Abstract
Insomnia, the most prevalent sleep-wake disorder, affects 6% to 10% of adults. It may result in interpersonal and occupational problems and has a deleterious effect on quality of life. Patients may experience difficulty with sleep onset, sleep maintenance, or both. Insomnia disorder is commonly comorbid with psychiatric, medical, and neurologic disorders, and insomnia and comorbid conditions have bidirectional relationships. Diagnosis should be based on patient interview, assessment with tools, and use of criteria. Selection of treatment for patients with insomnia should factor in efficacy for the patient’s specific complaint as well as other features such as safety profile and abuse liability. Cognitive-behavioral therapy for insomnia is a first-line recommendation by guidelines, but some patients are unable or unwilling to try it or may not respond to it. Older adults with insomnia disorder require careful consideration of medications’ risk-benefit profiles.
To cite: Rosenberg RP, Krystal AD. Diagnosing and treating insomnia in adults and older adults. J Clin Psychiatry. 2021;82(6):EI20008AH5C.
To share: https://doi.org/10.4088/JCP.EI20008AH5C
© Copyright 2021 Physicians Postgraduate Press, Inc.
aAtlanta School of Sleep Medicine and Technology and NeuroTrials Research, Inc., Georgia
bUniversity of California San Francisco
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
Insomnia, the most prevalent sleep-wake disorder, is defined as difficulty falling or staying asleep that is associated with significant impairment or distress in daytime function and occurs despite an adequate opportunity for sleep.1,2 This report, based on presentations given by Russell P. Rosenberg, PhD, and Andrew D. Krystal, MD, will address how to distinguish chronic insomnia from medical or psychiatric disorders and develop an effective evidence-based treatment plan for patients with insomnia.
Risk Factors for and Diagnosis of Insomnia
According to Dr Rosenberg, about one-third of adults report symptoms of insomnia, and 6%–10% meet diagnostic criteria for insomnia disorder.1 This condition may result in interpersonal and occupational problems1 and has a deleterious effect on quality of life.3 Decreased attention and concentration are common, and a higher rate of accidents is associated with insomnia.1 People who almost always experience difficulty falling asleep are more than twice as likely as people who never have issues initiating sleep to die from a motor vehicle injury.4 The presence of insomnia is a risk factor for cardiovascular disease, type 2 diabetes mellitus, gastroesophageal reflux disease, asthma, and thyroid disorders.5
Risk Factors for Insomnia
Dr Rosenberg explained that women are more likely than men to experience insomnia and that insomnia symptoms increase with age.6 He stated that the disorder is thought to be associated with hyperarousal throughout the day and night, as evidenced by neuroendocrine, autonomic, neuroimmunologic, electrophysiologic, and neuroimaging studies.7
Therefore, insomnia may result from the interplay between a genetic vulnerability for an imbalance between arousing and sleep-inducing brain activity, psychosocial or medical stressors, and perpetuating mechanisms including dysfunctional sleep-related behaviors, learned sleep-preventing associations, and a tendency to worry/ruminate.7
