INSIGHTS IN SCHIZOPHRENIA:
Perspectives From Experts on Relapse, the Role of Long-Acting Injectable Antipsychotics, and UZEDY® (risperidone)

Leslie Citrome, MD, MPH
Clinical Professor of Psychiatry and Behavioral SciencesNew York Medical CollegeValhalla, NY

Andrew Cutler, MD
Clinical Professor of PsychiatrySUNY Upstate Medical UniversityLakewood Ranch, FL

Thomas Gazda, MD
Founder and Medical DirectorPromises ScottsdaleScottsdale, AZ

Amber Hoberg, MSN, APRN, PMHNP-BC
GeroPsych Associates of Central TexasMorning Star Family Medicine PLLCSan Antonio, TX

Jonathan M. Meyer, MD
Voluntary Clinical ProfessorDepartment of PsychiatryUniversity of CaliforniaSan Diego, CA
Table of Contents
Introduction
Back to top
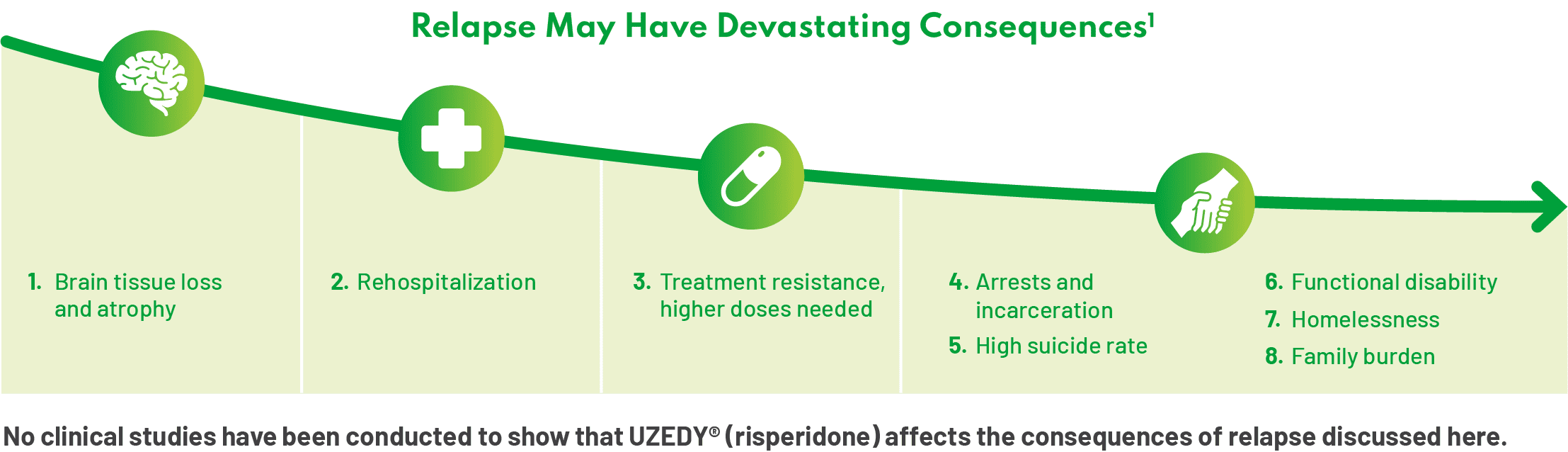
For patients living with schizophrenia, relapse can have devastating consequences.1 Nonadherence to antipsychotics and subsequent relapse are among the most significant disease-related challenges providers and patients may face.2 Underscoring the magnitude of this problem, it is estimated that two-thirds of patients with schizophrenia are partially adherent or nonadherent to antipsychotics, leading to negative outcomes.3-6 Prevention of relapse, therefore, is the fundamental focus of long-term management.
In March 2024, Teva convened a panel of experts in management of schizophrenia for a roundtable discussion to examine challenges and opportunities in the treatment of patients living with schizophrenia, with relapse prevention being a significant topic of interest. Embedded in this conversation are insights into causes of relapse, the consequences of successive relapse on antipsychotic response and patients’ lives, and approaches to treatment in patients with a history of relapse. – Andrew Cutler, MD
One treatment approach considered by this panel of experts was the use of long-acting injectable (LAI) antipsychotics, which, they agreed, continue to be underused by their psychiatry colleagues.7 They reviewed the American Psychiatric Association (APA) recommendations on use of LAIs for the treatment of patients with schizophrenia and explored reasons for clinician reluctance to employ this evidence-based strategy.8 The experts also emphasized the challenges with continuity of care and transitions between care settings that are particularly important for patients with schizophrenia, and the role that LAIs can play during such transitions. Additionally, they delved into other difficulties in managing patients living with schizophrenia that merit further attention from the psychiatry community. – Jonathan M. Meyer, MD
An LAI formulation discussed by the expert panel during this meeting was a subcutaneous risperidone LAI approved for adults with schizophrenia that uses the innovative SteadyTeq™ technology to provide a distinct pharmacokinetic profile, which includes (1) the ability to rapidly achieve therapeutic plasma levels within 24 hours of administration of a single dose, (2) sustained plasma levels for the duration of the dosing interval, and (3) the resulting streamlined initiation process, with no need for successive loading dose injections or oral supplementation.9-11 – Amber Hoberg, MSN, APRN, PMHNP-BC
No clinical studies have been conducted to show that UZEDY affects nonadherence or the consequences of relapse discussed here.
Consequences and Causes of Relapse in Patients With Schizophrenia
Back to top
What significant changes in patient characteristics have you observed with successive relapses?
There is evidence to support the progression of schizophrenia on the clinical and neurobiological levels. Successive relapses in schizophrenia can be dangerous. They not only affect patients’ lives—their sense of reality and self-esteem—but also have the potential to cause brain loss and atrophy, impact neuronal function, and may lead to cognitive impairment.1,12 Rehospitalization and treatment resistance represent other outcomes associated with successive relapse; patients also may begin to need higher doses of their medication.1 When treating schizophrenia, there needs to be a sense of urgency around preventing relapse. – Andrew Cutler, MD
We cannot underestimate the profound psychosocial effects of relapse on patients’ lives. I often use the idea of “burning bridges.” What I’ve seen in my patients is that with each successive relapse, they may burn bridges with their family, their treatment provider, the police. Patients may also be affected at school and in their workplace, with successive relapses leading to reduced attendance and higher rates of unemployment, respectively.13,14 – Thomas Gazda, MD
What factors may contribute to relapse in patients with schizophrenia?
Partial adherence or nonadherence to medication is one of the key factors predicting risk of relapse.2 In my experience, neglecting 1 or 2 doses may cause patients to miss subsequent ones, potentially leading to relapse. Other factors associated with increased risk of relapse include previous relapse, lack of insight, complexity of medication regimen, adverse effects and drug-drug interactions, substance use disorders, negative symptoms, and cognitive impairment.2,15-18 – Leslie Citrome, MD, MPH
Sometimes, external factors can contribute to a patient’s relapse.19 Recently, I had a patient who was quite stable on his medication. One day, due to circumstances out of his control, he was detained and missed several days of medication. Unfortunately, we tried to get him stable on the same medication, but it was no longer working for him.8,20 This patient’s experience underscores the various factors that can contribute to relapse in our patients with schizophrenia. – Amber Hoberg, MSN, APRN, PMHNP-BC
No clinical studies have been conducted to show that UZEDY affects adherence.
How does nonadherence, specifically, contribute to risk of relapse?
Although medication adherence is typically defined as ≥80% of medication taken over 12 months and/or <1 week of missed medications over 3 months, this research cutoff may be too low for patients with schizophrenia.21,22 In a 3-year longitudinal study of 341 patients who were treated with antipsychotics after first-episode psychosis, patients who missed more than 10% of their prescribed medication had a lower time to relapse than patients who were more adherent to their medication.23 – Leslie Citrome, MD, MPH
Another study looking at pharmacy refill and medical claims data for 4325 patients found that when these patients missed as few as 1 to 10 days of medication, the risk of hospitalization doubled. Six percent of patients with a maximum 0-day medication gap were hospitalized, versus 12% of patients with a maximum gap of 1 to 10 days.4 For me, these data are quite striking; it’s a call to action. – Andrew Cutler, MD
What contributes to nonadherence among patients with schizophrenia, and how can it be addressed?
