Parkinson disease (PD) is the fastest growing neurologic disease worldwide, and the majority of patients with PD will experience symptoms of psychosis during the course of the disease. Psychotic symptoms lead to poor outcomes and increase the already heavy burden on patients with PD and their caregivers. Treatment is available but must be initiated early for optimal outcomes. Therefore, clinicians need to be familiar with and vigilant for potential signs of psychosis. Once psychotic symptoms are detected, clinicians should follow a cautious management plan that includes a medical evaluation, medication review and reduction (while trying to avoid worsening motor symptoms), and possibly the addition of an antipsychotic. However, antipsychotics must be used with caution in this population because of the risk of increased mortality in elderly patients.
This CME activity is expired. For more CME activities, visit CMEInstitute.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
Abstract
Parkinson disease (PD) is the fastest growing neurologic disease worldwide, and the majority of patients with PD will experience symptoms of psychosis during the course of the disease. Psychotic symptoms lead to poor outcomes and increase the already heavy burden on patients with PD and their caregivers. Treatment is available but must be initiated early for optimal outcomes. Therefore, clinicians need to be familiar with and vigilant for potential signs of psychosis. Once psychotic symptoms are detected, clinicians should follow a cautious management plan that includes a medical evaluation, medication review and reduction (while trying to avoid worsening motor symptoms), and possibly the addition of an antipsychotic. However, antipsychotics must be used with caution in this population because of the risk of increased mortality in elderly patients.
J Clin Psychiatry 2018;79(suppl E1):ad18017su1c
To cite: Pahwa R, Kremens DE. Effective management approaches for psychosis in patients with Parkinson disease. J Clin Psychiatry. 2018;79(suppl E1):ad18017su1c
To share: https://doi.org/10.4088/JCP.ad18017su1c
© Copyright 2018 Physicians Postgraduate Press, Inc.
This supplement is derived from the planning teleconference “Effective Management Approaches for Psychosis in Patients With Parkinson Disease,” which was held in April 26, 2018. This report was prepared and independently developed by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by an educational grant from Acadia Pharmaceuticals Inc.
The teleconference was chaired by Rajesh Pahwa, MD, from the Movement Disorders Division, Department of Neurology, University of Kansas Medical Center, Kansas City. The faculty was Daniel E. Kremens, MD, JD, from the Movement Disorders Program, Department of Neurology, Thomas Jefferson University, Philadelphia, Pennsylvania.
Financial disclosure: Dr Pahwa is a consultant for or has received honoraria from AbbVie, Abide, Acadia, Acorda, Adamas, Alexza, Abbott, Global Kinetics, Ionis, Lundbeck, Neurocrine, Prilenia, Sunovion, Teva Neuroscience, and US WorldMeds; has received grant/research support from Abbott, AbbVie, Acorda, Adamas, Biogen, Bristol-Myers Squibb, Boston Scientific, Cala Health, Cavion, Intec, Jazz, Kyowa Hakko Kirin, Eli Lilly, National Institutes of Health/National Institute of Neurologic Disorders and Stroke, Parkinson Foundation, Parkinson Study Group, Pfizer, Prexton, Roche, Sunovion, and US WorldMeds; and is a member of the speakers bureaus for AbbVie, Acadia, Acorda, Adamas, Neurocrine, Sunovion, Teva Neuroscience, and US WorldMeds. Dr Kremens is a consultant for Prexton, Teva, UCB, Sunovion, Impax, Lundbeck, Acadia, US WorldMeds, Adamas, AbbVie, Merz, Allergan, Acorda, Kyowa Hakko Kirin, Neurocrine, GE Healthcare, and St. Jude Medical (now Abbott); is a member of the speakers bureaus for Teva, UCB, Impax, Lundbeck, Acadia, US WorldMeds, and Adamas; and has received research support from Acorda, Enterin, and Revance.
The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the commercial supporter.
Parkinson disease (PD) has been described as a pandemic because it is the fastest growing neurologic disorder and a leading cause of global disability.1 The global prevalence of PD is projected to more than double by 2040 as the population ages and life expectancies increase.1 More than half of patients with PD will experience hallucinations or delusions during the course of their illness, and most of these individuals will develop Parkinson disease psychosis (PDP).2,3
THE BURDEN OF PSYCHOSIS IN PATIENTS WITH PARKINSON DISEASE
Psychosis in patients with PD drastically increases the burden of this disease on caregivers4 and predicts reduced quality of life for the patient.5 PDP increases the risk of hospitalizations and placement in long-term care facilities.6,7 In one study6 of all PD admissions to a neurologic hospital over a 6-year period, investigators found that 24% of patients had been admitted due to psychosis alone and 25% had been admitted due to a combination of motor and psychiatric complications. Furthermore, they found drug-induced psychosis to be the greatest contributing factor to repeated and prolonged admissions. An analysis of Medicare claims data7 revealed that patients with PD and psychosis spent more than twice as many days in long-term care as PD patients without psychosis (179 days versus 83 days). Finally, the presence of psychosis greatly increases mortality in patients with PD.8
Patient Perspectives 
“I also get auditory Hallucinations. Door bell, road runner BEEP-BEEP, police sirens, etc. going off.’ ¦ I hear my name being called at random.”9
“I have had hallucinations and I was told it was probably one drug interacting with another. It was very scary. I saw whole families moving into my home and trying to take over. These hallucinations lasted for several weeks and followed a storyline you wouldn’ t believe. I had two bouts of them and the worst part was that I knew they weren’ t real but they still kept recurring.”10
“I keep seeing a shadowy female figure over my right shoulder. I have given her the identity of my estranged wife. I tried it because I got rid of her via the legal system in real life, so I thought that the identity of someone who was controllable in real life might make her controllable in the fantasy existence, and it works quite well. It normally takes a very short time, but just sometimes it can take about an hour or so on bad PD days. The important thing is to remember that it is a fantasy figure.”9
Caregiver Perspectives 
“[My husband] swears that the neighbor across the road has planted grape vines and put a row in our yard, [and] sees cows, people fishing in boats, thinks people have come and no one has.”11
“[My wife’s ] fixed delusion of infidelity is at its peak ‘ ¦ she believes I am having affair[s] with 2-3 women at one time.”12
“Today my mom got angry at me for an argument she hallucinated happened between me and my father. She was really hurt, and I couldn’ t tell her it had been a hallucination without it coming off like me and my dad were conspiring against her.”13
What Do PD Patients and Caregivers Need to Know Early on About Psychosis?