Patient Perspectives
Here, a person describes how nightly rumination about life circumstances contributed to insomnia, which has now become another source of worry itself:
“It feels like no matter how tired I am, whenever my head hits the pillow, my body is jolted awake by distressing thoughts and fears about my life and future. For context, I’m 24 and planning to go to law school in a few months. I fear that my lack of sleep will lead to irreversible health issues down the road (like dementia).”8
Diagnostic Criteria for Insomnia
Individuals with insomnia disorder may have trouble falling asleep, staying asleep, or both (Figure 1). To meet criteria for persistent insomnia disorder1 or chronic insomnia,2 symptoms must occur a minimum of 3 days per week for 3 months.1 Episodic insomnia1 (or short-term insomnia2) has the same criteria but occurs for less than 3 months. Criteria in both the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5),1 and the International Classification of Sleep Disorders, 3rd Edition (ICSD-3),2 include the following:
- Distress or impairment is caused by the insomnia
- The problem occurs despite adequate opportunity to sleep
- The difficulty cannot be better explained by other physical, mental, or sleep-wake disorders
- The problem cannot be attributed to substance use or medication
Figure 1. Manifestations of Insomnia
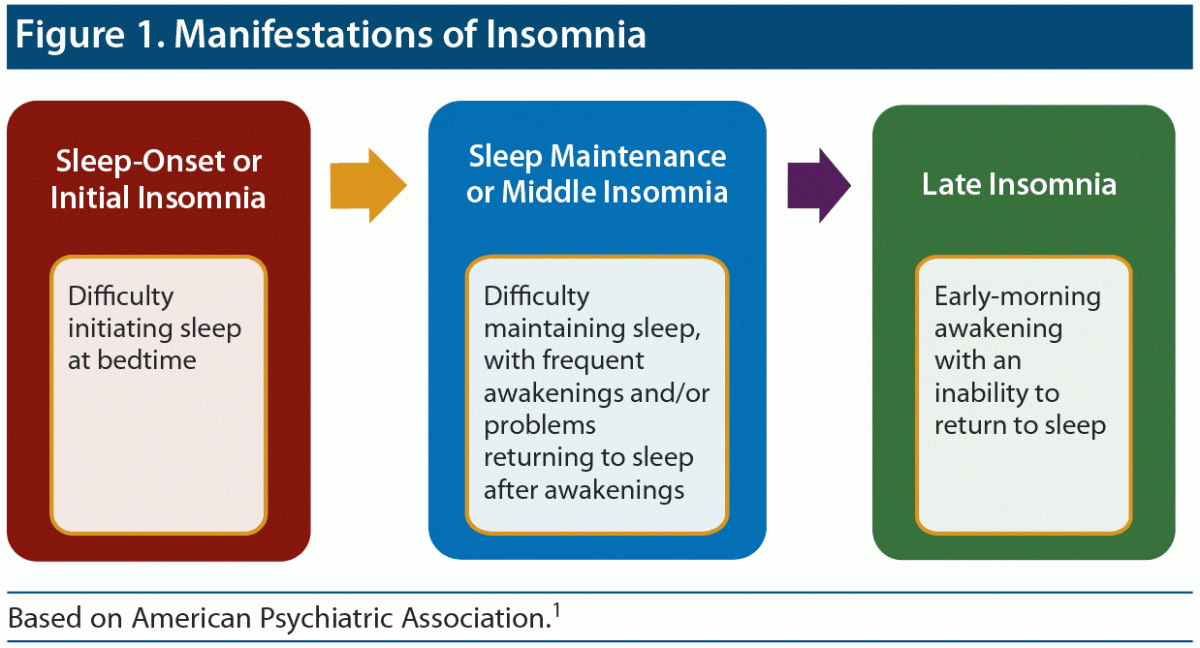
Based on American Psychiatric Association.1
Insomnia Interview and Assessment Tools
Dr Rosenberg stated that the cornerstone of the insomnia evaluation is a detailed history obtained during the patient interview.6,9 Key points that should be covered to ensure a thorough evaluation are the following: (1) the nature of the complaint (sleep onset, sleep maintenance, early-morning awakening, nonrestorative sleep quality, or a combination), (2) the patient’s sleep-wake schedule and routines, and (3) functional impairment (including safety issues) and distress. If possible, an interview with the patient’s bed partner, family member, or significant other is helpful. To see more details from Dr Rosenberg’s presentation, see the on-demand activity “Prevalence, Impact, and Burden of Insomnia and Discussing It With Patients” in this series at CMEInstitute.com. Dr Rosenberg also advised using tools such as the Epworth Sleepiness Scale,9 an actigraph,10 sleep diaries,9 and physical and mental status examinations to assess patients for insomnia. Polysomnography is used when the diagnosis is uncertain and there is reasonable clinical suspicion of sleep apnea or movement disorders, when initial treatment fails, or arousals from sleep occur with violent or injurious behavior.11
The Bidirectional Relationship Between Insomnia and Comorbid Disorders
Insomnia is commonly comorbid with psychiatric, medical, and neurologic disorders.12,13 Insomnia and comorbid conditions have a bidirectional relationship, ie, the status of each impacts the other, potentially affecting the treatment course and outcome.1 The National Comorbidity Survey Replication14 reported that 92% of people with insomnia in the past 12 months had at least 1 other disorder; the mean number of other disorders among those with insomnia was 3.2.
