multiple medication trials. His Clinical Global Impressions-Severity of Illness scale (CGI-S)2 score was 6 (severely ill). At the time of the dronabinol trial, he was receiving daily clozapine 775 mg (serum level = 650 ng/mL), risperidone 5 mg, lithium carbonate 900 mg (serum level = 1.18 mEq/L), and clonazepam 4 mg for months with no significant benefit.
On the basis of the patient’s report that he had felt calm when he took marijuana in high school, we added dronabinol 5 mg bid. Within 2 days, he became calm, cooperative, and logical (CGI-S score of 3, moderately ill; Clinical Global Impressions-Improvement scale [CGI-I]2 score of 1, very much improved). When the dronabinol was phased out 3 weeks later, his behavior deteriorated within 5 days to the pre-dronabinol state. Two weeks later, dronabinol treatment was restarted, and his behavior once again became calm, cooperative, and logical within 2 days. This excellent remission has continued for 3 months.
Case 2. Mr B, a 29-year-old man with DSM-IV chronic paranoid schizophrenia, had been suspicious, guarded, hostile, menacing, and paranoid for years in spite of treatment with multiple antipsychotics and mood stabilizers. At the time of the dronabinol trial, he was receiving olanzapine 25 mg/d and clonazepam 2 mg/d for months with minimal response and had a CGI-S score of 5, markedly ill.
On the basis of the patient’s report that marijuana "put me to sleep beautifully at night," we added dronabinol 5 mg bid. Within 2 days, Mr B became cooperative, only mildly irritable, and socially more appropriate (CGI-S score of 3, mildly ill; CGI-I score of 2, much improved). He said that a "weight was lifted from my head" and that he could be "relaxed for the first time." Regarding the resolution of his paranoia, he explained, "It helps me rest. I can get along with people and walk away from any situation now."
Case 3. Mr C, a 24-year-old man with DSM-IV schizoaffective disorder, bipolar type, had been extremely hypomanic, assaultive, and disorganized since admission several weeks earlier in spite of daily lithium carbonate 900 mg (serum level = 0.75 mEq/L), divalproex 2,500 mg (serum level = 107 μg/mL), chlorpromazine 300 mg, quetiapine 400 mg, and lorazepam 4 mg (CGI-S score of 6, severely ill).
On the basis of his history that "daily marijuana was the only thing that kept me straight and helped me concentrate," we added dronabinol 10 mg bid. Within 3 days, Mr C became calm and pleasant but was very hypersexual, making provocative comments to females (CGI-S score of 3, mildly ill; CGI-I score of 2, much improved). Fearing that the dronabinol was causing this hypersexuality, we stopped it. Within days, the patient resumed his baseline aggressive and disorganized behavior, becoming assaultive on virtually a daily basis and requiring continuous 1:1 and later 2:1 observations.
One month later, dronabinol was restarted at 5 mg bid. Within a day, he felt calmer, and the dose was raised to 10 mg bid. Three days later, he was pleasant, cooperative, and logical, and we were able to take him off all levels of observation (CGI-S score of 3, mildly ill; CGI-I score of 1, very much improved).
Case 4. Mr D, a 57-year-old man with DSM-IV chronic paranoid schizophrenia, had been guarded, suspicious, often frankly paranoid, loose in association, and hyperirritable for years in spite of multiple medication trials. At the time of the dronabinol trial, he was being treated with aripiprazole 20 mg/d with only minor improvement and had a baseline CGI-S score of 5, markedly ill.
On the basis of his stated history that "marijuana calmed me," we started dronabinol 5 mg bid, which brought about an immediate but partial improvement. When we raised the dose to 10 mg tid after 4 weeks, most of his symptoms resolved (CGI-S score of 2, borderline mentally ill; CGI-I score of 1, very much improved). He became calm, polite, and friendly if approached but still somewhat of a loner. Mr D explained, "The medication mellows me," and added, "But it doesn’ t give me a buzz like real marijuana." The staff noted that his anger, paranoia, and pacing were entirely gone and prepared him for discharge.
No patient had any significant side effects. The 4 nonresponders had similar diagnostic and symptom profiles to the 4 responders and received dronabinol 20 to 30 mg/d for 1 month without response (defined by standard clinical assessment and CGI-I and CGI-S ratings). Significantly, none of the nonresponders had a worsening of their psychosis or other effects; they simply had no change with the addition of dronabinol (Table 1).
