Patients with schizophrenia have increased prevalence rates for many cardiometabolic risk factors; the prevalence and severity of these risks increase after the institution of antipsychotic medication. Nearly 2 dozen different pharmacologic interventions have been trialed to prevent or attenuate antipsychotic-related cardiometabolic changes. Metformin (usually 1,000-1,500 mg/d) has emerged as the best-studied intervention; in short- and intermediate-duration randomized controlled trials, it has been shown to bring about improvements in weight and other anthropometric indices, in fasting sugar and other glycemic control indices, and in total cholesterol and other lipid metabolism indices. Topiramate and aripiprazole are other possible interventions with support in literature; besides improving metabolic outcomes, these drugs may improve indices of psychopathology, as well. Encouraging though the findings are, there are many unanswered questions that require attention in future research.
- The metabolic syndrome and its components are common in schizophrenia. Antipsychotic treatment plays a significant role in the genesis and worsening of these metabolic risks.
- Out of nearly 2 dozen pharmacologic interventions trialed, metformin (1,000-1,500 mg/d) has emerged as the best treatment option for improvement in anthropometric indices and indices of glucose and lipid metabolism. Blood pressure, however, does not change with metformin treatment.
- Topiramate and aripiprazole are other possible interventions; these may also improve psychopathology outcomes.

ABSTRACT
Patients with schizophrenia have increased prevalence rates for many cardiometabolic risk factors; the prevalence and severity of these risks increase after the institution of antipsychotic medication. Nearly 2 dozen different pharmacologic interventions have been trialed to prevent or attenuate antipsychotic-related cardiometabolic changes. Metformin (usually 1,000-1,500 mg/d) has emerged as the best-studied intervention; in short- and intermediate-duration randomized controlled trials, it has been shown to bring about improvements in weight and other anthropometric indices, in fasting sugar and other glycemic control indices, and in total cholesterol and other lipid metabolism indices. Topiramate and aripiprazole are other possible interventions with support in literature; besides improving metabolic outcomes, these drugs may improve indices of psychopathology, as well. Encouraging though the findings are, there are many unanswered questions that require attention in future research.
J Clin Psychiatry 2016;77(9):e1090-e1094
dx.doi.org/10.4088/JCP.16f11128
© Copyright 2016 Physicians Postgraduate Press, Inc.
Introduction
Schizophrenia is associated with an increased prevalence of many cardiometabolic risk factors. These risk factors are evident early during the course of the illness and increase in prevalence after antipsychotic exposure.1 Behavioral interventions, such as dietary guidance, exercise training, and combined diet and exercise (with psychoeducation and other behavioral elements included in the package) have not been shown to consistently improve cardiovascular health indices in patients with schizophrenia and other major mental illnesses2; furthermore, there are challenges to the implementation of behavioral interventions in such patients.3 The present article, the third in a series, examines whether pharmacologic interventions can mitigate the cardiometabolic risks.
Pharmacologic Interventions for Antipsychotic-Related Metabolic Changes
In one of the most recent and most extensive PRISMA-compliant meta-analyses on the subject, Mizuno et al4 searched several electronic databases and other sources and identified 41 published and 9 unpublished, randomized, placebo-controlled trials of pharmacologic interventions for antipsychotic-induced weight gain and other metabolic adverse effects in samples of patients (pooled N = 2,676) with schizophrenia as the commonest diagnosis.
These randomized controlled trials (RCTs) ranged from 4 to 24 (mean, 12) weeks in duration. The sample size in the individual studies was 2-207 (mean, 54). Most of the studies were conducted in North America (19 RCTs) and Asia (18 RCTs). Only 18 studies were clearly linked to industry funding. The pharmacologic interventions studied were amantadine, aripiprazole, atomoxetine, d-fenfluramine, dextroamphetamine, famotidine, fluoxetine, intranasal insulin, metformin, metformin with sibutramine, modafinil, nizatidine, orlistat, phenylpropanolamine, reboxetine, reboxetine with betahistine, rimonabant, rosiglitazone, sibutramine, telmisartan, topiramate, and zonisamide. Most of these agents were investigated in 1-3 RCTs; nizatidine and sibutramine were each investigated in 4 RCTs, and metformin was studied in monotherapy in 10 RCTs. One RCT investigated a drug that had a code number but no name.