Dr Rosenberg noted that, for years, insomnia was thought to occur secondary to various disorders; therefore, it did not receive the attention it warranted because the comorbid disorder seemed to play the primary role in the insomnia problem. It was believed that treatment for the other disorder(s) would resolve the insomnia.1 However, our thinking has evolved from that strategy toward the treatment of insomnia as a separate condition.13
Psychiatric Disorders and Insomnia
Insomnia and mental illnesses may share common causes, and insomnia may be a factor in someone developing a mental illness.15 Dr Rosenberg presented results from the National Comorbidity Survey Replication,16 which revealed that 4 sleep problems (difficulty initiating or maintaining sleep, early morning awakening, nonrestorative sleep) were significantly comorbid with 1 or more anxiety disorders, mood disorders, impulse-control disorders, or substance use disorders.
Patient Perspectives
Here, a person with insomnia and anxiety describes the impact of the disorders:
“I struggle out of bed, never feeling well-rested but unable to go back to sleep. I trudge through my day, anxious and dreading the most basic of chores and responsibilities. Do I check the mail? What if I have to talk to someone on the way? I don’t think I have the energy to buy groceries. If I do housework, what if I start something I can’t finish? I should do homework, but for some reason it’s scary. My brain is always in a fog. All day I want nothing more than a nap, but I tell myself to wait. Keep a schedule, stay awake until it’s time for bed. And then I can’t sleep. Anxious thoughts run through my head. I feel worthless. Everything hurts. I have to read a book until I pass out to stop the torrent of dread and general self-loathing that always hits me just when I’m trying to relax. Fatigue by day, insomnia by night. Awesome.”17
Medical Disorders and Insomnia
Dr Rosenberg stated that people with insomnia report higher rates of several medical conditions than do those without insomnia, and people with medical disorders have a higher prevalence of insomnia than people without those medical problems. In a survey18 of 772 individuals, results showed that people with chronic insomnia had a higher incidence of heart disease, high blood pressure, breathing problems, urinary problems, chronic pain, and gastrointestinal problems (Figure 2). He also cited a population-based study19 of 3,282 participants in which those with any medical disorders were more than twice as likely to have insomnia as those without medical disorders.