- Psychosis is common in PD as the disease progresses.
- The nonmotor symptoms the patient may begin experiencing may be related to PD.
- The Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale14 has a section that includes questions related to nonmotor PD symptoms.
- Psychosis symptoms are often underreported; therefore, it’s necessary to make patients aware that there are available treatment options.
RECOGNITION OF PSYCHOSIS IN PATIENTS WITH PARKINSON DISEASE
Clinicians often underrecognize the nonmotor symptoms of PD; this is particularly true of psychosis.15 A major contributor to the underrecognition of nonmotor symptoms, including PDP, is the failure of patients and caregivers to report these issues to their health care providers. Chaudhuri and colleagues16 conducted a study in which 242 patients with PD were asked to complete a 30-item self-report screening tool designed to detect nonmotor symptoms. This tool, the NMSQuest, assesses for a host of nonmotor symptoms, ranging from hallucinations and delusions to sleep and urinary problems. After completing the NMSQuest, patients were asked if any identified symptoms had previously been discussed during an appointment with their physician, and if not, why. The investigators found that hallucinations/delusions was the symptom domain most frequently not declared to physicians, with 41.5% of the patients experiencing hallucinations and 65.2% experiencing delusions not reporting these symptoms to their physicians.16
Although patients would admit to these symptoms on the survey, they would not report them to their physicians. Patients cited 3 explanations for not spontaneously declaring their nonmotor symptoms: (1) they did not realize the symptoms were related to PD, (2) they were embarrassed, and/or (3) their consultation time was focused on motor symptoms.16 The results of this study indicate several steps clinicians can take to improve the recognition of psychotic symptoms in patients with PD, including educating patients and caregivers early in the disease process about psychiatric symptoms, as well as screening patients for PDP symptoms at each visit.17,18 Furthermore, due to the sensitive nature of these symptoms, physicians should inquire about these issues in a non-threatening manner.19
Symptoms and Features
Psychosis associated with PD has a distinct clinical profile from other forms of psychosis.20 The National Institutes of Neurologic Disorders and Stroke and the National Institute of Mental Health formed a work group that developed diagnostic criteria for PDP (AV1).20 A central criterion is a primary PD diagnosis rendered prior to the onset of psychotic symptoms. In addition, because PDP is stable and progressive, diagnosis requires that the psychotic symptoms be recurrent or continuous for at least 1 month. Other medical or psychological causes of the symptoms must also be ruled out. The psychosis can occur with or without insight, PD treatment, or dementia.20 The characteristic symptoms required for a diagnosis of PDP include at least one of the following: hallucinations, illusions, a false sense of presence, or delusions.20
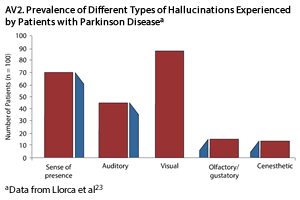
Hallucinations. A hallucination is an abnormal perception involving any sensory modality that occurs in the absence of external stimuli. Visual hallucinations are the most common symptoms in PDP. These can be simple or well formed, they often consist of people or animals, and the content is frequently recurrent.20 Auditory hallucinations are also common in PDP, but unlike the auditory hallucinations common in schizophrenia that tend to be perceived as threatening, those in PD are often vague and consist of music or sounds such as footsteps or whispers.21-23 Patients with PDP can also experience olfactory, gustatory, or somatic hallucinations, but these are less common (AV2).23 Often, patients with PDP will experience multimodal hallucinations. For example, they may both see and hear or be touched by a person who is not there. Hallucinations are usually brief and are more common at night, in low light, or in instances of compromised vision.21,22
Illusions. In PDP, illusions are considered a type of minor hallucination. Illusions are unique in that patients are misperceiving actual objects or stimuli.21 For example, a patient might see trees as people or a chair as a dog.