I think clinicians are aware that treatment nonadherence is an issue but often underestimate it in their own practice. In a recent survey, clinicians estimated that around 53% of patients nationwide were nonadherent to oral antipsychotics.24 Adherence is an issue inherent in treating patients with any chronic disorder; the rate of nonadherence in patients with schizophrenia is similar to that seen with other chronic conditions, such as asthma and hypertension.25,26 However, our patients with schizophrenia may also have cognitive problems, complex medication regimens, and substance use disorders that make adherence quite challenging.2,15,17 – Jonathan M. Meyer, MD
When I discuss adherence with my patients, I try to take the blame off them. I say, “Look, there are days when I don’t remember to take my medication. So, since the last time I saw you, how many doses have you missed?” – Amber Hoberg, MSN, APRN, PMHNP-BC
Additionally, clinicians must rely on evidence-based methods, such as pill counts and measurement of plasma antipsychotic levels to assess treatment adherence.27 Approaches to improving adherence that might prove useful include providing patients with a pill box, sending electronic reminders, and offering educational materials to increase awareness of nonadherence.28,29 – Jonathan M. Meyer, MD
The APA suggests that patients receive treatment with a long-acting injectable antipsychotic medication if they prefer such treatment or if they have a history of poor or uncertain adherence.8 – Leslie Citrome, MD, MPH
Chronic relapse can lead to treatment resistance. How do you confirm that your patients have reached this stage?
Treatment-resistant schizophrenia is defined as the persistence of symptoms despite ≥2 trials of antipsychotics of adequate dose and duration with documented adherence.8,30 It is critical to rule out pseudoresistance due to nonadherence by assessing plasma antipsychotic levels.27 Additionally, the Treatment Response and Resistance in Psychosis (TRRIP) guidelines state that, optimally, treatment resistance should only be determined after at least 1 failed trial of an LAI given for at least 6 weeks after it has achieved steady state.30 – Jonathan M. Meyer, MD
The TRRIP guidelines recommend that a person should not be considered treatment-resistant until they’ve had a trial of an LAI.30 – Andrew Cutler, MD
No clinical studies have been conducted to show that UZEDY affects adherence.
The Role of LAIs in the Treatment of Schizophrenia
Back to top
How do LAIs fit into the treatment algorithm for patients with schizophrenia?
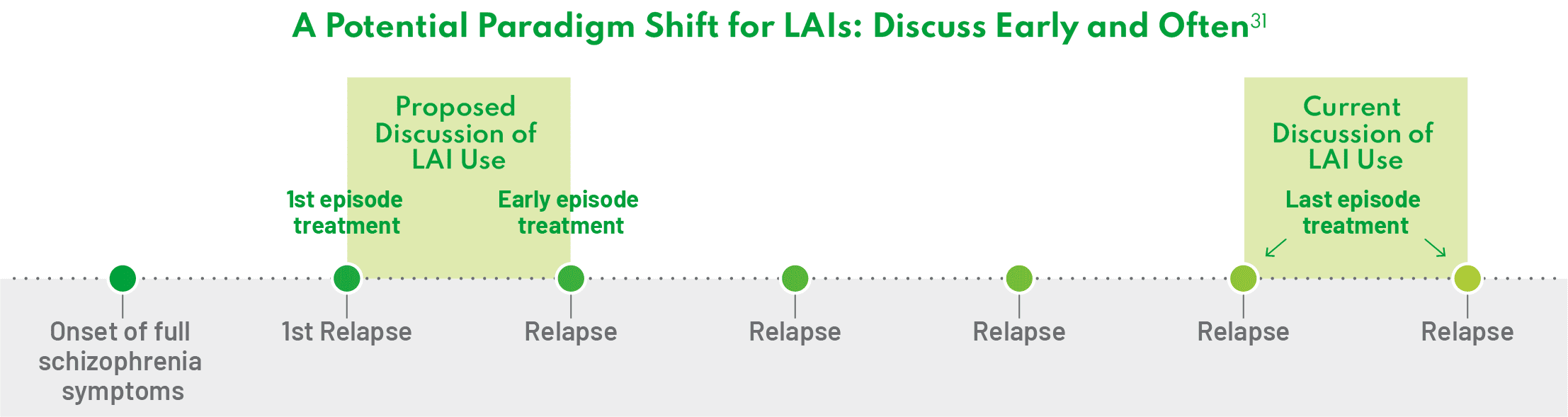
There is an old mindset that we have to address that views LAIs as a last resort for patients who have had successive relapses.31 But the standard of care is changing. We need to shift from a fail-first treatment paradigm to where patients are presented with all effective treatment options as early as possible.31 – Jonathan M. Meyer, MD
Evidence suggests that LAIs may be helpful early in the treatment process, at a time when the disease is most treatable.32 To that end, I discuss LAIs early and often with my patients with schizophrenia, and I think more clinicians should start the conversation about LAIs earlier in the course of the disease.32 Additionally, administration of LAIs after first hospitalization can prevent relapse and rehospitalization.32 Personally, I want to make sure every single one of my patients are aware that LAIs exist. – Leslie Citrome, MD, MPH
One way to shift the treatment paradigm is to present LAIs as one possible treatment option for schizophrenia after the patient’s first episode rather than an alternative option after treatment failure.31 In my opinion, it is crucial to offer and discuss all available treatment possibilities with patients; failure to do so is doing them a disservice. – Andrew Cutler, MD
What percentage of patients with schizophrenia are being treated with LAIs?
Although use of LAI formulations is normalized in other disease states (eg, diabetes, HIV), they are still underutilized by psychiatric clinicians, with data indicating that they use LAIs in <10% of their patients and have not offered an LAI to nearly 50% of patients with schizophrenia.7,33,34 One must educate patients about treatment options. If they are never informed about the LAI option, how can they consider it as a treatment option? – Jonathan M. Meyer, MD
The percentage of patients treated with LAIs varies between regions, care settings, and even clinical practices in the same city.35 Additionally, LAIs are more commonly used by assertive community treatment teams.36 – Leslie Citrome, MD, MPH
Why might LAIs be underused by clinicians?
First, clinicians may persist in the belief that all their patients are consistent in taking their medicine on a daily basis.37 Second, I think that the lack of LAI use in the healthcare setting may have been influenced by the introduction of atypical second-generation oral antipsychotics.38 Typical LAIs were generally reserved for patients who failed the newer oral atypical antipsychotics.39 Since then, the stigma that LAIs are reserved for more severely ill patients has persisted, reducing the uptake of atypical antipsychotic LAIs by clinicians.40 Educational materials demonstrating the efficacy and safety profile of LAIs and instructions for medication administration could increase the uptake of LAIs in clinical practices, as they may demystify the injection process quite a bit.41 Lastly, clinicians may be unsure of how to talk about LAIs with patients and teaching the techniques of motivation interviewing may help.37,42,43 – Leslie Citrome, MD, MPH
I also think clinicians are trying to put themselves in the patient’s shoes. Personal views on injections could affect whether a clinician offers an LAI. The clinician assumes that the patient does not want an injection, and they fear that offering a medication the patient opposes will hinder their relationship with the patient.44 – Andrew Cutler, MD
What are some reasons that patients might choose treatment with LAIs?
In my experience, LAIs may help reduce the uncertainty around how frequently my patients are or aren’t taking their medication. During discussions with patients, I can focus on psychosocial issues and functional goals, rather than devoting additional time to assessing whether or not my patient has been adherent. – Thomas Gazda, MD
Some of my patients expressed interest in the dosing schedule associated with LAIs. The patient can come into the office, receive their injection, and go about their life and business until their next scheduled injection. Of course, we monitor for possible side effects as well. With an LAI, my patients say they don’t have to think about taking their medication daily.46 One of my patients once said, “Taking a pill every day is a daily reminder of my condition.” – Andrew Cutler, MD
What information do HCPs need to better understand how LAIs can more effectively fit into their practice?