Figure 2. Prevalence of Medical Conditions in People With and Without Insomnia
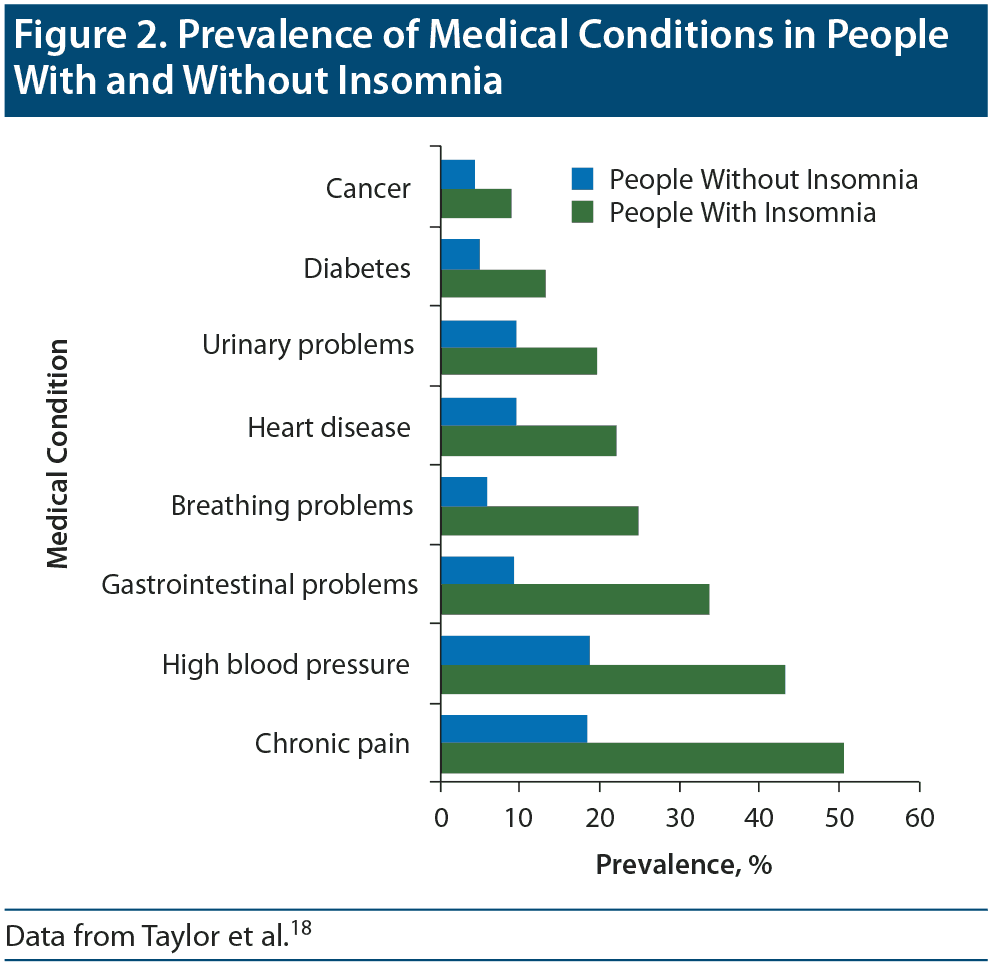
Data from Taylor et al.18
Neurologic Disorders and Insomnia
Dr Rosenberg reported on results from a large survey18 that showed that people with chronic insomnia had a higher incidence of neurologic disease than those without insomnia. He added that comorbid insomnia is common in patients with disorders such as Parkinson disease (PD) and Alzheimer disease (AD).20 According to Dr Rosenberg, insomnia may occur on its own or may occur because of factors associated with the neurologic condition, such as pain, depression, or medications.
Problems in the sleep-wake cycle have been found to influence the risk of developing AD through accumulation of amyloid-β (Aβ) in the brain.21,22 Once Aβ accumulates, increased wakefulness and altered sleep patterns develop. Thus, explained Dr Rosenberg, sleep disorders and neurodegenerative disease may influence each other in ways that have important implications for the treatment of both conditions. He also cited a review23 of insomnia as a risk factor in both PD and AD, indicating that insomnia and neurodegenerative disorders have a complex and bidirectional relationship.
Optimizing Treatment for Insomnia
Dr Krystal discussed new agents for insomnia that have emerged in recent years with highly specific pharmacologic effects.24 The newer agents include those with relatively limited negative effects on brain function, primarily affecting sleep systems and having reduced impact on other functions than older agents that have wide-ranging neural effects. According to Dr Krystal, this advancement paves the way for a new paradigm for managing insomnia in which clinicians aim to select interventions that best target the specific type of sleep problem of each patient.