In 13 RCTs, pharmacologic interventions were instituted along with antipsychotic medication with a view to prevent metabolic changes; in the remaining RCTs, the pharmacologic interventions were trialed for the attenuation of existing antipsychotic-induced metabolic changes. The primary antipsychotic was clozapine or olanzapine in 49 RCTs.
Data on weight change were available in 40 RCTs; 21 RCTs provided data on glucose metabolism, and 16, on lipid parameters. Data for meta-analysis were available from only the published studies. Only 6 RCTs showed a low risk of bias; in the rest, risk of bias was frequently unclear because of poor reporting on the relevant variables.
Important findings from this meta-analysis4 are presented in Tables 1 and 2. In summary, there was a reasonably strong evidence base to support the use of only 1 drug, metformin, for either prevention or attenuation of antipsychotic-induced metabolic changes. Metformin significantly prevented weight gain or reduced weight; it was associated with decrease in fasting blood sugar, glycosylated hemoglobin, fasting insulin levels, and insulin resistance. Metformin was also associated with a reduction in triglyceride levels. Not all of these changes were clinically significant, and there was evidence of heterogeneity as well as publication bias. Whereas topiramate was associated with substantial metabolic benefit, the evidence base comprised only 1 RCT. An interesting finding from a subgroup analysis was that the benefits in first-episode patients were larger in magnitude, thus encouraging the earlier use of pharmacologic interventions to prevent or treat antipsychotic-related metabolic complications.
Metformin for Antipsychotic-Related Metabolic Changes
Zheng et al5 described a PRISMA-compliant systematic review and meta-analysis of only metformin vs placebo RCTs; the advantage of this over the earlier meta-analysis4 is that, besides conducting an updated search of the English language database, the authors included Chinese language RCTs.
There were 21 RCTs (pooled N = 1,547) in all, 11 RCTs published in English and 10 in Chinese. Of these, 15 RCTs had been conducted in China. In 5 studies, metformin had been administered at the time of antipsychotic initiation to prevent antipsychotic-related metabolic changes; in the remaining 16 studies, the use of metformin was to attenuate antipsychotic-related metabolic changes. The sample size in these RCTs was 39-148 (median, 70). Of the 1,547 patients, 778 received metformin and 769 received placebo. Metformin was dosed at 250-1,750 (in most studies, 750-1,000) mg/d. Olanzapine and clozapine were the most common antipsychotics in these studies. The studies were 6-24 (median, 12) weeks in duration. In general, there seemed to be no evidence of publication bias.
Important findings from the meta-analysis are presented in Table 3. In summary, metformin significantly reduced body weight, body mass index, and waist circumference, but not the waist-hip ratio; it significantly reduced fasting glucose, glycosylated hemoglobin, fasting insulin, and insulin resistance; it reduced total cholesterol and triglycerides but not LDL cholesterol; it significantly increased HDL cholesterol; it did not affect blood pressure; and whereas it was significantly associated with nausea/vomiting and diarrhea, it did not increase the study discontinuation rate. Most effect sizes were > 0.50, indicating a medium to large treatment effect.
In subgroup analyses, metformin was superior to placebo for weight gain and BMI in both Chinese and non-Chinese studies, in prevention studies, in treatment studies in early (first year) as well as chronic schizophrenia patients, in younger as well as older patients (age cutoff, 30 years), in lower as well as higher doses (cutoff, 750 mg/d), and in short-term as well as intermediate-term studies (cutoff, 12 weeks). Largely similar results were obtained in subgroup analyses for fasting glucose, fasting insulin, total cholesterol, and triglyceride levels. In sensitivity analyses, metformin was generally superior to placebo on these outcome measures in olanzapine as well as clozapine studies, in high-quality RCTs, and in studies published in English.
Most analyses were characterized by high heterogeneity. Whereas the authors did not perform a meta-regression analysis, an examination of effect sizes showed that outcomes were substantially better in Chinese as compared with non-Chinese studies, in prevention as compared with treatment studies, and in younger as compared with older patients. Outcomes were also better in short-term relative to intermediate-term trials.