Sharing patient experiences with LAIs can help motivate clinicians to start the conversation about LAIs. – Leslie Citrome, MD, MPH
There are more data coming out displaying the features of LAIs that are relevant to both patients and providers, and I think helping people understand how to have a conversation about LAIs may be part of this. Improving HCPs’ education with communication about the role of LAIs is essential to increasing uptake in clinical practices.41 – Andrew Cutler, MD
The multidisciplinary team must also be aligned with treatment options for patients. For example, one of my patients seemed like the perfect candidate for an LAI, but he was hesitant when I proposed the treatment. However, he came back later to tell me that he would like to start treatment with the LAI. Over the course of a couple of days, he had spoken with the nursing staff who helped address some of his concerns. – Thomas Gazda, MD
When choosing an LAI for patients with schizophrenia, clinicians should consider, in addition to safety and efficacy, medication delivery and pharmacokinetic (PK) profiles, including the need for loading injections or prolonged oral supplementation.47,48 – Jonathan M. Meyer, MD
What are some of the challenges with continuity of care associated with transitions between care settings that are particularly important for patients with schizophrenia?
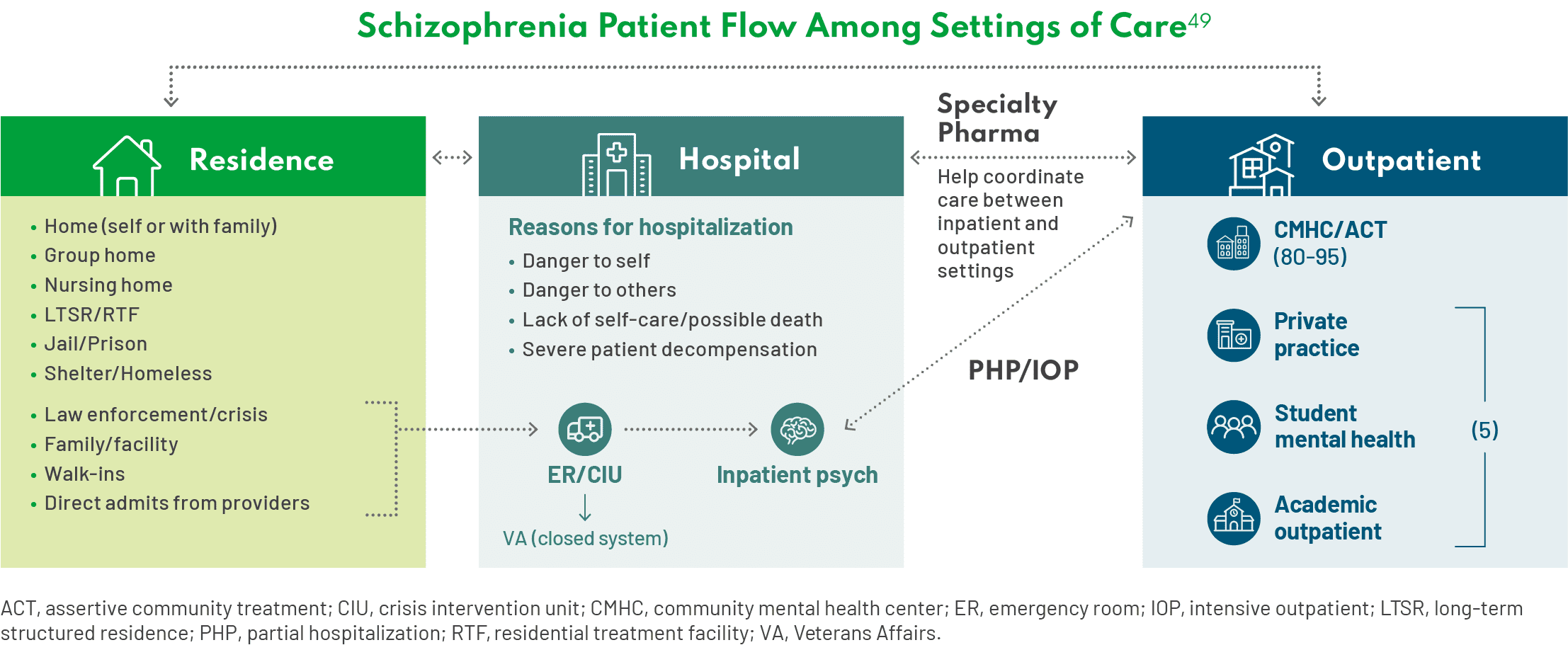
This isn’t the first time this has come up in this conversation, but there are several challenges with continuity of care in patients with schizophrenia. Patients with schizophrenia are going to shift between these settings of care—residence, hospital, and outpatient settings. Since deinstitutionalization began roughly 30 years ago, the numbers of patients in our practices coming from the jail setting have increased. In other cases, the patients are homeless. When patients transition between care settings, they may fall through the cracks and not receive consistent treatment.50 Ideally, there would be frequent, direct communication between the inpatient psychiatry unit and outpatient services, but in my experience, that rarely happens. – Thomas Gazda, MD
What role can LAIs play?
There is an anecdote I like to share about Medicaid managed care programs in Brooklyn, New York. There was a problem in some hospitals that had high readmission rates within 30 days. To address this issue, the health plan initiated a policy in which they would pay for treatment with LAIs, but they would not pay for readmissions that occurred within 30 days of discharge. Under this policy, readmissions rates went down.51* – Leslie Citrome, MD, MPH
*Data are from a retrospective cohort analysis of 1450 patients with a sole diagnosis of schizophrenia and 15,556 patients with schizophrenia, including patients with comorbid mood disorders.
Think of all the ways we can minimize dropping the ball when patients transition between care settings, based on what LAIs can offer. The literature documents that use of LAIs delays the time to psychiatric hospitalization after an initial schizophrenia diagnosis or to arrest/incarceration in a patient with a prior history of such events.52 – Jonathan M. Meyer, MD
Expert Perspectives on LAIs
Back to top

We need to acknowledge that our patients are not necessarily adherent to treatment, and it can be difficult for them due to the challenges posed by schizophrenia itself (eg, lack of insight, stigma, chaotic lifestyle). – Leslie Citrome, MD, MPH

Our patients with schizophrenia struggle with adherence, and how can we blame them? Schizophrenia is complicated enough. For providers, it is challenging and time-consuming to confirm how frequently our patients are taking their medication. With LAIs, I do not have to spend time trying to assess and confirm that my patient has taken their medication. If they show up for their injection, I know that my patient has taken their medication. I devote the time typically used to identify if my patient is taking their medication to understanding their additional mental health needs. – Thomas Gazda, MD

In my opinion, it is doing the patient a disservice if you do not offer and discuss all treatment options. LAIs are an important treatment option for patients with schizophrenia. They should be discussed as an option up front and not used just as an alternative to treatment failure. – Andrew Cutler, MD

For me, failure to discuss LAIs as an option with patients recently diagnosed with schizophrenia, and especially to a patient who is nonadherent or has relapsed, is below the standard of care: The APA suggests that patients receive treatment with an LAI if they prefer such treatment or if they have a history of poor or uncertain adherence. There should be open and ongoing discussion about treatment between HCPs and patients about their treatment options. – Jonathan M. Meyer, MD

It is critical to let the patient be a key driver of treatment decisions and allow them to determine their preference with a good understanding of the available treatment options. Avoid presuming patients won’t be interested in an injection; the patient can tell you their preference after they know everything that is available to them. – Amber Hoberg, MSN, APRN, PMHNP-BC
UZEDY® (risperidone): A Different LAI With SteadyTeq™ Technology
Back to top
What is UZEDY?
