Objective: Generalized anxiety disorder (GAD) is a prevalent and costly disorder for which many patients may prefer nontraditional treatment. A proof-of-concept study of was conducted to evaluate the acute effects of Swedish massage therapy (SMT) as a monotherapy for the treatment of subjects with GAD.
Methods: A randomized, single-masked, clinical trial was conducted between March 2012 and May 2013 at the Mood and Anxiety Disorders Program of Emory University. Forty-seven currently untreated subjects with a DSM-IV diagnosis of GAD were randomly assigned to twice-weekly SMT versus a light touch control condition for 6 weeks. The primary outcome measure was reduction in Hamilton Anxiety Rating Scale (HARS) scores after 6 weeks of treatment for SMT versus light touch, as determined by mixed model repeated-measures analysis of 40 evaluable subjects.
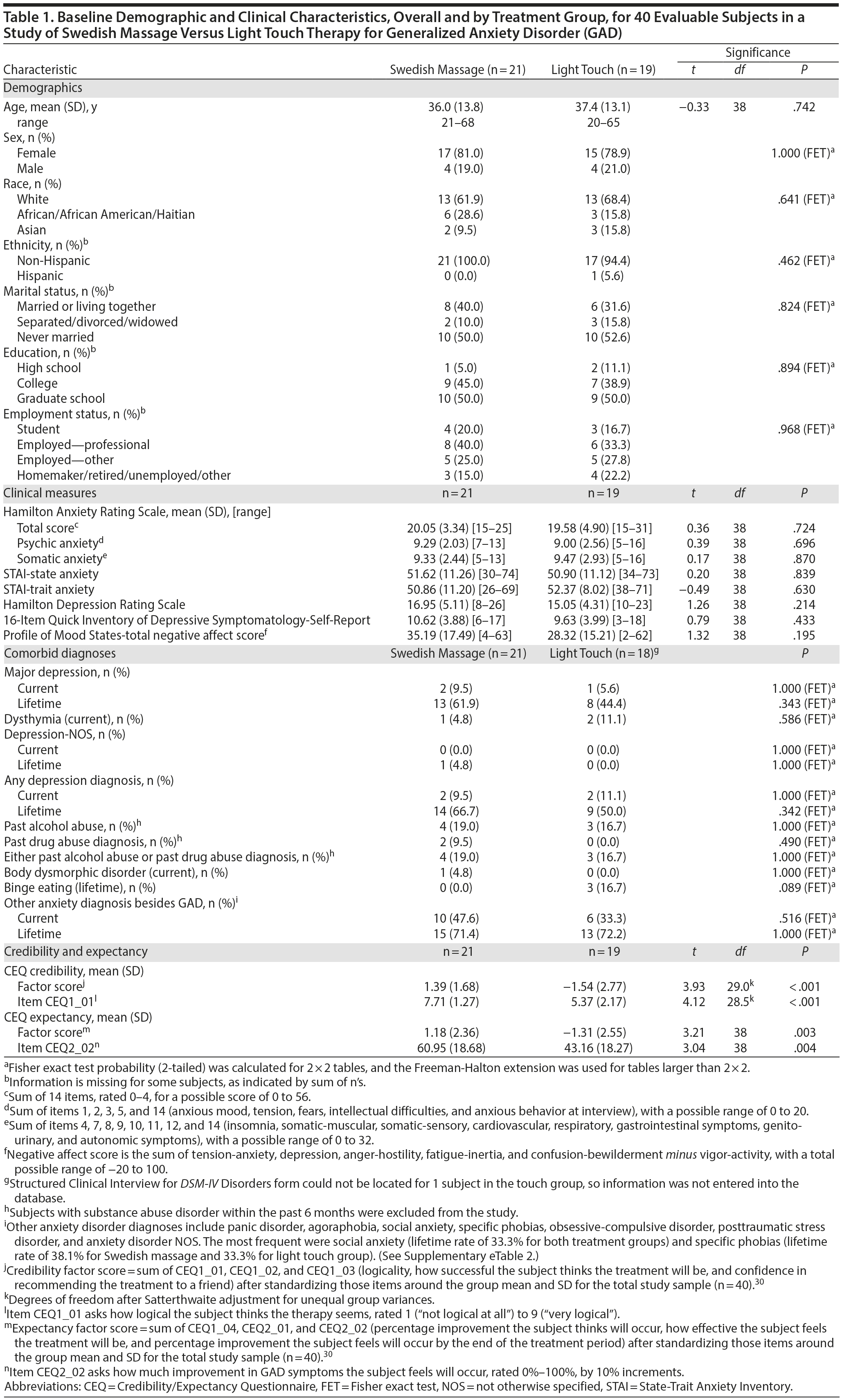
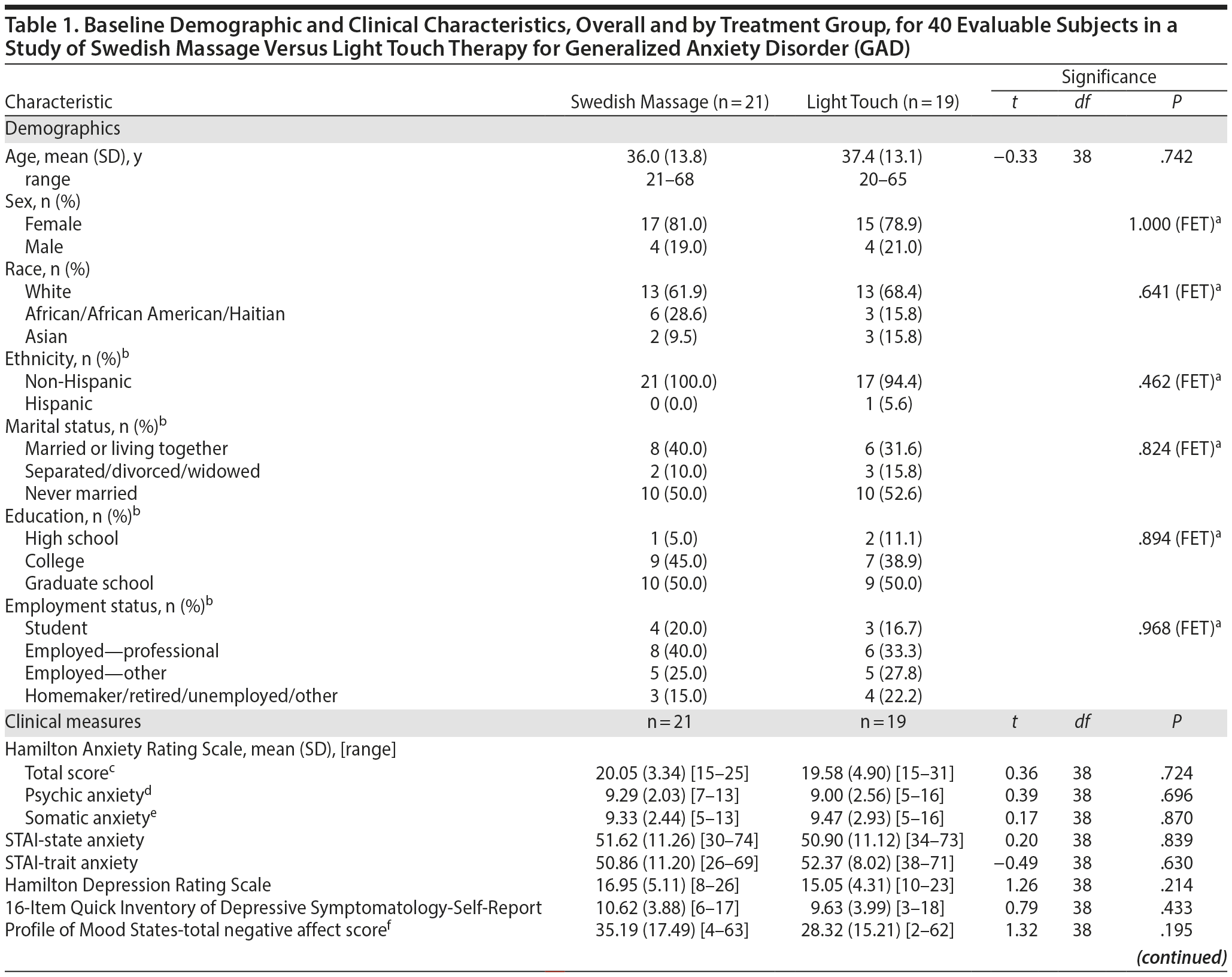
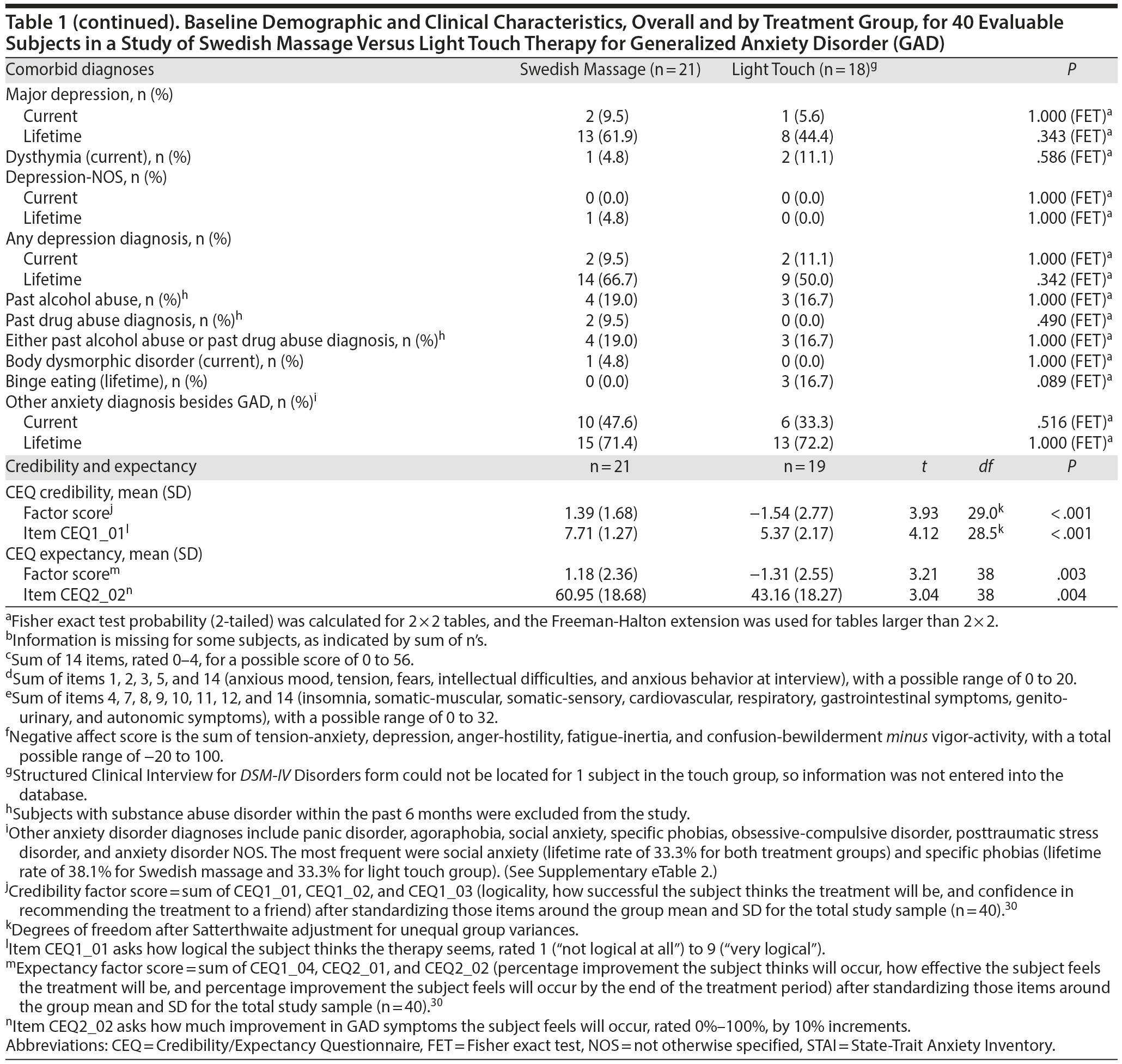
Results: Mean HARS baseline scores were 20.05 (SD = 3.34) for SMT and 19.58 (SD = 4.90) for light touch. At week 6, the difference in mean (standard error of the mean [SEM]) HARS score reduction was 3.26 points (SMT: −11.67 [1.09]; light touch: −8.41 [1.01]; t106 = −2.19; P = .030; effect size = −0.69). Treatment group differences were significant (P < .05) starting at the end of week 3.