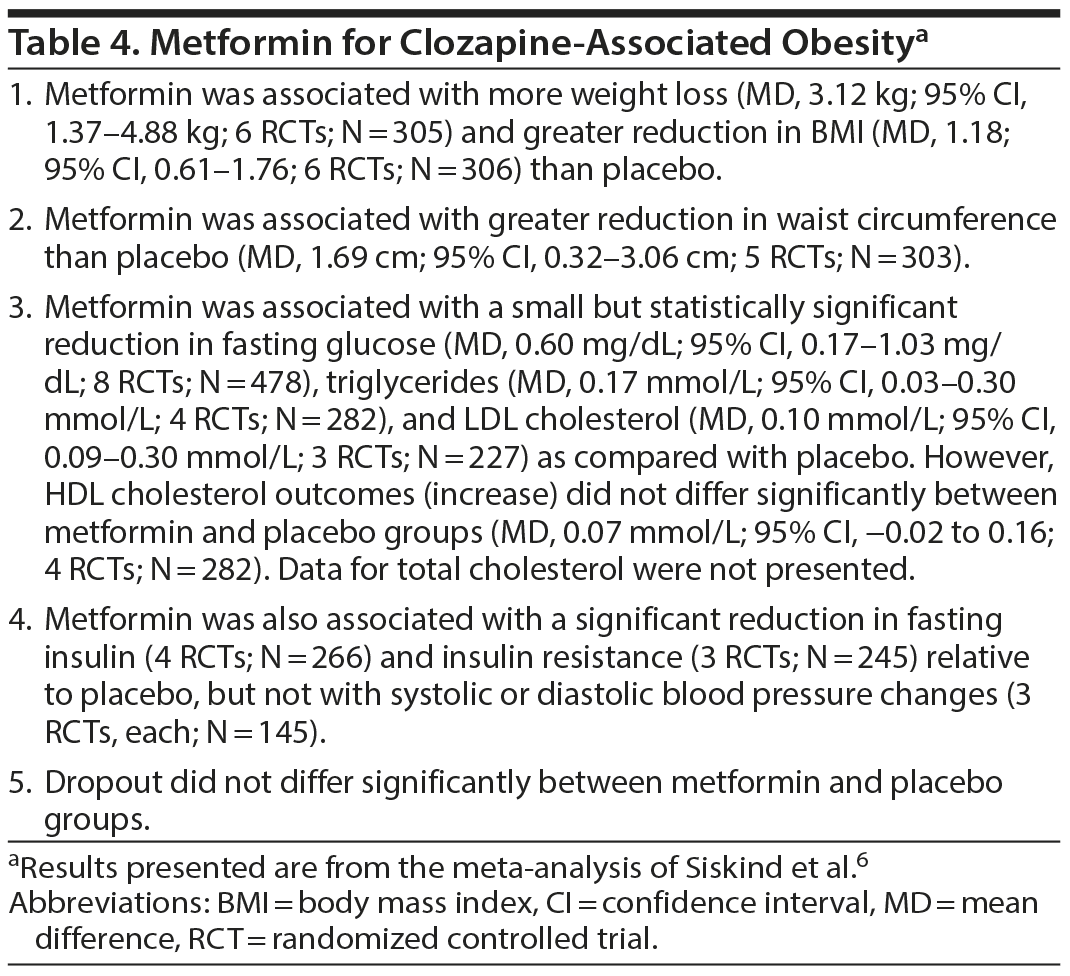
Metformin for Clozapine-Associated Obesity
In a very recent systematic review and meta-analysis, Siskind et al6 examined the benefits of metformin specifically for clozapine-associated obesity. They identified 8 placebo-controlled RCTs (3 from Chinese databases) with a pooled sample of 478 patients. These studies were 12-26 weeks in duration. Most studies dosed metformin at 1,000-1,500 mg/d. Important findings from the study are presented in Table 4. In summary, relative to placebo, metformin was associated with greater weight loss, reduction in BMI, and reduction in waist circumference; greater reduction in fasting glucose, triglycerides, and LDL cholesterol level; and greater reduction in fasting insulin and insulin resistance. However, metformin was not associated with an advantage for HDL cholesterol, systolic blood pressure, and diastolic blood pressure outcomes. Metformin was not associated with an increased risk of treatment dropout.