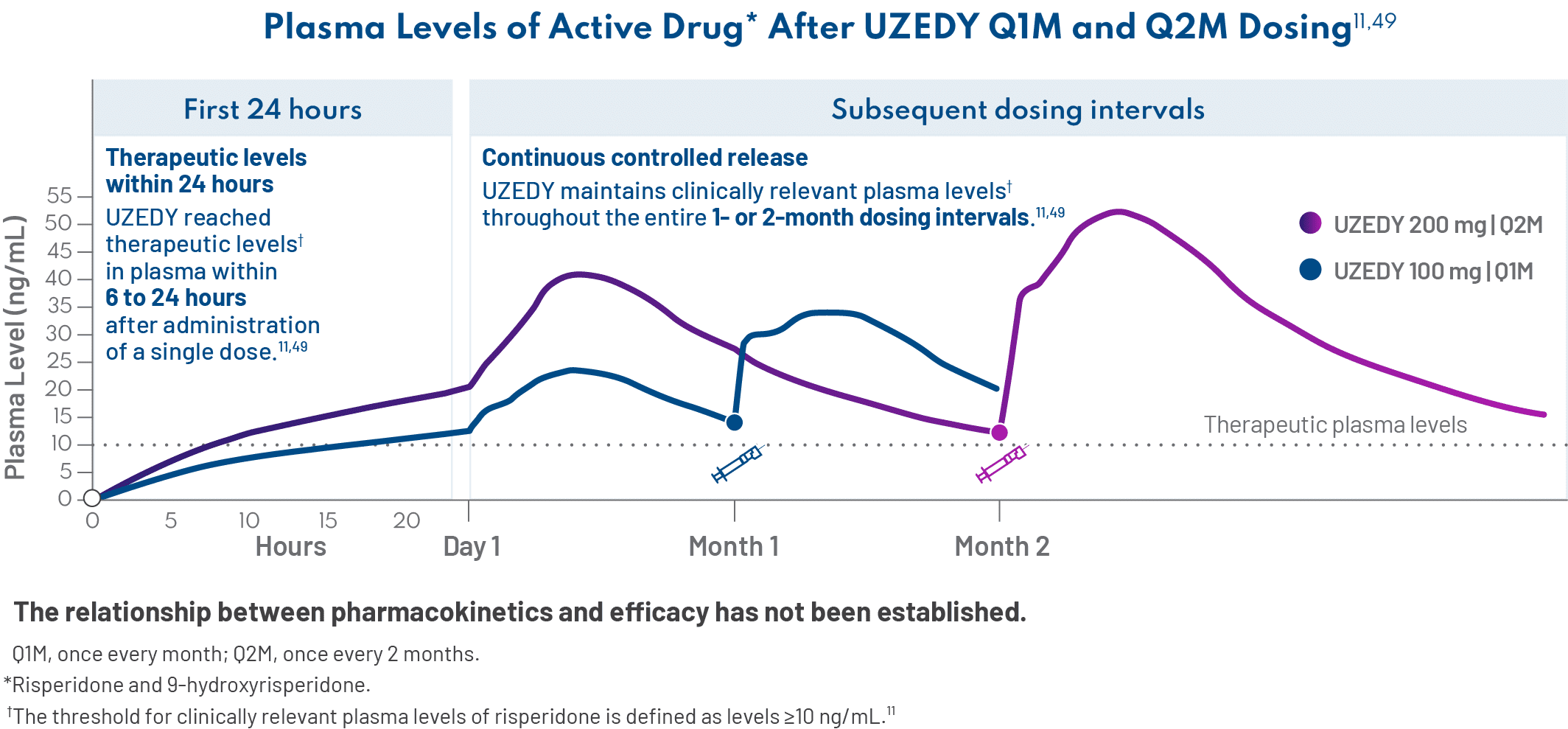
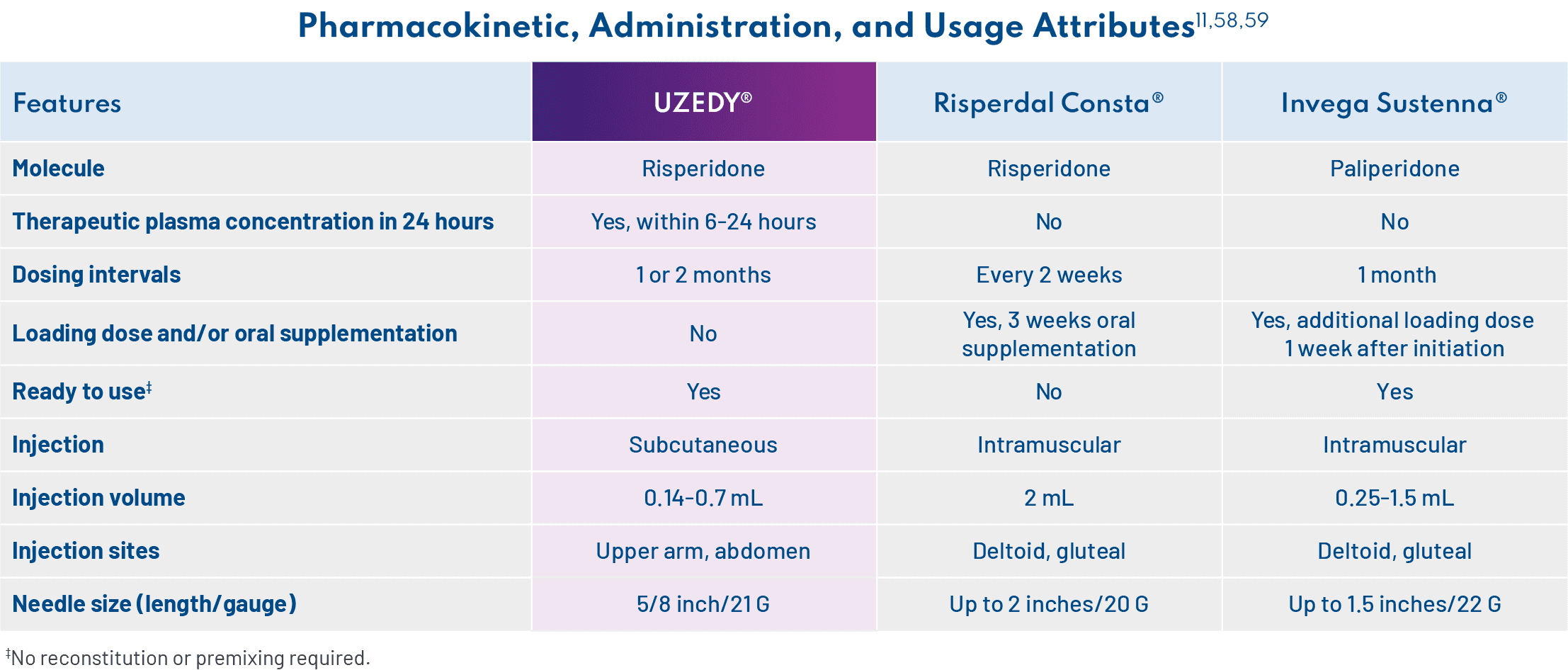
UZEDY is risperidone delivered via SteadyTeq™ technology.9,10 A World Health Organization essential medicine, risperidone is one of the most widely prescribed atypical antipsychotics, with a well-studied efficacy and safety profile.53,54 With SteadyTeq™, risperidone can be delivered subcutaneously, and therapeutic plasma levels are reached within 6 to 24 hours and sustained for the duration of the 1-month or 2-month dosing interval.11,55 – Andrew Cutler, MD
You know, when UZEDY was first approved, I thought, “Oh, it’s just another risperidone product.” But UZEDY is different. When I learned about the SteadyTeq™ technology and the attributes it conveys to the product, that’s when I realized the difference with this formulation and began to use it in my patients. – Amber Hoberg, MSN, APRN, PMHNP-BC
How does SteadyTeq™ differentiate UZEDY from other LAI products?