Dr Krystal outlined the differences between treatments and explained that awareness is needed of the specific effects of interventions and of the patient subpopulations who would optimally benefit from each type of treatment (Table 1).24–26
Table 1. Matching Insomnia Treatments to Patient Presentation
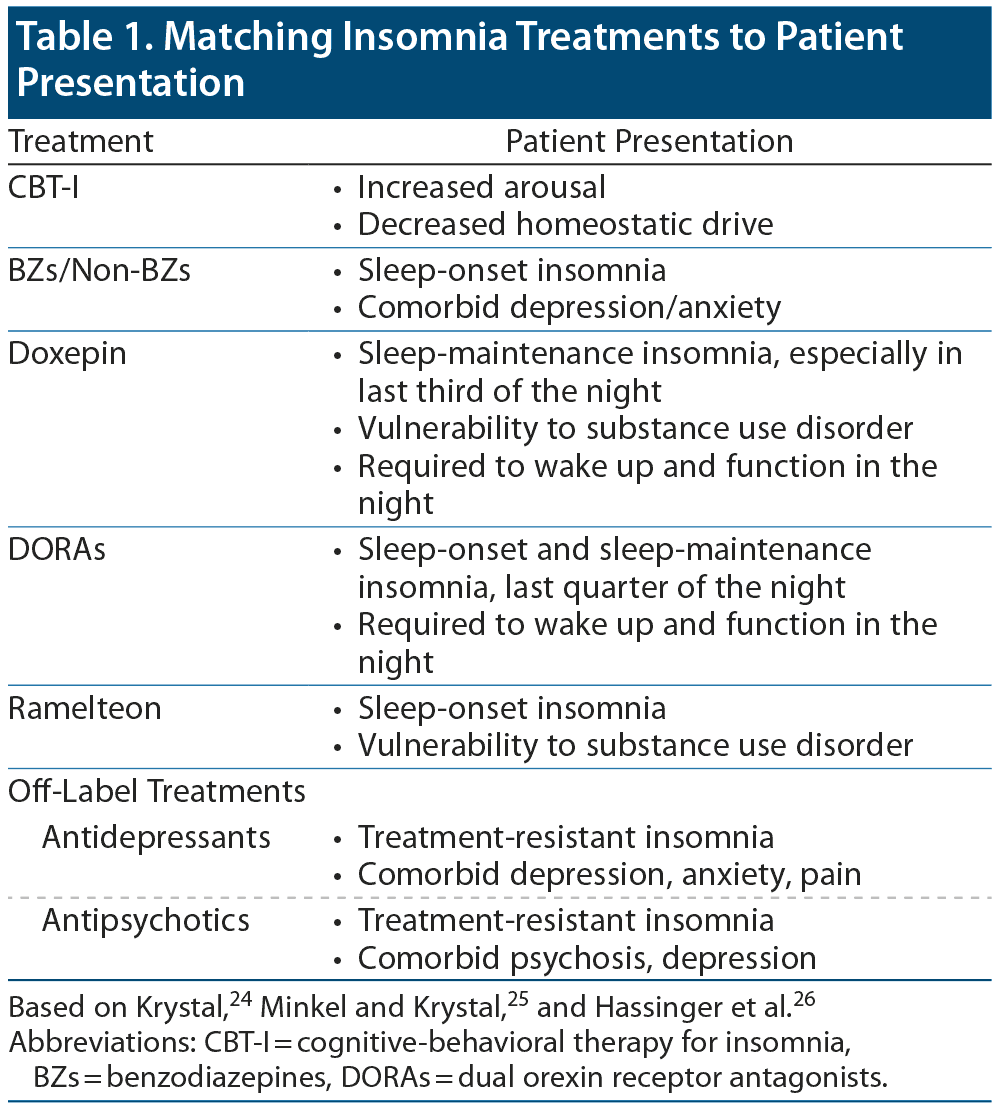
Based on Krystal,24 Minkel and Krystal,25 and Hassinger et al.26
Abbreviations: CBT-I = cognitive-behavioral therapy for insomnia, BZs = benzodiazepines, DORAs = dual orexin receptor antagonists.