Conclusion: This first monotherapy trial suggests that a complementary and alternative manual therapy, SMT, is an effective acute treatment for GAD.
Trial Registration: ClinicalTrials.gov identifier: NCT01337713
Acute Swedish Massage Monotherapy Successfully Remediates Symptoms of Generalized Anxiety Disorder:
A Proof-of-Concept, Randomized Controlled Study

ABSTRACT
Objective: Generalized anxiety disorder (GAD) is a prevalent and costly disorder for which many patients may prefer nontraditional treatment. A proof-of-concept study of was conducted to evaluate the acute effects of Swedish massage therapy (SMT) as a monotherapy for the treatment of subjects with GAD.
Methods: A randomized, single-masked, clinical trial was conducted between March 2012 and May 2013 at the Mood and Anxiety Disorders Program of Emory University. Forty-seven currently untreated subjects with a DSM-IV diagnosis of GAD were randomly assigned to twice-weekly SMT versus a light touch control condition for 6 weeks. The primary outcome measure was reduction in Hamilton Anxiety Rating Scale (HARS) scores after 6 weeks of treatment for SMT versus light touch, as determined by mixed model repeated-measures analysis of 40 evaluable subjects.
Results: Mean HARS baseline scores were 20.05 (SD = 3.34) for SMT and 19.58 (SD = 4.90) for light touch. At week 6, the difference in mean (standard error of the mean [SEM]) HARS score reduction was 3.26 points (SMT: −11.67 [1.09]; light touch: −8.41 [1.01]; t106 = −2.19; P = .030; effect size = −0.69). Treatment group differences were significant (P < .05) starting at the end of week 3.
Conclusion: This first monotherapy trial suggests that a complementary and alternative manual therapy, SMT, is an effective acute treatment for GAD.
Trial Registration: ClinicalTrials.gov identifier: NCT01337713
J Clin Psychiatry 2016;77(7):e883-e891
dx.doi.org/10.4088/JCP.15m10151
© Copyright 2016 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, Georgia
bAtlanta School of Massage, Atlanta, Georgia
*Corresponding author: Mark Hyman Rapaport, MD, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, 2nd Fl, 12 Executive Park Dr NE, Atlanta, GA 30329 ([email protected]).
Generalized anxiety disorder (GAD) has a lifetime prevalence rate of approximately 5.8% and is the most common anxiety disorder reported in primary care settings.1-3 It is characterized by feeling worried, apprehensive, or psychically tense most of the time about routine, everyday events and is often associated with irritability and restlessness. As described in DSM-IV and DSM-5, physical symptoms are common, and include insomnia, fatigue, muscle aches and tension, headaches, gastrointestinal complaints, and pain. Generalized anxiety disorder is frequently comorbid with other physical and psychiatric disorders, particularly major depressive disorder.4-6 It causes significant impairment in quality of life and work, and creates a significant economic burden both to the individual and to society.7
Although effective psychotherapies and pharmacotherapies exist to treat GAD, there are a number of significant barriers to receiving conventional treatment: the stigma of being diagnosed with a psychiatric disorder, the stigma of going to a mental health professional, the emotional challenge of participating in psychotherapy, the ambivalence people have about taking medication for anxiety, the cost of medication or therapy, and the burden of medication side effects. In fact, 43% of participants in the Coordinated Anxiety Learning and Management study endorsed using some form of complementary and alternative medicine therapy.8 Indeed, relief from anxiety and stress are 2 of the most common reasons that people seek out complementary and alternative treatments,9-11 with massage being one of the most frequently employed treatments for symptoms of anxiety.1,12 And Swedish massage therapy (SMT) is reputed to be the most widely used in community practice.
A decrease in symptoms of anxiety has been reported as a secondary outcome in a wide array of studies investigating the efficacy of massage for medical disorders (see Moyer et al13 for review), but the published literature on the use of massage as a primary treatment for GAD is limited to 1 case series14 and a single clinical trial15 in which massage was combined with conventional therapies. Massage has been demonstrated to modulate peripheral stress hormones and immune parameters,13,16,17 both of which are reported to be abnormal in patients with GAD.18-21
The contrast between the wide use of massage in the community to treat anxiety and the paucity of controlled data examining its efficacy for psychiatric symptoms led us to undertake a controlled clinical trial of SMT for GAD.9,13,22 On the basis of our previous research in normal volunteers,16,17 we hypothesized that twice-weekly SMT would be more effective than a twice-weekly light touch control condition in decreasing symptoms of GAD over 6 weeks.