False sense of presence. The false sense of presence commonly experienced by patients with PDP is the vivid sensation that someone is nearby when nobody is actually there. This sensation is also sometimes called a presence hallucination.20 The presence is usually perceived to be a human or an animal generally in the same room as the patient. Some patients perceive the identity of the presence, recognizing it, for example, to be a deceased loved one or pet, but for others the presence is unidentified.22,24 The false sense of presence most commonly occurs in combination with a hallucination of a different sensory modality, frequently visual.23
Delusions. Delusions are false, firmly held beliefs that the patient holds with no evidence, or with evidence to the contrary.20 These delusions can focus on jealousy, paranoia, or themes of abandonment, theft, or spousal infidelity.21 Delusions have a negative nature and can be far more disruptive and distressing to the patient than hallucinations.25
Risk Factors
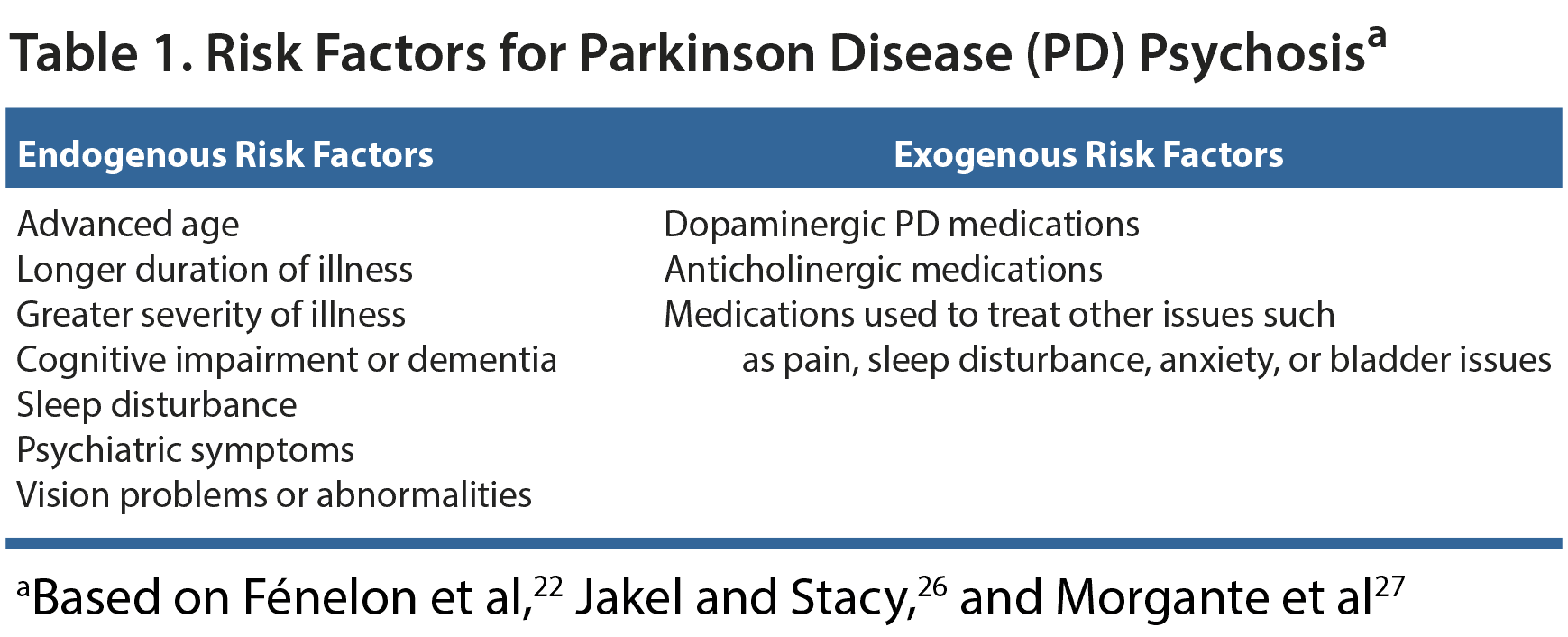
Not every patient with PD will develop psychosis. Certain risk factors may help physicians recognize patients that warrant close observation for signs of PDP. These factors can be both endogenous and exogenous (Table 1).22,26,27
Endogenous factors. Individuals who have experienced a late onset of PD have been found to be at a higher risk of developing psychotic symptoms.26 In addition, the prevalence of hallucinations can increase with the duration of and severity of motor impairments.22,26 Cognitive impairments are also associated with PDP risk. The presence and severity of cognitive symptoms have been found to be positively correlated with psychotic symptoms,26 and psychotic symptoms are experienced by as many as 70% of PD patients with dementia.22 Although PDP is extremely common in patients with dementia, it can also occur in patients without dementia. Clinicians must therefore screen all patients for signs of psychosis regardless of their cognitive status.
Other endogenous risk factors include the presence of psychiatric symptoms, particularly depression and anxiety, and sleep disturbance.22,27 In a study of 423 patients with newly diagnosed PD, Barrett and colleagues28 found that the patients who screened positive for REM sleep behavior disorder or excessive daytime sleepiness at baseline were significantly more likely to later screen positive for psychotic symptoms (P = .021 and P = .003 respectively). Interestingly, individuals who are blind can experience visual hallucinations, and, in patients with PD, visual abnormalities or visual deterioration may increase the risk of developing hallucinations.22 Patients with PD who experience hallucinations may have difficulties perceiving spatial relations, color and contrast discrimination, and object perception. They also commonly experience double vision.29 The hallucinations in PD may be a result of the brain attempting to make sense of defective visual information, or they may stem from the same underlying pathology.29
Exogenous risk factors. The main exogenous risk factor for psychosis is the medication used to treat the symptoms of PD. Initially, psychosis was thought to be caused by a surplus of dopamine as a result of treatment with common PD medication such as levodopa and dopamine agonists.21 However, as new research has been conducted, the pathophysiology of PDP is now understood to be more complex than simply an excess of dopamine. Although dopamine pathways are certainly involved, alterations in the serotonergic and cholinergic systems are known to also play a role in the development of psychosis.21 In one study,30 neither levodopa nor dopamine agonists were found to carry a significant risk of psychosis, but the risk associated with anticholinergic drugs was considerable (hazard ratio, 19.7 [95% CI, 2.39-1.63]; P = .006). Thus, the medications that can increase patients’ risk of psychosis will not only be those that are prescribed for their PD. They may, for example, have consulted their primary care doctor about pain, sleep issues, or bladder problems and received a prescription that is contributing to hallucinations.
Improving Recognition and Educating Patients
Because symptoms of psychosis are underreported by patients and caregivers,16 it is incumbent upon health care professionals to improve the recognition of PD psychosis. To facilitate this process, patients should receive education about PDP and available treatments early in the disease course.31 This can be done in a gentle, non-threatening manner using language such as the following:
“At some point in your disease process, particularly as the disease advances or as we change medication, you might experience some strange symptoms, such as hallucinations. You may see things that aren’ t there, and this is a common symptom in advancing Parkinson disease. There are treatments and ways to manage these symptoms, so I don’ t want you to be frightened, but it is important for you to know what to expect and for you to discuss these things with me if they begin to occur.”
Patients and caregivers must also be asked direct questions about the possible experience of psychotic symptoms at every visit. These questions should cover all types of sensory hallucinations, illusions, senses of presence, and delusions (AV3).32 Direct but non-threatening inquiries will help physicians identify the earliest possible signs of psychosis and initiate treatment.