Most analyses had low heterogeneity. The conclusions remained largely unchanged in various sensitivity analyses, including of higher quality studies and of studies that were > 3 months in duration. Publication bias could not be assessed as the number of studies in separate analyses was too small.
Other Metformin Studies
Wu et al7 described the effect of metformin (1,000 mg/d) on antipsychotic-induced dyslipidemia and other metabolic changes in a pooled analysis of two 24-week, placebo-controlled RCTs; one of these RCTs (n = 39) had previously been published and was part of 2 of the meta-analyses4,5 described earlier in this article; the other RCT (n = 162) was unpublished. The RCTs were similar in design. In analyses that controlled for age, sex, duration of illness, and antipsychotic drug used, metformin was associated with significantly greater benefits (relative to placebo) for weight loss, decrease in BMI, decrease in fasting insulin and insulin resistance, increase in HDL cholesterol, and decrease in total cholesterol, LDL cholesterol, and triglycerides. Fasting glucose was the only metabolic variable that did not differentiate the groups. Whereas some of the benefits appeared by 12 weeks of treatment, others were apparent only at the 24-week treatment endpoint. This suggests that an adequate metformin trial should last for at least 24 weeks.
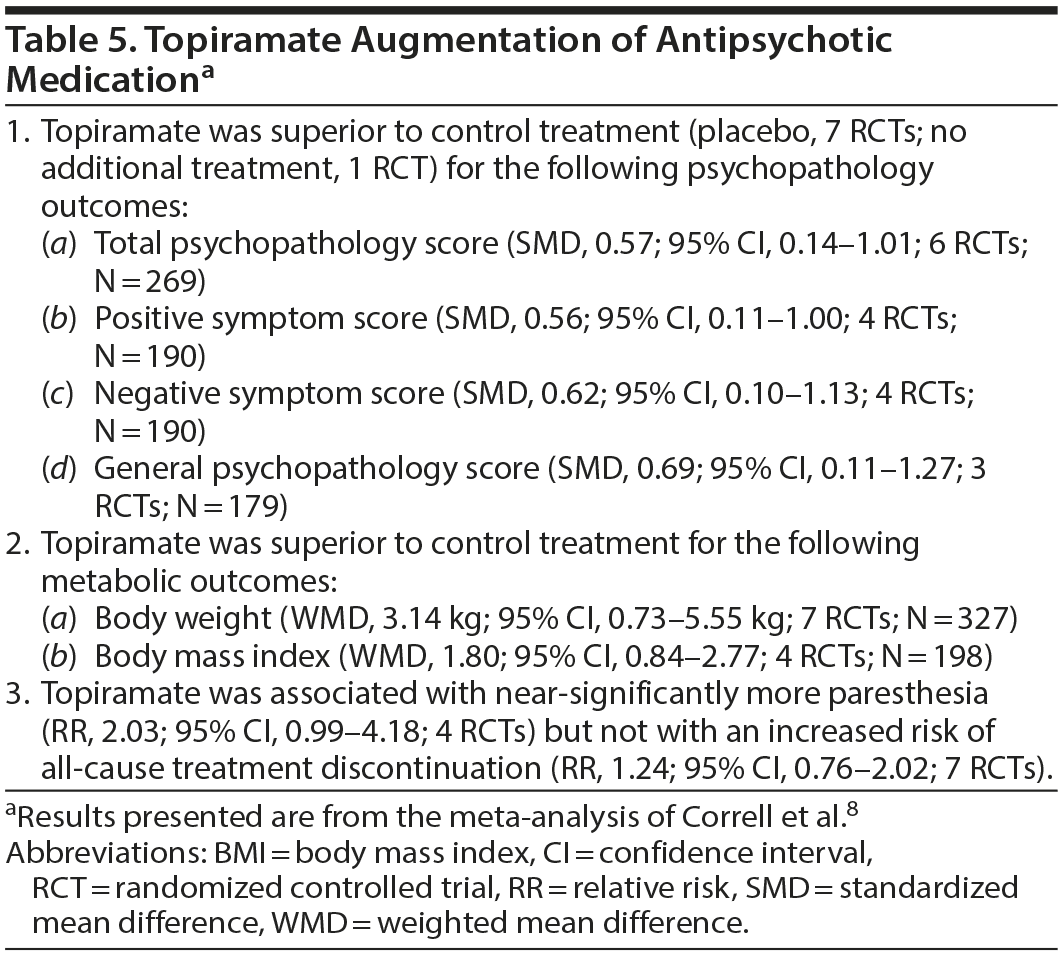
A Possible Place for Topiramate
Topiramate is emerging as a potential challenger to metformin. In a systematic review and meta-analysis of RCTs of topiramate (100-400 mg/d) augmentation of antipsychotic drugs in schizophrenia spectrum disorders, Correll et al8 identified 8 trials (pooled N = 439) lasting 8-24 (mean, 13.6) weeks. One study was open-label, and one was a crossover trial. One study included first episode patients, and the rest were conducted in chronically ill patients. Two studies initiated topiramate along with the antipsychotic; in the rest, topiramate was added to a stable dose of ongoing antipsychotic treatment.