METHODS
Trial Design and Participants
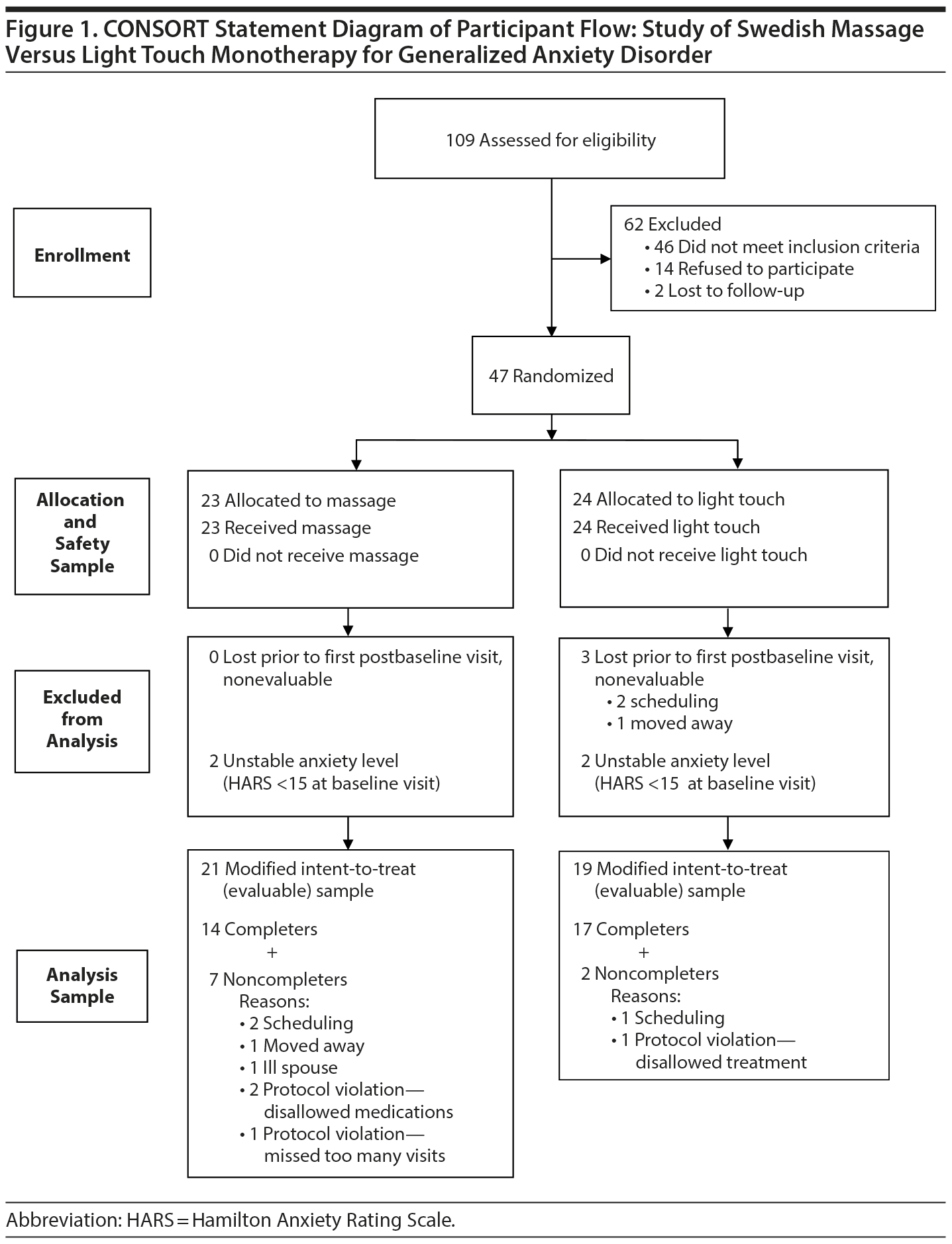
The study was a randomized, single-masked, 2-arm clinical trial comparing 6 weeks of twice-weekly SMT versus light touch as monotherapy for GAD. It was conducted at the Mood and Anxiety Disorders Program of Emory University in Atlanta, Georgia, between March 2012 and May 2013. Forty-seven subjects with GAD were recruited from the surrounding community by means of flyers, referrals, and word of mouth, and signed written informed consent was obtained. The Emory Institutional Review Board approved the study protocol. The study was registered at ClinicalTrials.gov (identifier: NCT01337713).

- Generalized anxiety disorder (GAD) is a common and highly comorbid disorder, and although almost half of the patients with GAD employ some type of complementary and alternative therapy as a component of their treatment, very little is known about the efficacy of these approaches.
- Our preliminary findings suggest that massage therapy may decrease both psychic and somatic symptoms of anxiety for patients with GAD.
To be included in the study, subjects had to be medically healthy as demonstrated by a normal medical history and physical examination, meet criteria for a primary diagnosis of current GAD as determined by the Structured Clinical Interview for DSM-IV (SCID),23 and have a Hamilton Anxiety Rating Scale (HARS)24 total score > 14. Subjects were permitted to have comorbid but secondary major depressive disorder, dysthymic disorder, or another anxiety disorder except obsessive-compulsive disorder. (The primary diagnosis was determined employing 2 criteria: [1] the subjects identified that the reason for seeking care was anxiety symptoms, and [2] the investigators assigned a primary diagnosis based on the SCID interview.) Exclusion criteria included current suicidal ideation, schizophrenia, bipolar disorder, borderline personality disorder, illicit drug use, current psychotropic medication use, current participation in psychotherapy, pregnancy, shift work, current dieting, active medical problems, excessive regular use of alcohol (more than two 5-oz glasses of wine or equivalents/d), or a history of binge drinking (more than 7 drinks/24-hour period) within the last 6 months.