Case Practice Question 
Case 1. Mr Smith is a 78-year-old man diagnosed with PD 15 years ago. His disease is now moderately advanced although he is otherwise in good health and shows no signs of dementia. Over the years, you have developed a good therapeutic relationship with Mr Smith and his visits are generally cordial. On his most recent visit, Mr Smith confides in you that he suspects his wife is having an affair and he believes that when she says she is going to visit their grandchildren, she is actually going to the neighbor’s house with whom she is having the affair.
What should you do?
- Because Mr Smith shows no signs of dementia, you have no reason to suspect that his fears are unfounded. You should assume that he is confiding in you as a friend and suggest he discuss these concerns with his wife.
- Recognize that Mr Smith is suffering from a delusion and alert him immediately that he has developed PDP.
- Ask Mr Smith if he has ever experienced sights, sounds, or other sensations that were not there, or if he ever has the impression that someone is nearby when no one is there.
Discussion of Case Practice Question 
Preferred response is c. Ask Mr Smith if he has ever experienced sights, sounds, or other sensations that were not there, or if he ever has the impression that someone is nearby when no one is there
Mr Smith’s concerns about his wife may be legitimate or may be a delusion, but a clinician probably cannot definitively determine this during an office visit without more information. Although PDP is more common in patients with dementia, the presence of dementia is not required and Mr Smith’s cognitive status, therefore, cannot exclude him from the diagnosis. Because delusions usually develop later in the course of PDP, the best action for the clinician to take is to question Mr Smith about other symptoms required for a diagnosis of PDP such as hallucinations, illusions, or sense of presence that he may not have reported during his visits.
Case Practice Question 
Case 2. Mrs Juarez is an 82-year-old woman who was diagnosed with PD 10 years ago. This is her first visit to your clinic, and she is accompanied by her daughter, Lisa. During Mrs Juarez’s examination, Lisa tells you that her mother recently saw her dead husband, Lisa’s father, standing by her bed. Mrs Juarez admits that this occurred but says she was too embarrassed to bring it up herself and she thinks that it was just her eyes playing tricks on her. Although Mrs Juarez is very dismissive of what she saw, the incident is clearly very distressing to her daughter.
Which of the following is the best way to address Mrs Juarez’s potential hallucination during this visit?
- Because the incident only occurred once and it was at night, tell Mrs Juarez that she probably was not experiencing a true hallucination. Rather, the low light might have tricked her eyes, or she may even have been dreaming.
- Ask if Mrs Juarez and her daughter are familiar with Parkinson disease psychosis, and if not, tell them that it is very common to experience some hallucinations in the course of PD. Assess Mrs Juarez for additional symptoms and provide information on what to expect and available treatments.
- Reassure both women that Mrs Juarez’s hallucination could have been caused by something as simple as being overtired or dehydrated and that they have no cause to worry unless the hallucinations recur.
Discussion of Case Practice Question 
Preferred response is b. Ask if Mrs Juarez and her daughter are familiar with Parkinson disease psychosis and, if not, tell them that it is very common to experience some hallucinations in the course of PD. Assess Mrs Juarez for additional symptoms and provide information on what to expect and available treatments
Because of the prevalence of PDP, clinicians should never be dismissive of any potential psychotic symptoms a patient experiences, as the options b. and c. suggest. All patients should receive education about PDP early in the course of illness, but because of the sensitive nature of psychotic symptoms, this topic should be approached gently, as presented in option b.
TREATMENT OF PARKINSON DISEASE PSYCHOSIS
Psychosis associated with PD is typically progressive; therefore, clinicians must decide both when and how to treat these symptoms. For most patients, the earliest signs of psychosis will be visual hallucinations, and initially patients will retain insight and be able to distinguish these symptoms from reality. These symptoms may occur occasionally, may not be too distressing to patients, and can often be managed with coping strategies.33 The hallucinations experienced at this stage of the disease process have sometimes been referred to as benign, but this term is misleading because it can be misunderstood as indicating a positive prognosis.17 A study by Goetz and colleagues17 found that after 3 years, 96% of PD patients (n = 48) with “benign” hallucinations at baseline had progressed to either hallucinations with loss of insight or delusions, signifying that the experience of hallucinations, even with retained insight, should not be considered benign. Indeed, the majority of patients who experience mild hallucinations will eventually develop more severe psychotic symptoms, which contribute to negative outcomes and therefore necessitate treatment.26
Implications of PD Pathology for Treatment
In PD, motor symptoms such as rigidity, bradykinesia, and tremor are thought to result from degeneration of dopamine neurotransmission within the nigrostriatal pathway, whereas behavioral symptoms such as psychosis are believed to originate from overactivation of the ventral dopaminergic pathway.34 This creates a treatment conundrum because medications that are intended to reduce motor symptoms by providing the brain with extra dopamine may induce or exacerbate psychosis, and medications that attempt to alleviate psychosis by blocking dopamine can worsen motor symptoms.
The serotonergic system (5-HT) is also involved in PD pathology and treatment. Patients with PD have been found to have decreased serotonin function,34 and patients with PD and psychosis have been found to have lower cerebral serotonin levels than those without.21 There is up-regulation of the 5-HT2A receptors in the cerebral cortex due to loss of serotonin signaling from medial raphe. Up-regulated 5-HT2A receptors on the glutamate neurons are presumed to increase signaling to the ventral dopaminergic pathways, which results in delusions and hallucinations.35 Hallucinogenic effects of drugs such as LSD are known to be mediated through serotonin receptors,36 providing further evidence of the role of the serotonergic system in the pathology of PDP and another potential pathway for treatment.
Initial Assessment
All patients with PD who exhibit symptoms of psychosis should first be given a complete medical evaluation to rule out other potential causes or triggers (AV4).18,26 Relatively minor medical issues such as dehydration or urinary tract infections can contribute to psychotic symptoms. Therefore, patients should receive routine blood work, a urinalysis, and an assessment of their vitamin B12 and folate levels. Any issues identified should be treated and the patient monitored to see if the psychotic symptoms persist.26
Medication Review and Adjustment
Medications that a patient is taking for a variety of non-PD related issues can contribute to symptoms of psychosis. If a patient continues to experience symptoms of psychosis after a medical evaluation, the patient’s medications should be reviewed. Polypharmacy is common in PD, and patients may, for example, be taking sedatives, anxiolytics, antidepressants, narcotic pain medications, antihistamines, or anticholinergics.18,25,26 These medications should be reduced, or discontinued, if possible.