Important findings from their meta-analysis are presented in Table 5. In summary, topiramate augmentation was associated with significant benefits with regard to total, positive, negative, and general psychopathology symptoms in schizophrenia. Topiramate was also associated with significant reduction in weight and BMI. Data on other metabolic outcomes were insufficient for meta-analysis. The drug was not associated with an increased risk of study discontinuation.
Heterogeneity was high in most analyses. However, the benefits of topiramate for psychopathology and metabolic outcomes were not mediated or moderated by RCT duration, age, sex, inpatient status, baseline severity of psychopathology, baseline BMI, or dose of topiramate. Importantly, in clozapine-treated patients, topiramate had greater psychopathology benefits but resulted in less weight loss.
Other Possible Pharmacologic Interventions
Nearly 2 dozen pharmacologic interventions have been investigated in the meta-analyses described in this article. There are other possible interventions, as well. For example, melatonin and ramelteon have been found to attenuate some cardiometabolic risk factors in antipsychotic-treated patients with major mental illness.9
In both pediatric10 and adult11 samples, the 677T allele of the methylene tetrahydrofolate reductase gene has been associated with an increased risk of antipsychotic-related metabolic syndrome and of specific elements thereof. This raises the intriguing possibility that the administration of methylfolate supplements may prevent or attenuate the metabolic syndrome in children and adults who receive antipsychotic drugs. The issue merits study because the 677T allele is present in about half of the population.12
There is presently no information about the safety and efficacy of conventional (approved) weight loss treatments in antipsychotic-receiving patients with schizophrenia; these treatments include lorcaserin, liraglutide, the phentermine-topiramate combination, the naltrexone-bupropion combination, and others. However, an important limitation of focusing on weight alone is that there is no evidence that other criteria that define the metabolic syndrome will meaningfully attenuate, as shown with metformin.
Schizophrenia is associated with higher rates of smoking than those observed in the general population, and many studies have examined smoking cessation treatments in schizophrenia; a consideration of this body of research is important but is out of the scope of the present review.
The Many Unknowns
The meta-analyses reviewed in this article provide guidance for pharmacologic interventions (most importantly, metformin) to prevent or treat antipsychotic-related metabolic complications. There are, however, many unknowns. For example, we do not know the best dose of a particular drug and whether there is a dose-dependent effect. We do not know for how long these medications need to be continued. There is no information on the long-term (beyond 6 months) safety and efficacy of these interventions. We do not know whether adding diet and exercise to a pharmacologic strategy would have additive or synergistic benefits, or no additional benefit. We do not know whether specific subgroups of patients benefit less or more; an example is in patients with a genetic mutation for a metabolic process. Given the differences in the vulnerability to and the prevalence of metabolic abnormalities in different ethnic groups, it is important to determine whether the benefits of metformin and other pharmacologic interventions vary with ethnicity. Last, but not least, it is necessary to know whether addressing cardiometabolic risk factors through the use of metformin makes a difference to hard outcomes such as the incidence of ischemic heart disease events, cerebrovascular disease events, and mortality related thereto; one wonders, though, how such a study would be funded.