Randomization and Masking
Randomization lists were created by a statistician at Emory who was not otherwise involved with the study, using randomly permuted block sizes of 4 or 6 subjects. Randomization was stratified by sex to balance the distribution of men and women in the 2 treatment groups. Two groups of sealed envelopes, 1 for women and 1 for men, were prepared based on the stratified randomization lists and kept in a locked box with access by only the study coordinator. At the baseline visit, after confirming the patient met all criteria for randomization, the study coordinator determined the treatment assignment by selecting the next sealed randomization envelope, based on male/female stratification. The coordinator opened the randomization envelope and showed the randomization to the therapist, and both coordinator and therapist signed off on the randomization in the randomization log, which was kept in a locked location by the study coordinator. Staff conducting outcome assessments were masked to subjects’ treatment intervention, and participants were asked not to disclose their assigned treatment to those staff members. The study coordinator and therapists performing the interventions did not discuss subjects’ treatment assignment with other staff at any time during the study. The study statistician conducted primary outcome analyses using masked treatment codes.
Interventions
Swedish massage therapy and light touch treatments occurred between 12:00 pm and 6:00 pm. At the start of each visit, the study coordinator obtained information from the subject about changes in health or pregnancy status, use of prescription or over-the-counter medication, illicit substance use, and any new life events. After this interview, the subject was escorted to the therapy room.
Interventions lasting 45 minutes were performed by licensed massage therapists from the Atlanta School of Massage, who adhered to a script that standardized their interactions with subjects and followed manualized treatment protocols for both interventions. Conversation between the participant and the massage therapist was kept to a minimum. The room was dimly lit, and a sound machine was used to mask unwanted noises. Each session began with the subject draped with a sheet and in a prone position on a massage table while the therapist worked slowly down the body from the shoulders to the feet. The subject then turned over to the supine position and the therapist continued the protocol from the feet back up to the shoulders. Swedish massage techniques included effleurage (slow, rhythmic, continuous stroking), petrissage (slow, rhythmic kneading of underlying muscles), and tapotement (various forms of percussive touching/tapping). The same therapists performed the control light touch treatment, which consisted of the light laying on of hands, in the same sequence and for the same amount of time. Quality control was maintained by audio taping and quarterly reliability sessions, weekly discussion of issues arising during intervention sessions, periodic spot checks of protocol adherence in conducting the SMT and light touch techniques, and feedback from participants about consistency across therapists.
Baseline and Outcome Assessments
Prior to starting the first intervention session, baseline assessments were made by a study psychiatrist using the HARS and 28-item Hamilton Depression Rating Scale (HDRS),25 and the participant completed the Spielberger State-Trait Anxiety Inventory (STAI)26 as well as the Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR),27 Profile of Mood States (POMS),28 and Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q).29 After every subsequent intervention (numbers 2 through 12), the subject completed the QIDS-SR, then met with a study psychiatrist who conducted a HARS interview and assessed the subject for suicidality. After the 12th intervention session, participants completed the STAI (state anxiety scale only), QIDS-SR, POMS, and Q-LES-Q, and the psychiatrist assessed the subject with the HDRS in addition to the HARS. The HARS consists of 14 items scored 0-4 and can be divided into 2 subscales comprising a psychic anxiety component (items 1-6 and 14) and a somatic anxiety component (items 7-13).
Credibility and Expectancy Assessments
After being informed of their treatment assignment, prior to the first intervention session, the coordinator read treatment descriptions, and the participant filled out a Credibility/Expectancy Questionnaire (CEQ).30 Three CEQ items assess the subject’s opinion about the credibility of the assigned treatment to be effective, and 3 items assess the subject’s expectation that the intervention will improve his or her symptoms.30
Statistical Analysis
Analyses comparing the 2 treatment groups were based on a modified intent-to-treat sample of 40 evaluable subjects—ie, those with at least 1 postbaseline visit. Comparisons across treatment groups at baseline were made by analysis of variance (ANOVA) for continuous measures and χ2 tests for categorical variables. Mixed model repeated-measures analysis was carried out to test treatment group differences in change from baseline to the end of week 6 for the 2 assessments performed after every intervention, the primary outcome measure (HARS) and the QIDS-SR. Models included subjects as a random effect and treatment group and visit number as fixed effects. An autoregressive covariance structure was used because it provided the best fit to the data. Changes in scores obtained only at baseline and completion of 6 weeks of treatment (HDRS, POMS, STAI-state anxiety, and Q-LES-Q) were analyzed by ANOVA, with baseline level as covariate when appropriate. Treatment response was defined as an improvement of ≥ 50% in HARS score from baseline to last visit. Comparison of response rates between treatment groups was made for all evaluable subjects and for study completers. Safety was evaluated by review of data about adverse events gathered at every study visit. Credibility and expectancy factor scores prior to the start of treatment were computed,30 compared for SMT versus light touch, and analyzed in relation to retention and primary outcome within treatment group. Statistical analyses were carried out using SAS software (SAS Institute Inc, 2001). A 2-tailed α level of .05 was used to determine statistical significance. Standardized treatment effect sizes and detailed parameters are provided for key outcome assessments as a guide for designing future studies.
RESULTS
Study Participants
As shown in Figure 1, 109 subjects were screened for this study, 47 were randomized, and 40 subjects had at least 1 intervention session and were included in the analysis. Demographic characteristics and baseline mood scores are shown in Table 1; evaluable samples randomized to SMT (n = 21) versus light touch (n = 19) did not differ on any variable.