If psychotic symptoms persist, clinicians should attempt to reduce the patient’s PD medications without worsening the patient’s motor symptoms. The recommended strategy is to reduce or eliminate the drugs that hold the least potential for clinical benefit or are most likely to cause hallucinations.18 Thus, clinicians should adjust anticholinergics, amantadine, dopamine agonists, monoamine oxidase B inhibitors, and catechol-O-methyltransferase (COMT) inhibitors. Finally, if psychotic symptoms persist, clinicians should attempt to adjust the patient’s levodopa dose. These treatment adjustments will often lead to an exacerbation of motor symptoms.18,25,26 Patients must decide if they believe the improvement in psychosis is an acceptable trade-off for the decline in motor symptoms.25
Nonpharmacologic Management
Patients may find nonpharmacologic strategies helpful for dealing with symptoms of psychosis, particularly early in the disease process while insight is still retained. Clinicians should educate patients on helpful coping strategies.18 A study33 by Barnes et al identified a number of coping strategies frequently used by patients with PD who were experiencing psychotic symptoms. The most common strategy was an action-oriented approach. Patients would blink, rub their eyes, or turn on a light to make hallucinations or illusions go away. They would also try to interact with the things they saw by trying to touch or talk to them. Additionally, many patients used a cognitive approach in which they rationalized the symptoms, told themselves they were not real, and tried to inspect them more closely. Emotional coping was common as well and involved the patients discussing their feelings and fears with others to relieve the emotional burden. Humor was an effective coping strategy, too; many patients found the best way to deal with their hallucinations was to laugh and joke about them with friends and family.37 Psychosocial treatments have not been well studied in PDP, but they may be considered early in the disease process before insight is lost.18
Pharmacologic Treatment
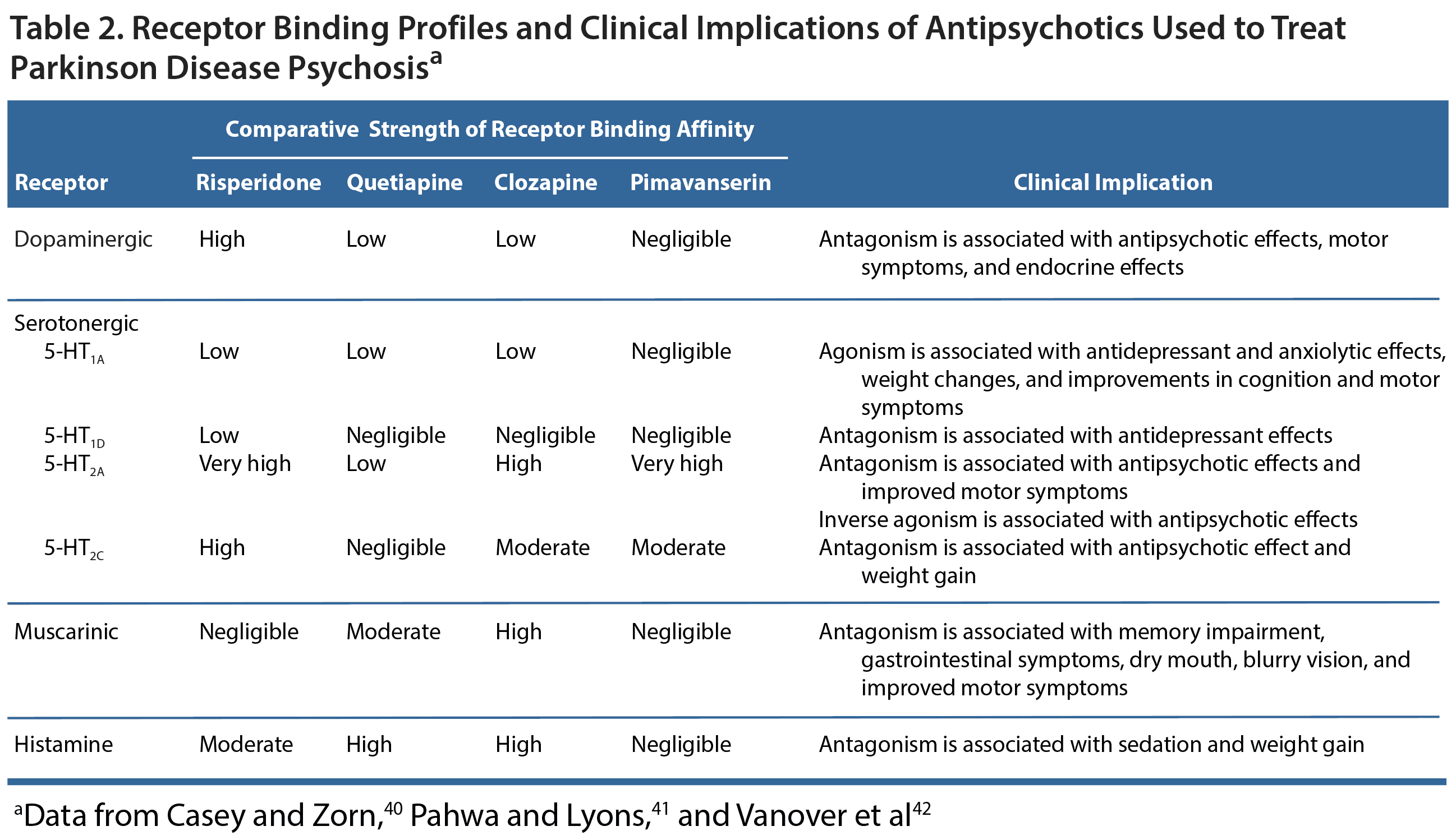
If a patient has been assessed for medical issues, had non-PD medications discontinued, and had PD treatments reduced as much as possible yet psychotic symptoms persist, the clinician should consider treatment with an antipsychotic. This must be done carefully because all atypical antipsychotics are associated with a risk of increased mortality,38 especially in elderly patients with dementia-related psychosis.21,39 The receptor binding profiles of antipsychotics are an important consideration in PD patients because the symptom profiles of these patients are quite complex and the pharmacology of antipsychotic treatment can have serious clinical implications (Table 2).40-42 Namely, the dopamine-blocking action of antipsychotics will often exacerbate motor symptoms. The degree of motor symptom worsening generally corresponds with the degree to which an antipsychotic blocks dopamine. Thus, the agents with the greatest dopamine blocking properties,43 including typical antipsychotics such as haloperidol and atypical antipsychotics such as risperidone, have the greatest risk of exacerbating motor symptoms.21 Clozapine is used off-label to treat PDP and has the most available evidence of efficacy,39 but pimavanserin is the only agent approved for use in PDP.44 Although quetiapine is often used off-label, this use is based more on clinical experience rather than strong evidence suggesting its efficacy.39
Clozapine is a serotonergic receptor antagonist and a weak dopamine receptor antagonist that has consistently been shown in double-blind, placebo-controlled trials to effectively treat PDP without worsening motor symptoms.41 Because of the risk of agranulocytosis, regular blood testing is required. The risk of agranulocytosis is actually minimal, but the required blood tests limit this treatment’s use. Quetiapine has a similar structure to clozapine, but regular blood tests are not required. Quetiapine is not a recommended treatment strategy because controlled trials have failed to find that the medication is any more effective than placebo for controlling PDP.39 In some instances, patients experienced worsening motor symptoms.