For me, UZEDY can be a good option. This technology allows subcutaneous delivery of an LAI antipsychotic in a manner that rapidly reaches therapeutic concentrations in plasma within 6 to 24 hours of administration of a single dose, and these levels are sustained for the duration of the dosing interval.11 The result is a streamlined initiation process with no loading dose or oral supplementation required.9 – Andrew Cutler, MD
Importantly, if a dose of UZEDY is missed, the next dose can be administered as soon as possible without a loading dose or oral supplementation, so reinitiation is streamlined as well.11 – Jonathan M. Meyer, MD
In addition to the PK-related features, UZEDY has a number of attributes relating to administration and usage, including needle size and injection site, which are important for providers and patients to understand. For example, one of my patients initially rejected injections but was more open to them after learning about these features. I explained to him that I could inject the drug into the back of his arm, and he agreed to try it. The nurses I work with who administer the drug have spoken positively about the ready-to-use prefilled syringe, which does not require mixing or reconstitution.11 In terms of storage, UZEDY can be stored in the refrigerator or at room temperature for 90 days, which is especially important for HCPs who work in clinics that administer a lot of injections or are small and have limited refrigerator storage (if refrigerated, UZEDY must sit at room temperature for at least 30 minutes before injection).11 – Amber Hoberg, MSN, APRN, PMHNP-BC
This information should not be construed to imply any difference in safety, efficacy, or other clinical outcome.
All trademarks referenced are properties of their respective owners.
Efficacy and Safety Data for UZEDY
Back to top
What are some of the key elements from the RISE study design?
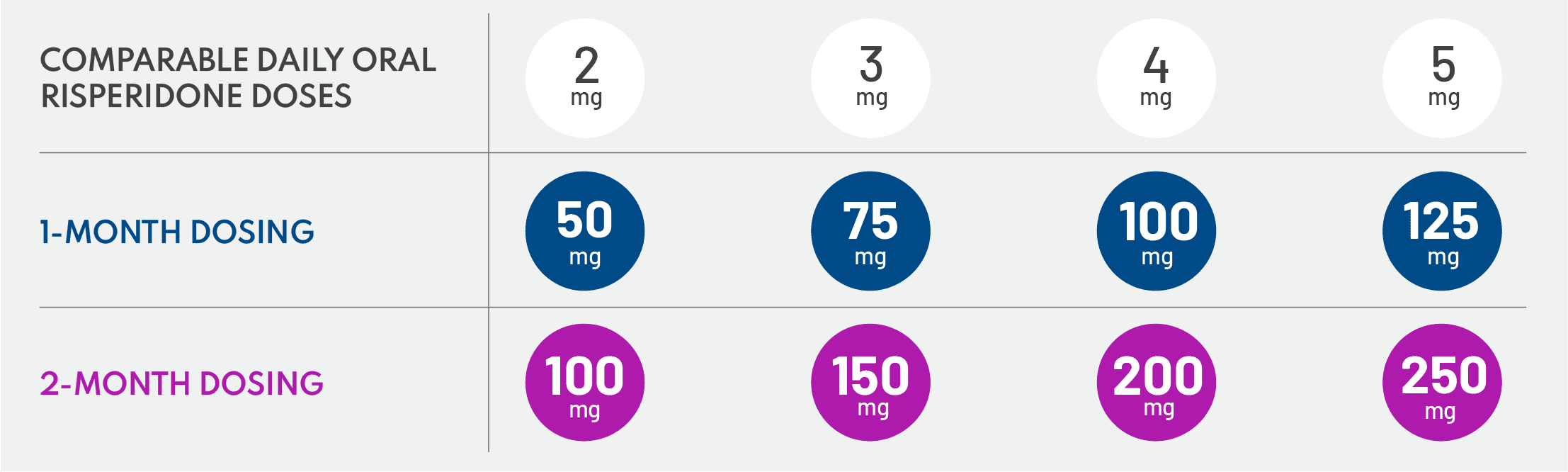
The safety and efficacy of UZEDY were evaluated in the phase 3 RISE trial, which was the largest and longest trial of a risperidone LAI in schizophrenia to date.10,11,49,58,60 RISE was a double-blind, placebo-controlled, randomized relapse prevention study that compared UZEDY Q1M or Q2M vs placebo in patients with schizophrenia.10 Importantly, participants who enrolled in the trial must have had ≥1 episode of relapse in the past 24 months.10 After a 4-week screening period, 544 patients were stabilized on 2 mg to 5 mg of oral risperidone per day for 12 weeks.10 Stabilized patients were then randomized to UZEDY Q1M or Q2M or placebo.10 Those who were randomized to either UZEDY arm received a dose strength that corresponded to the oral dose of risperidone on which they were stabilized.11 The primary endpoint was time to impending relapse, and the trial was halted when 90 relapse events were recorded across all study patients.10 It is worth noting that patients who were randomized in the study were quite stable, with Positive and Negative Syndrome Scale scores around 61, and this may explain why there were patients still on placebo who had not relapsed when the study ended.10 – Jonathan M. Meyer, MD
It is also worth highlighting that this is not an acute study and that the primary endpoint is not a rating scale; it is time to impending relapse.10 – Andrew Cutler, MD
What are the efficacy results from the clinical trials with UZEDY?
Results from RISE showed that adult patients treated with UZEDY Q1M or Q2M experienced a statistically significant delay in time to relapse; 29% of patients receiving placebo experienced impending relapse compared with 7% of patients receiving UZEDY Q1M at study termination.49 Furthermore, UZEDY Q1M significantly reduced the risk of relapse by 80% compared with placebo.49 – Andrew Cutler, MD
Relapse was defined as
- Clinical Global Impression – Improvement (CGI-I) score ≥5, AND
- Any key Positive and Negative Syndrome Scale (PANSS) item (conceptual disorganization, hallucinatory behavior, suspiciousness, and unusual thought content) >4 with ≥2-point increase from randomization on any individual key PANSS items, OR
- ≥4-point increase from randomization in combined score of key PANSS items
- Hospitalization due to worsening of psychotic symptoms
- Clinical Global Impression – Severity of Suicidality (CGI-SS) score ≥4 on part 1 and/or ≥6 on part 2
- Violent behavior resulting in self-injury, injury to another person, or property damage
How consistent is the safety profile of UZEDY with the known safety profile of oral risperidone, and what long-term safety results have been demonstrated?