Treatment Outcomes
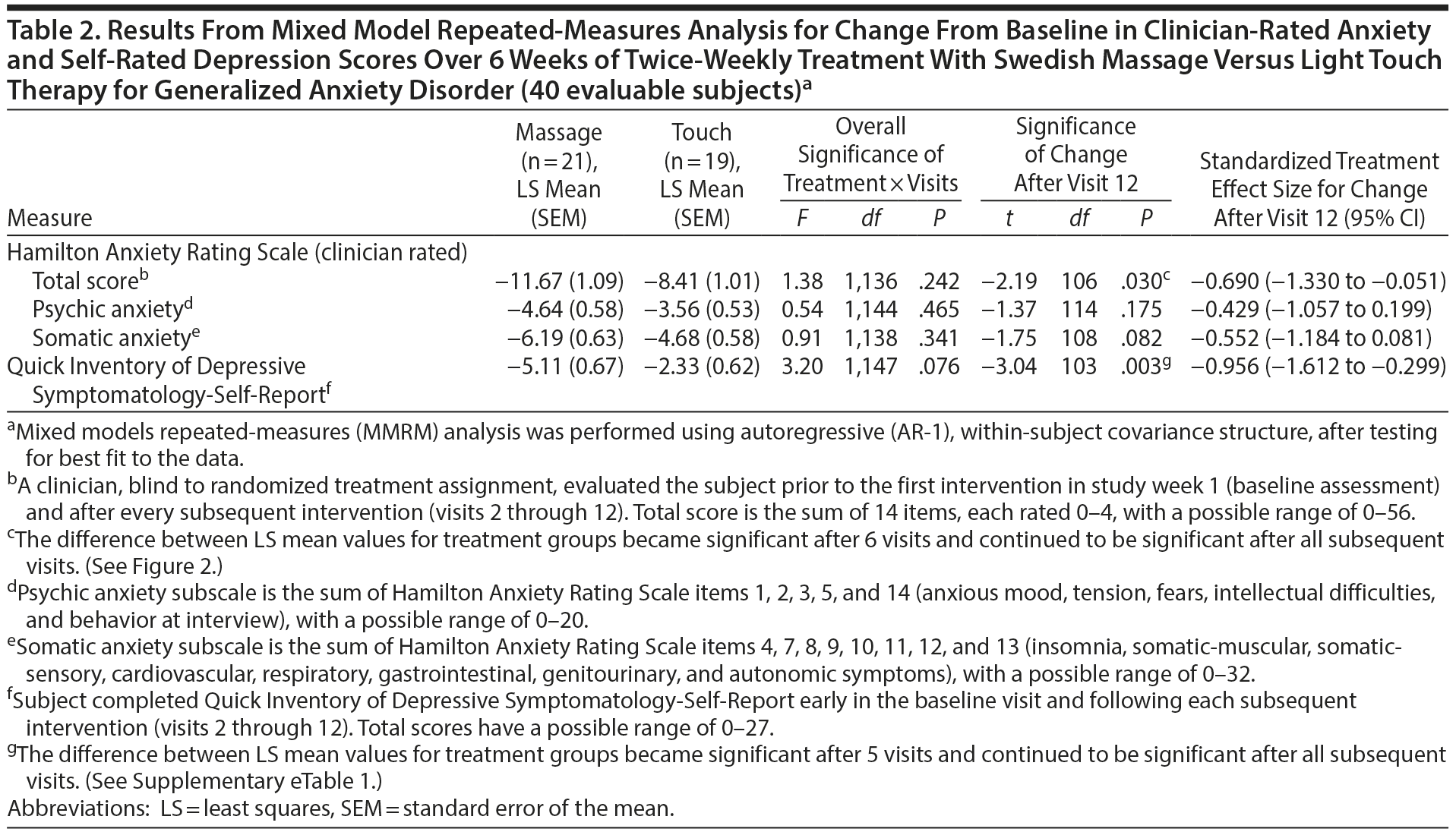
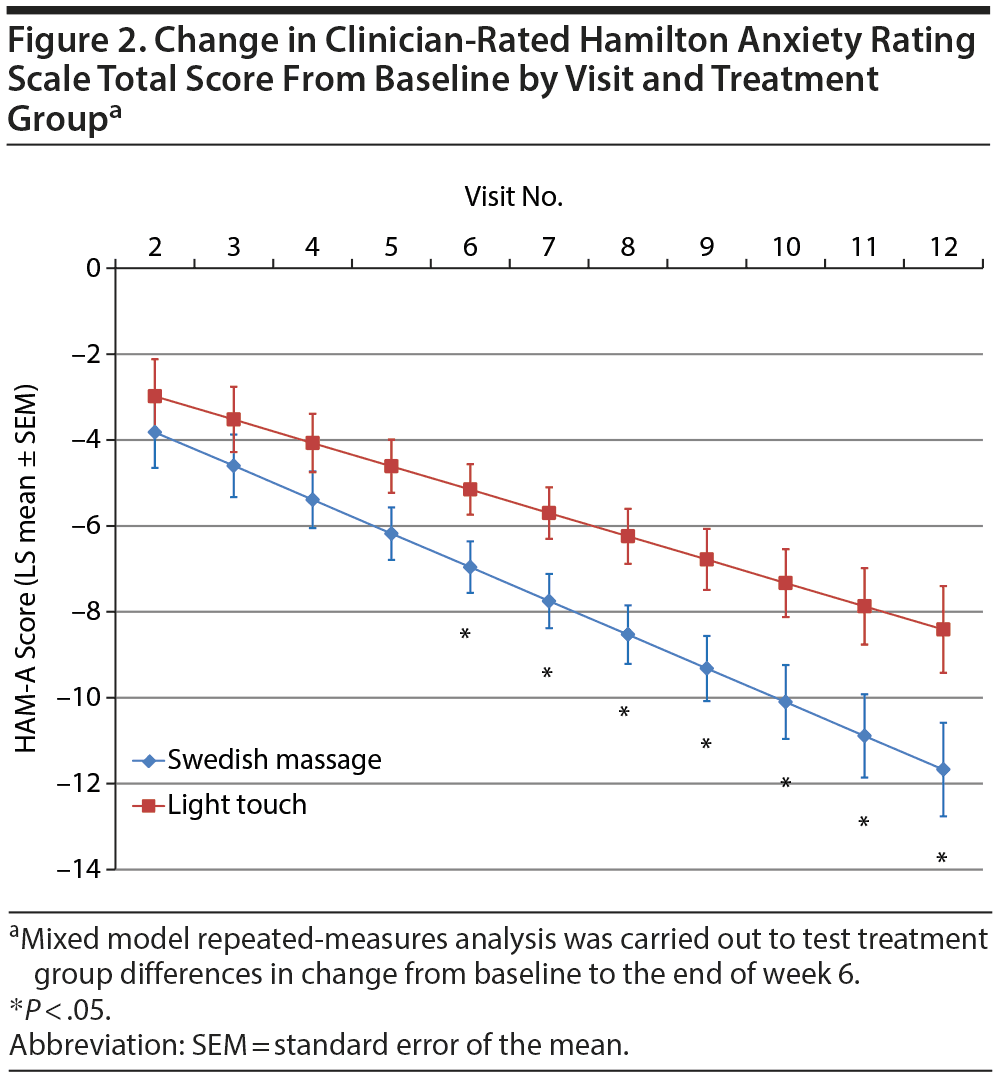
Results from mixed model repeated-measures analysis (Table 2) showed that, while the overall difference in slopes (treatment-by-visits interaction term) was not significant, the test of the primary study hypothesis was statistically and clinically significant: reduction in clinician-rated HARS scores after 12 treatment sessions (end of week 6) was 11.67 points (SEM = 1.09) for the SMT group compared to 8.41 (SEM = 1.01) points for the light touch group (t106 = −2.19; P = .030), with a standardized treatment effect size of −0.690 (95% CI, −1.330 to −0.051). Treatment group differences became significantly different after 6 treatments (end of week 3; Figure 2). Within-group effect size (change/baseline SD) over 6 weeks of treatment was twice as high for the SMT as for the light touch group (−3.49 vs −1.72). Differences in improvement on the HARS total score were accompanied by nonsignificant but moderate effect size differences favoring SMT on both the psychic anxiety and the somatic anxiety subscales (effect size = −0.429 and −0.552, respectively; Table 2). The treatment response rate (for ≥ 50% reduction from baseline in HARS