Cognitive-Behavioral Therapy for Insomnia
A first-line treatment for insomnia is CBT-I, or cognitive-behavioral therapy for insomnia.9,27–29 Dr Krystal explained that CBT-I targets factors such as sleep drive dysregulation, sleep-related anxiety, and sleep-interfering behaviors, thereby restoring homeostatic regulation of sleep and helping to diminish maladaptive sleep-related thoughts through cognitive restructuring.30
Certain features of patient presentation can suggest usefulness of CBT-I. Dr Krystal cited a study31 that tested the hypothesis that biomarkers of homeostatic sleep drive and arousal would be predictors and correlates of CBT-I response. Results suggested that both decreased homeostatic sleep drive and elevated arousal can be effectively targeted with CBT-I. Results from trials32,33 in patients with insomnia and depression indicated that CBT-I combined with antidepressant therapy is beneficial for people who have insomnia occurring with depression, although other treatments are needed to improve depression symptoms. Finally, those with objectively determined short sleep duration (< 6 hours) are less likely to improve with CBT-I than those who sleep at least 6 hours.34,35
Benzodiazepines and Nonbenzodiazepines
Dr Krystal outlined the mechanisms and effects of benzodiazepines (BZs) and nonbenzodiazepines (non-BZs). Commonly prescribed BZs that are approved by the United States Food and Drug Administration (FDA) for the treatment of insomnia are triazolam, flurazepam, estazolam, quazepam, and temazepam. Zolpidem, zaleplon, and eszopiclone are non-BZs. Evidence indicates that both classes are useful when nonspecific effects are desired, such as for patients with comorbid anxiety, depression, or pain, or for patients with sleep-onset difficulties.24 Eszopiclone and zolpidem CR also have evidence for benefit in sleep maintenance.25
Selective H1 Histamine Receptor Antagonists
Evidence supports that histamine drives wakefulness via H1 receptors.36 As a result, blocking the H1 histamine receptors has the potential to enhance sleep.25 However, Dr Krystal noted, the antihistamines that clinicians have used for years were not specific H1 antihistamines; they had other pharmacologic effects that either detracted from or added to their sleep effects and also led to side effects that are not linked specifically to the H1 mechanism.25
Doxepin, a tricyclic antidepressant used in dosages of 75–150 mg/d, is approved for the treatment of insomnia in dosages of 3–6 mg/d; this agent is a more potent and selective H1 antagonist than other medications referred to as “antihistamines.”25 Unique effects of selective H1 antihistamines are evident in the profile of doxepin 3–6 mg, which has stronger effects on sleep maintenance than on sleep onset37 and has therapeutic effects in the last third of the night, without significant adverse effects in the morning.25 It would be useful in abuse-prone patients with sleep maintenance difficulties because it does not have abuse liability.
Selective Orexin Antagonists
Dr Krystal discussed the FDA-approved hypocretin-orexin receptor antagonists suvorexant and lemborexant.38 These medications have a highly specific effect, blocking only the 2 orexin receptors, which are important mediators of wakefulness.24 Because they block both orexin receptors, these agents are sometimes referred to as dual orexin receptor antagonists (DORAs).