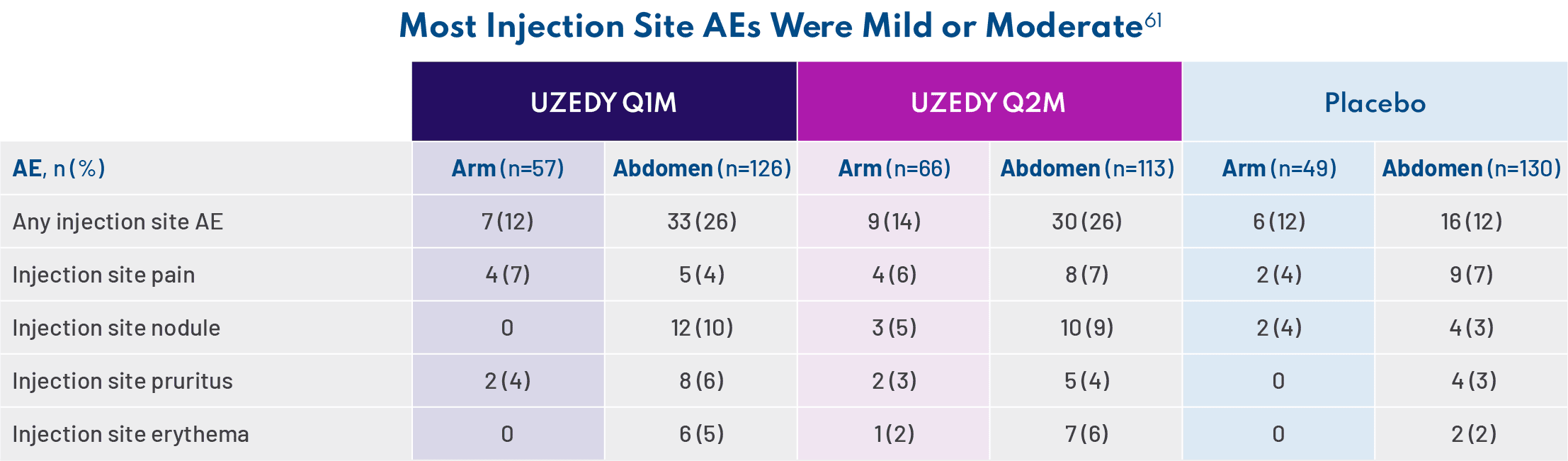
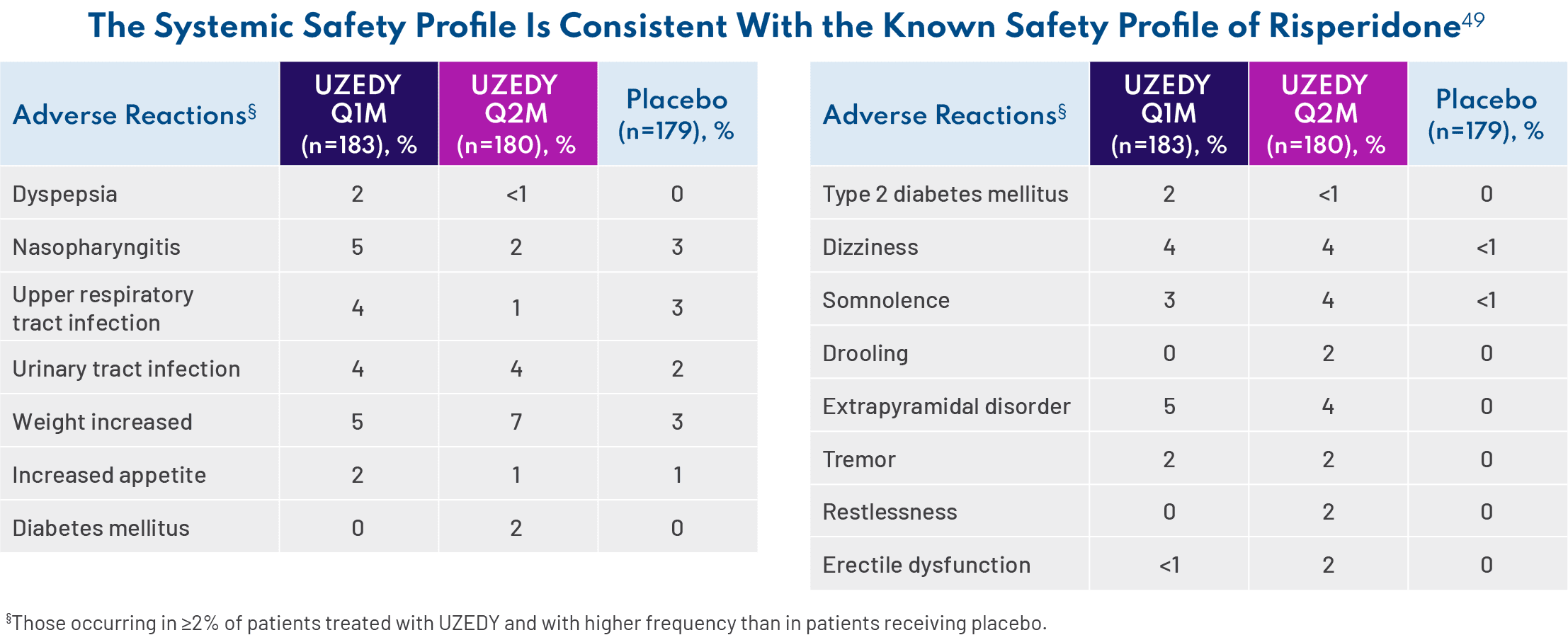
Because oral risperidone is so widely prescribed, we are very familiar with its safety profile in adults with schizophrenia established in three 4- to 8-week, double-blind, placebo-controlled trials.11 UZEDY is risperidone, so it is not surprising that the safety profile of UZEDY is consistent with that of the corresponding oral risperidone dose of 2 mg to 5 mg daily. The most common adverse reactions (>5% and twice placebo) in three 4- to 8-week, double-blind, placebo-controlled trials of oral risperidone were parkinsonism, akathisia, dystonia, tremor, sedation, dizziness, anxiety, blurred vision, nausea, vomiting, upper abdominal pain, stomach discomfort, dyspepsia, diarrhea, salivary hypersecretion, constipation, dry mouth, increased appetite, increased weight, fatigue, rash, nasal congestion, upper respiratory tract infection, nasopharyngitis, and pharyngolaryngeal pain.11 Since UZEDY is delivered as subcutaneous injections, one should also examine injection site reactions. The most common injection site reactions with UZEDY (≥5% and greater than placebo) were pruritus and nodule. In the UZEDY clinical trials, most injection site adverse events (AEs) and nodules were mild or moderate.61 The AEs occurring in 5% of patients taking UZEDY and more than placebo were nasopharyngitis, weight increased, and extrapyramidal disorder.49 – Jonathan M. Meyer, MD
It is important to note that the discontinuation rate was similar in the placebo and UZEDY arms—11% vs 13%, respectively.49 If a patient experienced an AE, it usually didn’t lead to discontinuation of treatment. Long-term safety was assessed in SHINE, a phase 3, multicenter, randomized, double-blind, parallel-group study in which 336 adults with schizophrenia were stabilized on oral risperidone for 12 weeks, randomized to UZEDY Q1M or Q2M, and followed for 56 weeks.62 Results from SHINE were consistent with the data from the RISE study, with no new safety signals.62 – Andrew Cutler, MD
Using UZEDY: Initiation, Dosing, and Administration
Back to top
What are the dosing intervals and strengths for UZEDY?
Dosages correspond to 2 mg/day to 5 mg/day oral risperidone, with the option of a 1-month or 2-month dosing interval, which allows for individualization of patient care.11 Selection of dosing intervals is based on patient needs and clinician judgment, and patients can start at any dose with either the 1-month or 2-month schedule. I recommend the 2-month dosing interval for patients who miss appointments or have an unstable living situation. I appreciate the flexibility to offer my patients either option. – Amber Hoberg, MSN, APRN, PMHNP-BC
In my patients who are stable on a medication with a biweekly or monthly injection schedule, if it is appropriate to switch them to UZEDY, I prefer to start them with a 1-month dosing interval to minimize changes in their current dosing schedule. – Jonathan M. Meyer, MD
In rural areas, where you may provide care to patients in a region the size of Massachusetts, it can be challenging to see patients on a monthly or more frequent basis. With the 2-month dosing interval option, I know that my patients are being treated between visits, and I can do an outreach, if needed. – Thomas Gazda, MD
How can patients transition from oral risperidone to UZEDY?
Even though patients were stabilized on oral risperidone for 12 weeks in the RISE study, patients are not required to be stabilized on oral risperidone prior to initiating UZEDY, although tolerability should be established for patients who have never taken risperidone.11 This is a critical point to understand, particularly for less-experienced providers. – Amber Hoberg, MSN, APRN, PMHNP-BC
I agree. I would like to emphasize that patients do not need to be stabilized on oral risperidone. Tolerability should be established for patients who have never taken risperidone, and the time needed to establish tolerability is determined by the clinician.11 – Andrew Cutler, MD
To transition patients from oral risperidone, you select the comparable risperidone dose, select a 1-month or 2-month dosing interval, and administer UZEDY the day after their last dose of oral therapy.11 – Amber Hoberg, MSN, APRN, PMHNP-BC
How is UZEDY administered?