total score) was 1.42 times higher for all subjects treated with SMT than those treated with light touch (52.4% vs 36.8%), and 1.62 times higher for SMT than for light touch study completers (57.1% vs 35.3%); neither result was statistically significant (P = .324 and .224, respectively).
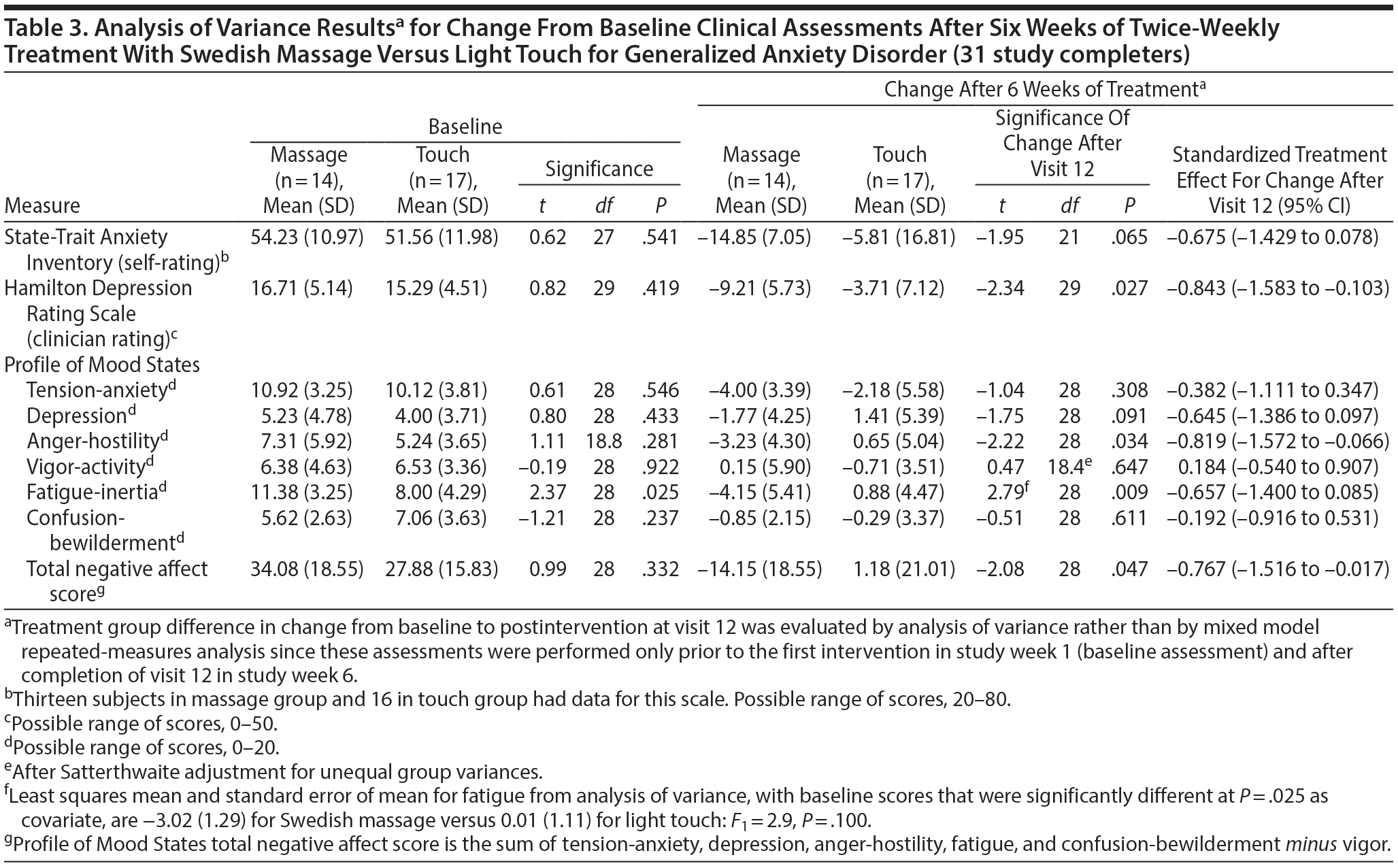
Secondary measures of improvement in mood symptoms by the end of 12 sessions were also greater for the SMT than for the light touch group for self-rated QIDS-SR depression scores (effect size = −0.956; P = .003; Table 2) as well as for STAI-state anxiety (effect size = −0.675; P = .065) and clinician-rated HDRS scores (effect size = −0.843; P = .027) based on ANOVA for study completers (Table 3). Reduction in POMS total negative affect scores at study completion was significantly greater for SMT than for light touch subjects (effect size = −0.767; P = .047), reflecting large effect size differences on the anger-hostility subscale (effect size = −0.819; P = .034) as well as the fatigue-inertia (effect size = −0.657; P = .009) and depression (effect size = −0.645; P = .091) subscales (Table 3). Negligible treatment differences occurred on the Q-LES-Q total score and overall sense of well-being item.