According to Dr Krystal, data suggest that the DORAs have robust effects on sleep onset and sleep maintenance,39–41 improving sleep in the last quarter of the night without substantial morning impairment. This effect is like that of doxepin 3–6 mg for the same reason: they block a single wake-promoting system that is active in the last quarter of the night while others are not (hypocretin/orexin release drives histamine release in the last quarter of the night, so blocking either achieves the same effect).42 Also like doxepin 3–6 mg, the DORAs do not prevent awakenings and do not raise the arousal threshold; they have a relatively limited impact on systems other than sleep and, as a result, are associated with fewer side effects and less impairment than the BZs and non-BZs in patients’ ability to arise and function. Unlike doxepin, the DORAs improve sleep onset, but they also have abuse liability and are listed as controlled substances by the Drug Enforcement Administration.43,44
Selective Melatonin Receptor Agonists
Dr Krystal discussed agents that produce pharmacologic effects through agonism of melatonin receptors, including the hormone melatonin (available “over the counter” as a supplement) and ramelteon.24
Melatonin appears to have a greater effect on patients with circadian rhythm disorders than on patients with insomnia; however, meta-analyses suggest that melatonin has a modest therapeutic effect on sleep onset in patients with insomnia.24,45 Dr Krystal stated that ramelteon has been shown to be effective for sleep-onset difficulties.24,37 The agent has relatively limited adverse effects, especially regarding motor and cognitive impairment, and is without abuse potential.46
Antidepressants
The antidepressants that are most often used off-label for the treatment of insomnia include trazodone, mirtazapine, amitriptyline, and higher-dose doxepin.24 According to Dr Krystal, despite their relatively widespread use for the treatment of insomnia, limited data exist on the efficacy and safety of these drugs.24 For example, he noted, in a 14-day study,47 trazodone 50 mg/d failed to show sustained therapeutic effect versus placebo.25 Dr Krystal also mentioned the side effects of antidepressants due to their nonselective mechanisms of action, which vary depending on the drug. Adverse effects may include weight gain, orthostatic hypotension, dry mouth, constipation, blurred vision, urinary retention, cognitive impairment, cardiotoxicity, sexual dysfunction, and potentially delirium.26
Antipsychotics
Dr Krystal discussed the antipsychotics often used off-label for insomnia, including quetiapine, olanzapine, risperidone, and lurasidone.24 Limited data on the efficacy and safety of these drugs are available in the treatment of insomnia. Their side effect profiles, inferred from use in psychiatric conditions, can be quite significant, including extrapyramidal side effects (eg, tardive dyskinesia, parkinsonism), weight gain, insulin resistance, orthostatic hypotension, dry mouth, constipation, and sexual dysfunction.26 According to Dr Krystal, like antidepressants, antipsychotics are most beneficial for patients with insomnia in whom nonspecific effects could be advantageous, such as patients with comorbid mania or a psychotic condition, or for patients with treatment-resistant insomnia.24
Case Practice Question
A 58-year-old attorney specializing in family law presents to the clinic with a complaint of difficulty staying asleep. He began having sleep maintenance problems when he attended law school. He would often wake feeling that he was unprepared for class, and then he would stay awake studying for hours in the middle of the night. The insomnia remitted during his late 30s, but he relapsed with both sleep onset and sleep maintenance difficulties when his law partner decided to leave the practice. Currently, he has mild depression and coronary artery disease. He has used zolpidem 10 mg, and, while it helps him initiate sleep, he awakens about 3:30 am or 4:00 am and has difficulty getting back to sleep until 1 or 2 hours have passed. Which pharmacologic agent is best for you to consider next?
| a. Benzodiazepine | |
| b. Antihistamine | |
| c. Dual orexin receptor antagonist | |
| d. Antipsychotic |
Discussion of Case Practice Question
Preferred response: c. Dual orexin receptor antagonist
Explanation: Insomnia is often comorbid with depression and heart disease. This patient may have significant adverse events when taking a benzodiazepine and has had an inadequate response to a nonbenzodiazepine. Antihistamines are not reliably used for insomnia treatment, and their effect is mostly limited to sleep maintenance. The safety of antipsychotics is unproven. DORAs have been shown to improve sleep onset and maintenance and are well tolerated by all adult age groups.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
Overview
Drs Rosenberg and Krystal identify key steps in the diagnosis and treatment of insomnia disorder.
Target Audience
Psychiatrists, primary care clinicians, and neurologists; nurse practitioners and & physician assistants in psychiatry, primary care, and neurology settings
Learning Objectives
- Routinely include sleep history and use screening tools as part of patient assessment
- Develop an effective evidence-based treatment plan for patients with insomnia
Support Statement
Supported by an educational grant from Eisai Inc.