Pimavanserin was approved based on the results of a 6-week, placebo-controlled study of 199 patients with PDP.44,45 All patients went through a 2-week lead-in phase during which they received psychosocial treatment adapted for PD in order to elicit a placebo response prior to baseline. Once treatment was initiated, the active treatment group received 40 mg (equivalent to 34 mg of the active drug) of pimavanserin daily.45 The groups were evaluated after 15, 29, and 43 days of treatment. Primary outcomes were assessed using the PD-adapted Scale for Assessment of Positive Symptoms (SAPS-PD),46 which is a 9-item scale that covers individual symptoms as well as global hallucinations and delusions. After 6 weeks of treatment, the patients receiving pimavanserin had experienced a 37% improvement in psychotic symptoms from baseline compared with a 14% improvement in the placebo group (P = .0006).
Several secondary outcome measures, including Clinical Global Impressions-Severity (CGI-S) and -Improvement (CGI-I) scores, caregiver burden, sleep quality, and daytime wakefulness, significantly improved compared to the placebo group (P ≤ .0446 for all measures).45 Pimavanserin was generally well-tolerated; serious adverse events occurred in 11% of the pimavanserin group and 4% of the placebo group. The most common treatment-emergent adverse events were urinary tract infections, falls, hallucinations, peripheral edema, nausea, and confusional states.45
One of the most promising findings of this study was that the patients who were treated with pimavanserin experienced a reduction in their psychotic symptoms without any worsening in motor function.45 Pimavanserin is a selective 5-HT2A inverse agonist that lacks activity at dopaminergic, muscarinic, adrenergic, and histaminergic receptors.47 This agent’s action at serotonergic receptors is noteworthy because the binding of 5-HT2A receptors is increased in the neocortex of patients with PD, and visual hallucinations are thought to be associated with an increased number of 5-HT2A receptors in visual processing areas.41 As an inverse agonist, pimavanserin binds to and decreases the activity of 5-HT2A receptors, thereby improving symptoms of psychosis. Additionally, because dopaminergic receptors are not affected, motor symptoms should remain unchanged.47
Clinical Points 
- Ask all patients with PD about possible signs of psychosis at every visit.
- Be sensitive to the fact that patients and caregivers may find the topic of psychosis embarrassing or distressing.
- Give all patients with PDP a complete medical evaluation to eliminate other potential causes of psychosis prior to changing or adding medications.
- Reduce or eliminate all non-essential PD medications prior to starting a trial of an antipsychotic.
- Select an antipsychotic that is likely to alleviate psychosis without worsening motor symptoms.
Pimavanserin 34-mg capsules and 10-mg tablets are approved to treat delusions and hallucinations associated with PD.48,49 The lower dose should be used when pimavanserin is coadministered with strong CYP3A4 inhibitors (eg, ketoconazole). After reports raised concerns regarding the safety of pimavanserin, the US Food and Drug Administration evaluated the evidence50 but did not add new warnings. Post-approval adverse effects that have been reported include somnolence, rash, urticaria, and reactions consistent with angioedema.49 Current clinical trial database evidence suggests that patients with PDP are about 5 times more likely to respond (according to the SAPS-PD) to pimavanserin than to discontinue it due to an adverse event.51 Small studies reporting patient experience so far indicate that about half have improved,52,53 but more evidence is needed. With any pharmacologic agent, clinicians, patients, and caregivers must discuss potential benefits and risks before deciding to initiate treatment.
Case Practice Question ![]()
Case 3.1 Mr Cohen is a 73-year-old man with PD who has been having visions of dogs running around his house. These visions initially occurred only once a week, but he now has them daily. He has even started buying food to feed the dogs and is putting food in a bowl on the ground. Mr Cohen’s caregiver is upset and is arguing with him that there are no dogs. Mr Cohen’s primary care doctor checked his laboratories and urine, which were normal, and asked him to follow up with his neurologist.
What would be the best line of treatment?
- Start the patient on haloperidol.
- Reduce PD medications.
- Start risperidone.
- Do nothing.
Discussion of Case Practice Question 
Preferred response is b. Reduce PD medications
Because Mr Cohen’s hallucinations are upsetting to his caregiver and have become disruptive, doing nothing is no longer an option. Neither haloperidol nor risperidone would be the best line of treatment because both of these medications are strong dopamine blockers and would, therefore, lead to worsening motor symptoms. Reducing Mr Cohen’s PD medications is the best choice because his hallucinations may resolve without the need for adding any additional treatments.