Concluding Notes
Patients prescribed antipsychotic medications should be regularly screened for metabolic changes. Among nearly 2 dozen treatments that have been trialed for treating or preventing antipsychotic-related adverse metabolic changes, metformin is the best studied and is associated with the best evidence of short- to intermediate-term efficacy for improving weight, glycemic, and lipid (but not blood pressure) outcomes in patients with schizophrenia. There seems to be a signal for better outcomes with an earlier institution of metformin. Topiramate is emerging as another possible treatment; besides reduced weight, indices of psychopathology may also improve. Finally, in patients who need better antipsychotic control, aripiprazole augmentation is also a possible choice because metabolic outcomes may also be improved13,14; however, its role as an augmentation agent requires better research.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Andrade C. Cardiometabolic risks in schizophrenia and directions for intervention, 1: magnitude and moderators of the problem. J Clin Psychiatry. 2016;77(7):e844-e847. PubMed doi:10.4088/JCP.16f10997
2. Andrade C. Cardiometabolic risks in schizophrenia and directions for intervention, 2: nonpharmacological interventions. J Clin Psychiatry. 2016;77(8):e964-e967.
3. Ward MC, White DT, Druss BG. A meta-review of lifestyle interventions for cardiovascular risk factors in the general medical population: lessons for individuals with serious mental illness. J Clin Psychiatry. 2015;76(4):e477-e486. PubMed doi:10.4088/JCP.13r08657
4. Mizuno Y, Suzuki T, Nakagawa A, et al. Pharmacological strategies to counteract antipsychotic-induced weight gain and metabolic adverse effects in schizophrenia: a systematic review and meta-analysis. Schizophr Bull. 2014;40(6):1385-1403. PubMed doi:10.1093/schbul/sbu030
5. Zheng W, Li XB, Tang YL, et al. Metformin for weight gain and metabolic abnormalities associated with antipsychotic treatment: meta-analysis of randomized placebo-controlled trials. J Clin Psychopharmacol. 2015;35(5):499-509. PubMed doi:10.1097/JCP.0000000000000392
6. Siskind DJ, Leung J, Russell AW, et al. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PLoS One. 2016;11(6):e0156208. PubMed doi:10.1371/journal.pone.0156208
7. Wu RR, Zhang FY, Gao KM, et al. Metformin treatment of antipsychotic-induced dyslipidemia: an analysis of two randomized, placebo-controlled trials [published online ahead of print January 26, 2016]. Mol Psychiatry. 2016. PubMed doi:10.1038/mp.2015.221
8. Correll CU, Maayan L, Kane J, et al. Efficacy for psychopathology and body weight and safety of topiramate-antipsychotic cotreatment in patients with schizophrenia spectrum disorders: results from a meta-analysis of randomized controlled trials. J Clin Psychiatry. 2016;77(6):e746-e756. PubMed doi:10.4088/JCP.15r10373
9. Wang HR, Woo YS, Bahk WM. The role of melatonin and melatonin agonists in counteracting antipsychotic-induced metabolic side effects: a systematic review [published online ahead of print July 4, 2016]. Int Clin Psychopharmacol. 2016. PubMed doi:10.1097/YIC.0000000000000135
10. Devlin AM, Ngai YF, Ronsley R, et al. Cardiometabolic risk and the MTHFR C677T variant in children treated with second-generation antipsychotics. Transl Psychiatry. 2012;2:e71. PubMed doi:10.1038/tp.2011.68
11. Ellingrod VL, Miller DD, Taylor SF, et al. Metabolic syndrome and insulin resistance in schizophrenia patients receiving antipsychotics genotyped for the methylenetetrahydrofolate reductase (MTHFR) 677C/T and 1298A/C variants. Schizophr Res. 2008;98(1-3):47-54. PubMed doi:10.1016/j.schres.2007.09.030
12. Farah A. The role of L-methylfolate in depressive disorders. CNS Spectr. 2009;14(suppl 2):2-7. PubMed doi:10.1017/S1092852900003473
13. Srisurapanont M, Suttajit S, Maneeton N, et al. Efficacy and safety of aripiprazole augmentation of clozapine in schizophrenia: a systematic review and meta-analysis of randomized-controlled trials. J Psychiatr Res. 2015;62:38-47. PubMed doi:10.1016/j.jpsychires.2015.01.004
14. Galling B, Roldán A, Rietschel L, et al. Safety and tolerability of antipsychotic co-treatment in patients with schizophrenia: results from a systematic review and meta-analysis of randomized controlled trials. Expert Opin Drug Saf. 2016;15(5):591-612. PubMed
This PDF is free for all visitors!