Clinicians should read the “Preparation and Administration Instructions” in the Prescribing Information before administering UZEDY. In short: Whip. Inspect. Inject. The nurses I work with have said that these instructions help them remember how to administer UZEDY. Inspecting to ensure that the bubble is at the cap is critical, and it is important that you do not expel the bubble.11 Many nurses who are familiar with intramuscular injections think that bubbles must be removed, so this administration process may be a bit foreign to them. My nurses who have administered UZEDY in group homes really appreciate the lack of reconstitution with this drug. – Amber Hoberg, MSN, APRN, PMHNP-BC
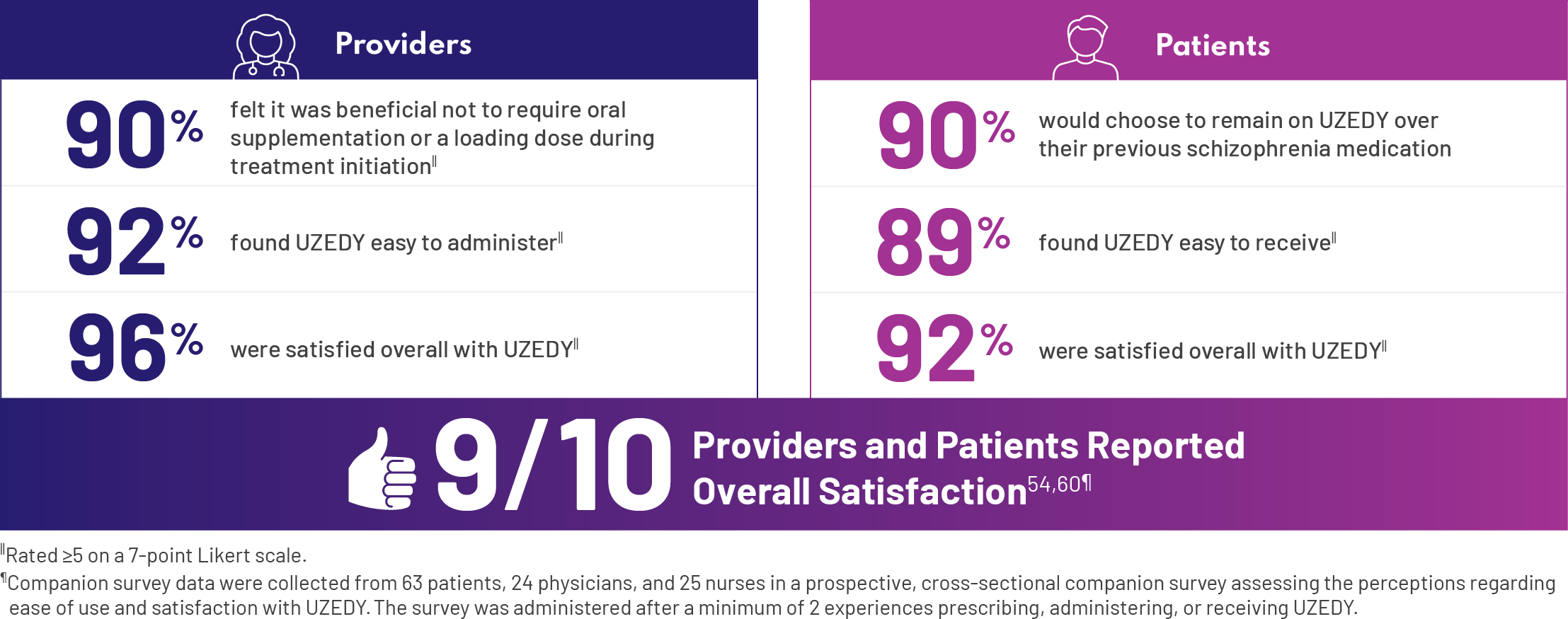
How satisfied were providers and patients with UZEDY?
The attributes of UZEDY that we’ve been discussing—they’re not just theoretical. I can tell you based on my own experience as a provider that these are important. However, there are also data from a companion survey that examined patient and HCP perceptions of UZEDY. I was impressed that a high percentage of patients would choose to remain on UZEDY over their previous schizophrenia medication.63 – Andrew Cutler, MD
Get more information on how UZEDY may support your patients, particularly in transitions of care.
References
Back to top
- Nasrallah HA. 10 devastating consequences of psychotic relapses. Curr Psychiatr. 2021;20(5):9-12.
- Alvarez-Jimenez M, Priede A, Hetrick SE, et al. Risk factors for relapse following treatment for first episode psychosis: a systematic review and meta-analysis of longitudinal studies. Schizophr Res. 2012;139(1-3):116-128.
- Kaplan G, Casoy J, Zummo J. Impact of long-acting injectable antipsychotics on medication adherence and clinical, functional, and economic outcomes of schizophrenia. Patient Prefer Adherence. 2013;7:1171-1180.
- Masand PS, Roca M, Turner MS, Kane JM. Partial adherence to antipsychotic medication impacts the course of illness in patients with schizophrenia: a review. Prim Care Companion J Clin Psychiatry. 2009;11(4):147-154.
- Ascher-Svanum H, Zhu B, Faries DE, Furiak NM, Montgomery W. Medication adherence levels and differential use of mental-health services in the treatment of schizophrenia. BMC Res Notes. 2009;2:6.
- Ahn J, McCombs JS, Jung C, et al. Classifying patients by antipsychotic adherence patterns using latent class analysis: characteristics of nonadherent groups in the California Medicaid (Medi-Cal) program. Value Health. 2008;11(1):48-56.
- Velligan DI, Weiden PJ, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46.
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. 3rd ed. Washington, DC: American Psychiatric Association; 2021.
- Merenlender Wagner A, Elgart A, Perlstein I, et al. Phase 1 open-label study assessing the pharmacokinetics and safety of TV-46000, a novel long-acting subcutaneous injectable formulation of risperidone. Presented at: European Congress of Neuropathology; October 2-5, 2021; Lisbon, Portugal. Poster P.0463.
- Kane JM, Harary E, Tohami O, et al. Subcutaneous risperidone (TV-46000) efficacy and safety in schizophrenia: a phase 3, randomized, double-blind, relapse prevention study (RISE study). Presented at: Psych Congress 2021; October 29-November 1, 2021; San Antonio, TX.
- UZEDY® (risperidone) extended-release injectable suspension Current Prescribing Information. Parsippany, NJ: Teva Neuroscience, Inc.
- Holding JC, Tarrier N, Gregg L, Barrowclough C. Self-esteem and relapse in schizophrenia: a 5-year follow-up study. J Nerv Ment Dis. 2013;201(8):653-658.
- John A, Friedmann Y, DelPozo-Banos M, Frizzati A, Ford T, Thapar A. Association of school absence and exclusion with recorded neurodevelopmental disorders, mental disorders, or self-harm: a nationwide, retrospective, electronic cohort study of children and young people in Wales, UK. Lancet Psychiatry. 2022;9(1):23-34.
- Lin D, Joshi K, Keenan A, et al. Associations between relapses and psychosocial outcomes in patients with schizophrenia in real-world settings in the United States. Front Psychiatry. 2021;12:695672.
- Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10(2):1-7.
- Bergé D, Mané A, Salgado P, et al. Predictors of relapse and functioning in first-episode psychosis: a two-year follow-up study. Psychiatr Serv. 2016;67(2):227-233.
- Loots E, Goossens E, Vanwesemael T, Morrens M, Van Rompaey B, Dilles T. Interventions to improve medication adherence in patients with schizophrenia or bipolar disorders: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(19):10213.
- Uçok A, Polat A, Cakir S, Genç A. One year outcome in first episode schizophrenia. Predictors of relapse. Eur Arch Psychiatry Clin Neurosci. 2006;256(1):37-43.
- San L, Bernardo M, Gómez A, Peña M. Factors associated with relapse in patients with schizophrenia. Int J Psychiatry Clin Pract. 2013;17(1):2-9.
- Bronson J, Berzofsky M. Indicators of Mental Health Problems Reported by Prisoners and Jail Inmates, 2011-12. Bureau of Justice Statistics. 2017;6:1-16. Accessed July 16, 2025. https://bjs.ojp.gov/content/pub/pdf/imhprpji1112.pdf
- Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. 2014;5:43-62.
- Brissos S, Veguilla MR, Taylor D, Balanzá-Martinez V. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198-219.
- Pelayo-Terán JM, Gajardo Galán VG, de la Ortiz-García de la Foz V, et al. Rates and predictors of relapse in first-episode non-affective psychosis: a 3-year longitudinal study in a specialized intervention program (PAFIP). Eur Arch Psychiatry Clin Neurosci. 2017;267(4):315-323.
- Velligan D, Jain R. Determining Clinician Factors for Implementing LAIs and Defeating Barriers (DECIDE): results from a survey of US-practicing psychiatric clinicians. Presented at: PSYCH Symposium. November 17, 2023. London, England.