Case Practice Question ![]()
Case 3.2 Mr Cohen’s treatment regimen consisted of 1 mg of pramipexole 3 times a day and 25/100 mg of carbidopa/levodopa, also 3 times a day. To treat his hallucinations, Mr Cohen’s pramipexole dose was reduced to 0.5 mg/3 times a day. His motor symptoms increased and he could not sleep. His pramipexole dose was brought back up, and his hallucinations were unchanged.
What should be the next line of treatment?
- Add 34 mg/d of pimavanserin.
- Add 17 mg/d of pimavanserin.
- Add 1 mg of risperidone at bedtime.
- No medication change.
Discussion of Case Practice Question 
Preferred response is a. Add 34 mg/d of pimavanserin
As in Mr Cohen’s previous visit, doing nothing or adding risperidone are not viable options because the hallucinations have become disruptive, and risperidone is likely to lead to a worsening of motor symptoms. Pimavanserin is the only antipsychotic approved to treat psychosis in patients with PD, and the recommended dose is 34 mg/d.
Disclosure of off-label usage: The chair has determined that, to the best of his knowledge, haloperidol, risperidone, quetiapine, and clozapine are not approved by the US Food and Drug Administration for the treatment of Parkinson disease psychosis.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Dorsey ER, Bloem BR. The Parkinson pandemic—a call to action. JAMA Neurol. 2018;75(1):9-10. PubMed CrossRef
2. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010;67(8):996-1001. PubMed CrossRef
3. Fénelon G, Alves G. Epidemiology of psychosis in Parkinson’s disease. J Neurol Sci. 2010;289(1-2):12-17. PubMed CrossRef
4. Schrag A, Hovris A, Morley D, et al. Caregiver-burden in Parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord. 2006;12(1):35-41. PubMed CrossRef
5. McKinlay A, Grace RC, Dalrymple-Alford JC, et al. A profile of neuropsychiatric problems and their relationship to quality of life for Parkinson’s disease patients without dementia. Parkinsonism Relat Disord. 2008;14(1):37-42. PubMed CrossRef
6. Klein C, Prokhorov T, Miniovitz A, et al. Admission of Parkinsonian patients to a neurological ward in a community hospital. J Neural Transm (Vienna). 2009;116(11):1509-1512. PubMed CrossRef
7. Hermanowicz N, Edwards K. Parkinson’s disease psychosis: symptoms, management, and economic burden. Am J Manag Care. 2015;21(suppl):s199-s206. PubMed
8. Forsaa EB, Larsen JP, Wentzel-Larsen T, et al. What predicts mortality in Parkinson disease? a prospective population-based long-term study. Neurology. 2010;75(14):1270-1276. PubMed CrossRef
9. Anonymous. Does anyone have hallucinations from their PD meds. Heal – Park Mov. April 2013. Available at https://healthunlocked.com/parkinsonsmovement/posts/977500/does-anyone-have-hallucinations-from-their-pd-meds. Accessed August 1, 2018.
10. Anonymous. Does anyone worry about Lewy Body Disease? Park Found Forums. November 2016. Available at http://forum.parkinson.org/topic/21599-does-anyone-worry-about-lewy-body-disease/. Accessed August 1, 2018.
11. Anonymous. Need help – Dad having a serious delusion. Park Found Forums. August 2016. Available at http://forum.parkinson.org/topic/21363-need-help-dad-having-a-serious-delusion/. Accessed August 1, 2018.
12. Anonymous. Maximum dose of Sinemet. Park Found Forums. February 2013. Available at http://forum.parkinson.org/topic/14075-maximum-dose-of-sinemet/?page=2&tab=comments#comment-119029. Accessed August 1, 2018.
13. Anonymous. Drifting from myself…… Park Found Forums. June 2016. Available at http://forum.parkinson.org/topic/21064-drifting-from-myself/?page=2&tab=comments#comment-113281. Accessed August 1, 2018.
14. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society UPDRS Revision Task Force. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170. PubMed CrossRef
15. Mehndiratta M, Garg RK, Pandey S. Nonmotor symptom complex of Parkinson’s disease—an under-recognized entity. J Assoc Physicians India. 2011;59:302-308, 313. PubMed
16. Chaudhuri KR, Prieto-Jurcynska C, Naidu Y, et al. The nondeclaration of nonmotor symptoms of Parkinson’s disease to health care professionals: an international study using the nonmotor symptoms questionnaire. Mov Disord. 2010;25(6):704-709. PubMed CrossRef
17. Goetz CG, Fan W, Leurgans S, et al. The malignant course of “benign hallucinations” in Parkinson disease. Arch Neurol. 2006;63(5):713-716. PubMed CrossRef
18. Martinez-Ramirez D, Okun MS, Jaffee MS. Parkinson’s disease psychosis: therapy tips and the importance of communication between neurologists and psychiatrists. Neurodegener Dis Manag. 2016;6(4):319-330. PubMed CrossRef
19. Carlat DJ. The psychiatric review of symptoms: a screening tool for family physicians. Am Fam Physician. 1998;58(7):1617-1624. PubMed
20. Ravina B, Marder K, Fernandez HH, et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord. 2007;22(8):1061-1068. PubMed CrossRef
21. Goldman JG, Vaughan CL, Goetz CG. An update expert opinion on management and research strategies in Parkinson’s disease psychosis. Expert Opin Pharmacother. 2011;12(13):2009-2024. PubMed CrossRef
22. Fénelon G, Mahieux F, Huon R, et al. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123(pt 4):733-745. PubMed CrossRef
23. Llorca PM, Pereira B, Jardri R, et al. Hallucinations in schizophrenia and Parkinson’s disease: an analysis of sensory modalities involved and the repercussion on patients. Sci Rep. 2016;6(1):38152. PubMed CrossRef
24. Fénelon G, Soulas T, Cleret de Langavant L, et al. Feeling of presence in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2011;82(11):1219-1224. PubMed CrossRef