Before our prior report,1 cannabinoid stimulation had generally been associated with worsening of psychosis.3 However, not all patients with schizophrenia worsen with cannabinoids, and it is hypothesized that a genetic predisposition makes the chance of psychosis more likely.4 Now, it appears that a predisposed subset of patients with schizophrenia may actually improve with cannabinoid stimulation.
It is well recognized that schizophrenia is not 1 illness but a syndrome of many disorders, with the final common denominator of psychotic behavior. We suspect that in a small subset of these patients, the etiology of their psychosis is low endogenous endocannabinoid brain function, so that cannabinoid stimulation would improve their behavior. It is also possible that the known soothing effects of cannabinoid stimulators such as marijuana caused a novel but nonspecific calming in our patients, which led to the symptom improvement. Indeed, calming was a significant element in the improvement of most of the responders. However, 3 of the 4 responders were already being treated with benzodiazepines without significant improvement. Moreover, a recent report5 supports a specific mechanism. Self-administration of the cannabinoid-1 receptor agonist WIN55,212-2 in rats attenuated phencyclidine-induced deficits, and the authors concluded that cannabinoid consumption can ameliorate schizophrenia-like behavior caused by phencyclidine use.
Before proceeding to a double-blind, controlled study, we need to identify a subgroup who is more likely to respond in order to achieve statistical significance, since only 4 of our 8 patients responded to dronabinol. It is intuitive that the inclusion criterion of a good past response to marijuana made the responders more likely to respond to dronabinol. However, we do not know whether the criterion of refractoriness made them more or less likely to respond. Conceivably, the very lack of response to standard treatments might self-select psychoses due to unusual etiologies, such as low endocannabinoid functioning. Biomarkers might help sort out likely responders. Recent studies of the catechol-O-methyltransferase genetic functional polymorphism have shown that carriers of the valine158 allele were more likely to develop psychosis later in life if they used marijuana in their youth6 and more likely to develop hallucinations after cannabis exposure,7 whereas carriers of the methionine/methionine genotype were protected. Perhaps this biomarker can identify which patients are more likely to tolerate endocannabinoid stimulation and, thereby, receive any benefits of such stimulation without having an exacerbation of their psychosis. In addition, it would be valuable to use rating scales to attempt to determine how much of the responses are due to nonspecific calming and how much to a true lysis of psychosis.
References
1. Schwarcz G, Karajgi B, McCarthy R. Synthetic Δ9-tetrahydrocannabinol (dronabinol) can improve the symptoms of schizophrenia. J Clin Psychopharmacol. 2009;29(3):255-258. PubMed doi:10.1097/JCP.0b013e3181a6bc3b
2. Guy W. CGI: Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology, Revised. US Dept of Health, Education, and Welfare publication (ADM) 76-338. Rockville, MD: National Institute of Mental Health; 1976:218-222.
3. D’ Souza DC, Abi-Saab WM, Madonick S, et al. Δ9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biol Psychiatry. 2005;57(6):594-608. PubMed doi:10.1016/j.biopsych.2004.12.006
4. Henquet C, Di Forti M, Morrison P, et al. Gene-environment interplay between cannabis and psychosis. Schizophr Bull. 2008;34(6):1111-1121. PubMed doi:10.1093/schbul/sbn108
5. Spano MS, Fadda P, Frau R, et al. Cannabinoid self-administration attenuates PCP-induced schizophrenia-like symptoms in adult rats. Eur Neuropsychopharmacol. 2010;20(1):25-36. PubMed doi:10.1016/j.euroneuro.2009.09.004
6. Caspi A, Moffitt TE, Cannon M, et al. Moderation of the effect of adolescent-onset cannabis use on adult psychosis by a functional polymorphism in the catechol-O-methyltransferase gene: longitudinal evidence of a gene ×— environment interaction. Biol Psychiatry. 2005;57(10):1117-1127. PubMed doi:10.1016/j.biopsych.2005.01.026
7. Henquet C, Rosa A, Delespaul P, et al. COMT ValMet moderation of cannabis-induced psychosis: a momentary assessment study of "switching on" hallucinations in the flow of daily life. Acta Psychiatr Scand. 2009;119(2):156-160. PubMed doi:10.1111/j.1600-0447.2008.01265.x
Author affiliations: Rockland Psychiatric Center, Orangeburg (both authors); and Stroud Center, Columbia University Medical Center, and Department of Psychiatry, New York University School of Medicine (Dr Schwarcz), New York. Potential conflicts of interest: None reported. Funding/support: None reported.
doi:10.4088/JCP.10l06035yel
© Copyright 2010 Physicians Postgraduate Press, Inc.