- Bidwal M, Lor K, Yu J, Ip E. Evaluation of asthma medication adherence rates and strategies to improve adherence in the underserved population at a Federally Qualified Health Center. Res Social Adm Pharm. 2017;13(4):759-766.
- Villalva CM, Alvarez-Muiño XLL, Mondelo TG, Fachado AA, Fernández JC. Adherence to treatment in hypertension. Adv Exp Med Biol. 2017;956:129-147.
- Bright CE. Measuring medication adherence in patients with schizophrenia: an integrative review. Arch Psychiatr Nurs. 2017;31(1):99-110.
- El-Mallakh P, Findlay J. Strategies to improve medication adherence in patients with schizophrenia: the role of support services. Neuropsychiatr Dis Treat. 2015;11:1077-1090.
- Saber E, Abdel-Kader NM, Sayied NE, Mohammed AA. Effect of psycho educational program on improving of medication adherence among schizophrenic patients. IOSR J Nurs Health Sci. 2018;7(6):69-79.
- Howes OD, McCutcheon R, Agid O, et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174(3):216-229.
- Stahl SM. Long-acting injectable antipsychotics: shall the last be first? CNS Spectr. 2014;19(1):3-5.
- Stevens GL, Dawson G, Zummo J. Clinical benefits and impact of early use of long-acting injectable antipsychotics for schizophrenia. Early Interv Psychiatry. 2016;10(5):365-377.
- Morieri ML, Avogaro A, Fadini GP. Long-acting injectable GLP-1 receptor agonists for the treatment of adults with type 2 diabetes: perspectives from clinical practice. Diabetes Metab Syndr Obes. 2020;13:4221-4234.
- Edwards GG, Miyashita-Ochoa A, Castillo EG, et al. Long-acting injectable therapy for people with HIV: looking ahead with lessons from psychiatry and addiction medicine. AIDS Behav. 2023;27(1):10-24.
- Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493.
- Yee MR, Espiridon E, Oladunjoye AO, Millsaps U, Vora A, Harvey N. The use of long-acting injectable antipsychotics (LAI) in the serious mental illness (SMI) patients enrolled in an assertive community treatment (ACT) program. Cureus. 2021;13(4):e14490.
- Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24.
- Conley RR, Kelly DL. Second-generation antipsychotics for schizophrenia: a review of clinical pharmacology and medication-associated side effects. Isr J Psychiatry Relat Sci. 2005;42(1):51-60.
- Biagi E, Capuzzi E, Colmegna F, et al. Long-acting injectable antipsychotics in schizophrenia: literature review and practical perspective, with a focus on aripiprazole once-monthly. Adv Ther. 2017;34(5):1036-1048.
- Schwartz S, Carilli C, Mian T, Ruekert L, Kumar A. Attitudes and perceptions about the use of long-acting injectable antipsychotics among behavioral health practitioners. Ment Health Clin. 2022;12(4):232-240.
- Potkin S, Bera R, Zubek D, Lau G. Patient and prescriber perspectives on long-acting injectable (LAI) antipsychotics and analysis of in-office discussion regarding LAI treatment for schizophrenia. BMC Psychiatry. 2013;13:261.
- Lindenmayer JP, Glick ID, Talreja H, Underriner M. Persistent barriers to the use of long-acting injectable antipsychotics for the treatment of schizophrenia. J Clin Psychopharmacol. 2020;40(4):346-349.
- SCOPE. Clinical education videos. Accessed June 10, 2024. https://www.scopeengage.com/clinical-education-videos
- Potkin S, Bera R, Zubek D, Lau G. Patient and prescriber perspectives on long-acting injectable (LAI) antipsychotics and analysis of in-office discussion regarding LAI treatment for schizophrenia. BMC Psychiatry. 2013;13:261.
- Vita A, Fagiolini A, Maina G, Mencacci C, Spina E, Galderisi S. Achieving long-term goals through early personalized management of schizophrenia: expert opinion on the role of a new fast-onset long-acting injectable antipsychotic. Ann Gen Psychiatry. 2023;22(1):1.
- Blackwood C, Sanga P, Nuamah I, et al. Patients’ preference for long-acting injectable versus oral antipsychotics in schizophrenia: results from the patient-reported medication preference questionnaire. Patient Prefer Adherence. 2020;14:1093-1102.
- Kishimoto T, Hagi K, Kurokawa S, Kane JM, Correll CU. Long-acting injectable versus oral antipsychotics for the maintenance treatment of schizophrenia: a systematic review and comparative meta-analysis of randomised, cohort, and pre-post studies. Lancet Psychiatry. 2021;8(5):387-404.
- Gardner KN, Nasrallah HA. Managing first-episode psychosis: rationale and evidence for nonstandard first-line treatments for schizophrenia. Curr Psychiatry. 2015;14(7):38-45.
- Data on file. Teva Neuroscience, Inc. Parsippany, NJ.
- Farr AM, Smith DM, Pesa JA, Cao Z. Follow-up care among Medicaid patients with schizophrenia treated with antipsychotics in the inpatient setting. J Health Econ Outcomes Res. 2014;2(1):29-40.
- MacEwan JP, Kamat SA, Duffy RA, et al. Hospital readmission rates among patients with schizophrenia treated with long-acting injectables or oral antipsychotics. Psychiatr Serv. 2016;67(11):1183-1188.
- Alphs L, Benson C, Cheshire-Kinney K, et al. Real-world outcomes of paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: a randomized, open-label, review board-blinded 15-month study. J Clin Psychiatry. 2015;76(5):554-561.
- World Health Organization. WHO Model List of Essential Medicines – 22nd list, 2021. Accessed May 29, 2025. https://www.who.int/publications/i/item/WHO-MHP-HPS-EML-2021.02
- Kane JM, Mychaskiw MA, Lim S, et al. Treatment patterns among patients in the United States utilizing long-acting injectable antipsychotic agents: an analysis of a commercial claims database. Presented at: Psych Congress 2021; October 29-November 1, 2021; San Antonio, TX.
- Perlstein I, Merenlender Wagner A, Gomeni R, et al. Population pharmacokinetic modeling and simulation of TV-46000: a long-acting injectable formulation of risperidone. Clin Pharmacol Drug Dev. 2022;11(7):865-877.
- Rainier MK. Risperidone long-acting injection: a review of its long term safety and efficacy. Neuropsychiatr Dis Treat. 2008;4(5):919-927.
- Citrome L, Suett M, Franzenburg KR, et al. TV-46000, a long-acting subcutaneous risperidone injectable, demonstrated improved patient-centered outcomes in patients with schizophrenia. Presented at: Psych Congress 2021; October 29-November 1, 2021; San Antonio, TX.
- Risperdal Consta® current Prescribing Information. Titusville, NJ: Janssen Pharmaceuticals, Inc.
- Invega Sustenna® current Prescribing Information. Titusville, NJ: Janssen Pharmaceuticals, Inc.
- Perseris® current Prescribing Information. North Chesterfield, VA: Indivior, Inc.
- Correll CU, Kane JM, Suett M, et al. Efficacy and safety of TV-46000, subcutaneous long-acting risperidone, by injection site (upper arm/abdomen): post hoc analysis of the RISE study. Presented at: Psych Congress; October 29-November 1, 2021; San Antonio, TX.
- Kane JM, Harary E, Tohami O, et al. Long-term safety, tolerability, and effectiveness of a long-acting subcutaneous antipsychotic (LASCA) agent (TV-46000) in patients with schizophrenia: a phase 3, randomized, double-blind study (SHINE). Presented at: Schizophrenia International Research Society; May 11-May 15, 2023; Toronto, Canada.
- Robinson DG, Suett M, Wilhelm A, et al. Patient preferences and treatment experiences with TV-46000, a long-acting subcutaneous injectable risperidone formulation. Presented at: Psych Congress 2021; October 29-November 1, 2021; San Antonio, TX.

© 2025 Teva Neuroscience, Inc.
All rights reserved. RIS-41413 July 2025