25. Friedman JH. Parkinson disease psychosis: update. Behav Neurol. 2013;27(4):469-477. PubMed CrossRef
26. Jakel RJ, Stacy M. Parkinson’s disease psychosis. J Park Restless Legs Syndr. 2014;4:41-51.
27. Morgante L, Colosimo C, Antonini A, et al. PRIAMO Study Group. Psychosis associated to Parkinson’s disease in the early stages: relevance of cognitive decline and depression. J Neurol Neurosurg Psychiatry. 2012;83(1):76-82. PubMed CrossRef
28. Barrett MJ, Blair JC, Sperling SA, et al. Baseline symptoms and basal forebrain volume predict future psychosis in early Parkinson disease. Neurology. 2018;90(18):e1618-e1626. PubMed CrossRef
29. Davidsdottir S, Cronin-Golomb A, Lee A. Visual and spatial symptoms in Parkinson’s disease. Vision Res. 2005;45(10):1285-1296. PubMed CrossRef
30. Sawada H, Oeda T, Yamamoto K, et al. Trigger medications and patient-related risk factors for Parkinson disease psychosis requiring anti-psychotic drugs: a retrospective cohort study. BMC Neurol. 2013;13(1):145. PubMed CrossRef
31. Hermanowicz N, Alva G, Pagan F, et al. The emerging role of pimavanserin in the management of Parkinson’s disease psychosis. J Manag Care Spec Pharm. 2017;23(suppl):S2-S8. PubMed CrossRef
32. Fénelon G, Soulas T, Zenasni F, et al. The changing face of Parkinson’s disease-associated psychosis: a cross-sectional study based on the new NINDS-NIMH criteria. Mov Disord. 2010;25(6):763-766. PubMed CrossRef
33. Barnes J, Connelly V, Boubert L, et al. Behavioural coping patterns in Parkinson’s patients with visual hallucinations. J Neuropsychol. 2013;7(2):326-334. PubMed CrossRef
34. Joutsa J, Johansson J, Seppפnen M, et al. Dorsal-to-ventral shift in midbrain dopaminergic projections and increased thalamic/raphe serotonergic function in early Parkinson disease. J Nucl Med. 2015;56(7):1036-1041. PubMed CrossRef
35. Stahl SM. Parkinson’s disease psychosis as a serotonin-dopamine imbalance syndrome. CNS Spectr. 2016;21(5):355-359. PubMed CrossRef
36. Sadzot B, Baraban JM, Glennon RA, et al. Hallucinogenic drug interactions at human brain 5-HT2 receptors: implications for treating LSD-induced hallucinogenesis. Psychopharmacology (Berl). 1989;98(4):495-499. PubMed CrossRef
37. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry. 1989;154(05):672-676. PubMed CrossRef
38. Ballard C, Isaacson S, Mills R, et al. Impact of current antipsychotic medications on comparative mortality and adverse events in people with Parkinson disease psychosis. J Am Med Dir Assoc. 2015;16(10):898.e1-898.e7. PubMed CrossRef
39. Wilby KJ, Johnson EG, Johnson HE, et al. Evidence-based review of pharmacotherapy used for Parkinson’s disease psychosis. Ann Pharmacother. 2017;51(8):682-695. PubMed CrossRef
40. Casey DE, Zorn SH. The pharmacology of weight gain with antipsychotics. J Clin Psychiatry. 2001;62(suppl 7):4-10. PubMed
41. Pahwa R, Lyons KE. A new perspective in the treatment of Parkinson’s disease psychosis. US Neurol. 2016;12(2):93-97. CrossRef
42. Vanover KE, Weiner DM, Makhay M, et al. Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N‘ ²-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther. 2006;317(2):910-918. PubMed CrossRef
43. Mauri MC, Paletta S, Maffini M, et al. Clinical pharmacology of atypical antipsychotics: an update. EXCLI J. 2014;13:1163-1191. PubMed
44. Nuplazid [package insert]. San Diego, CA: Acadia Pharmaceuticals; 2018.
45. Cummings J, Isaacson S, Mills R, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383(9916):533-540. PubMed CrossRef
46. Schubmehl S, Sussman J. Perspective on pimavanserin and the SAPS-PD: novel scale development as a means to FDA approval [published online ahead of print June 14, 2018]. Am J Geriatr Psychiatry. PubMed CrossRef
47. Bozymski KM, Lowe DK, Pasternak KM, et al. Pimavanserin: a novel antipsychotic for Parkinson’s disease psychosis. Ann Pharmacother. 2017;51(6):479-487. PubMed CrossRef
48. Han DH. FDA approves new Nuplazid formulation, dosage strength. MPR. http://www.empr.com/news/nuplazid-pimavanserin-parkinson-psychosis-capsule-new-strength/article/777194/. Published June 29. 2018.
49. NUPLAZID- pimavanserin tartrate capsule NUPLAZID- pimavanserin tartrate tablet, coated. DailyMed. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1e6bea44-57d6-4bac-9328-46e1ee59f83b&audience=consumer. Updated July 6, 2018. Accessed August 22, 2018.
50. Webster P. Pimavanserin evaluated by the FDA. Lancet. 2018;391(10132):1762. PubMed CrossRef
51. Citrome L, Norton JC, Chi-Burris K, et al. Pimavanserin for the treatment of Parkinson’s disease psychosis: number needed to treat, number needed to harm, and likelihood to be helped or harmed. CNS Spectr. 2018;23(3):228-238. PubMed CrossRef
52. Friedman JH. Pimavanserin for psychotic symptoms in people with Parkinsonism: a second chart review [published online ahead of print July 13, 2018]. Clin Neuropharmacol. PubMed CrossRef
53. Mahajan A, Bulica B, Ahmad A, et al. Pimavanserin use in a movement disorders clinic: a single-center experience [published online ahead of print July 21, 2018]. Neurol Sci. PubMed CrossRef
This PDF is free for all visitors!