Learning Objective
After completing this educational activity, you should be able to:
- Routinely include sleep history and use screening tools as part of patient assessment
- Develop an effective evidence-based treatment plan for patients with insomnia
Release, Review, and Expiration Dates
This Academic Highlights activity was published in September 2021 and is eligible for AMA PRA Category 1 Credit™ through December 31, 2023. The latest review of this material was August 2021.
Statement of Need and Purpose
Clinicians are not vigilant enough in documenting patients’ sleep histories. Because insomnia frequently accompanies psychiatric or medical illnesses and has a variety of negative consequences, clinicians must routinely screen patients for sleep-onset or sleep-maintenance insomnia. Therefore, education is needed to address clinicians’ incomplete understanding and assessment of insomnia. In addition, many clinicians are failing to provide recommended treatments for insomnia because they have reservations about the safety and appropriateness of some agents. Lack of understanding about the differences between treatments can prevent them from explaining and providing insomnia treatment options to patients. Education for clinicians about both approved and unapproved treatments is warranted to improve their management of insomnia.
Disclosure of Off-Label Usage
The faculty members have determined that, to the best of their knowledge, trazodone, mirtazapine, amitriptyline, quetiapine, olanzapine, risperidone, lurasidone, and melatonin are not approved by the US Food and Drug Administration for the treatment of insomnia.
Review Process
The faculty members agreed to provide a balanced and evidence-based presentation and discussed the topics and CME objectives during the planning sessions. The faculty’s submitted content was validated by CME Institute staff, and the activity was evaluated for accuracy, use of evidence, and fair balance by the Chair and a peer reviewer who is without conflict of interest.
Acknowledgment
This activity is derived from the teleconference series “Current Management Approaches for Insomnia,” which was held in June, September, and October 2020 and supported by an educational grant from Eisai, Inc. The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the commercial supporter.
Faculty Affiliation
Russell P. Rosenberg, PhD
Atlanta School of Sleep Medicine and Technology and NeuroTrials Research, Inc., Georgia
Andrew D. Krystal, MD
Department of Psychiatry and Behavioral Sciences, University of California San Francisco
Financial Disclosure
The faculty for this CME activity and the CME Institute staff were asked to complete a statement regarding all relevant personal and financial relationships between themselves or their spouse/partner and any commercial interest. The Accreditation Council for Continuing Medical Education (ACCME) defines a commercial interest as any entity producing, marketing, re-selling, or distributing health care goods or services consumed by, or used on, patients. The ACCME defines relevant financial relationships as financial relationships in any amount occurring within the past 12 months that create a conflict of interest. The CME Institute has resolved any conflicts of interest that were identified. No member of the CME Institute staff reported any relevant personal financial relationships. Faculty financial disclosure is as follows:
Dr Rosenberg is a consultant and member of the speakers/advisory boards for Jazz, Eisai, and Harmony BioSciences and has received grant/research support from Jazz, Eisai, and Avadel. Dr Krystal is a consultant for or receives research support from Adare, Axsome Therapeutics, Big Data, Eisai, Evecxia Therapeutics, Ferring, Galderma, Harmony Biosciences, Idorsia, Janssen, Jazz, Millenium, Merck, Neurocrine Biosciences, Pernix Therapeutics, Otsuka, Sage Therapeutics, and Takeda and has received grant/research support from Janssen, Axsome Therapeutics, Reveal Biosensors, The Ray and Dagmar Dolby Family Fund, and the National Institutes of Health.
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this enduring material for a maximum of 1.00 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Nurses Credentialing Center (ANCC) and the American Academy of Physician Assistants (AAPA) accept certificates of participation for educational activities certified for AMA PRA Category 1 Credit™ from organizations accredited by the ACCME.
To obtain credit for this activity, study the material and complete the CME Posttest and Evaluation.
This PDF is free for all visitors!