Role of Credibility and Expectancy
Credibility and expectancy were significantly higher for SMT than for light touch as an effective treatment for GAD symptoms, based on factor scores and key items loading on those factors (Table 1). The change in HARS total scores during the study had a nonsignificant, negative correlation with CEQ credibility and expectancy factor scores for subjects assigned to SMT, and the change in HARS total scores had a nonsignificant, positive correlation for those in the light touch group (Supplementary eTable 3). Neither CEQ factor score was significantly correlated with early study termination, although dropouts from the light touch group (2 subjects) had higher credibility and expectancy factor scores (P = .054 and .062, respectively) than study completers (Supplementary eTable 4).
Safety
No serious adverse events occurred within either treatment group during the course of treatment. Acne occurred more often within the light touch group, most likely stemming from the use of a massage lubricant, which was modified once this adverse effect was noted.
DISCUSSION
At the end of 6 weeks, subjects with GAD who received twice-weekly manualized SMT demonstrated greater statistically and clinically significant improvement on clinician-rated and self-report ratings of both anxiety and depression than subjects receiving light touch. The effect sizes observed for the anxiety measures were moderate, and those for the depression ratings were large. Greater improvement in symptoms of anxiety and depression with SMT were observed as early as the end of week 3 and continued throughout the remainder of this acute treatment trial (Figure 2, Supplementary eTable 1). These results were not merely due to a decrease in the somatic symptoms of anxiety, since the effect size was −0.429 for the psychic anxiety subscale and −0.552 for the somatic anxiety subscale of the HARS. The moderate to large effect size observed for the POMS depression, anger-hostility, and fatigue subscales further support this postulate that SMT exerts effects on both psychological and physiological symptoms. Taken as a whole, these findings suggest that SMT causes clinically significant improvement in symptoms of anxiety and depression in subjects with GAD.
We did not find significant changes in measures of quality of life over the 6 weeks of this study, which is consistent with what is observed in most short-term treatment trials of GAD.31,32 There were no serious adverse events and no notable adverse events in this study, demonstrating that SMT and light touch are well tolerated interventions, even in patients suffering from pathological anxiety.
Our findings suggest that a manualized SMT intervention may be a clinically useful monotherapy for patients with GAD. These findings, if replicated in a larger study, have important ramifications for both patients and providers. It would be the first manual monotherapy treatment demonstrated to be effective for a psychiatric disorder. Second, it suggests that the SMT treatment protocol does not have to be personalized for individual subjects to be effective; thus it could be disseminated more easily. Third, it would help argue for the acceptance of SMT by insurers as a valid and reimbursable treatment for patients with GAD. And fourth, it may increase the number of patients seeking treatment for GAD since they could choose to work with a complementary and alternative medicine therapist rather than a traditional mental health provider. Our findings also give credence to the postulate that SMT may be an effective treatment for some forms of depression.33 Thus, although further research is needed, SMT may represent a transdiagnostic treatment intervention effective for patients who are classified as presenting with a variety of comorbid anxiety and mood disorders.
The only prior randomized controlled study15 of massage therapy for GAD found that, after 12 weeks, massage was not superior to receiving thermotherapy or lying quietly on a massage table. The difference in effectiveness of massage between the 2 studies may be explained by differences in study designs. Sherman and colleagues15 allowed concomitant medication and psychotherapy treatment, used an individualized (as opposed to manualized) treatment approach, and used less intensity of treatment (intervention sessions of shorter duration, with study duration of 12 versus 6 weeks with fewer total numbers of sessions). The other published study14 of massage for GAD is an open trial of SMT for GAD, which employed a qualitative outcome interview and reported that SMT decreased anxiety and improved self-confidence. We believe that our study adds significantly to the extant literature.
Although rigorous investigation of massage therapy is relatively nascent, there are well-conducted randomized trials demonstrating that massage can ameliorate depression in human immunodeficiency virus-infected patients,33 knee pain,34 and back pain.35 Both preclinical studies and clinical studies investigating the effects of massage on exercise-induced muscle damage demonstrate that massage can profoundly decrease local inflammation and promote muscle healing.36-39 Studies in healthy volunteers also suggest that massage and touch can modulate peripheral hormone and immune function.16,17 This work indicates that there is merit to further investigation of the biology of massage.
A significant strength of this study was our development of manualized approaches to both SMT and light touch that were carefully monitored for fidelity. Other strengths include use of masked assessors and the consistency of effects across a variety of self-report and clinician-rated measures. A limitation common to all manual therapy trials and with most psychotherapy trials is the inability to mask the subject to the treatment assignment. However, our assessments of expectancy and credibility did not identify significant correlations between these constructs and either treatment outcome or treatment discontinuation. (Although it is intriguing to note that the 2 subjects who prematurely discontinued light touch had higher initial expectancy and credibility scores.) The relatively small sample size of the study produced large confidence intervals around the effect size estimates, yet the consistency of effect sizes across self-report and clinician-rated measures (for anxiety and depression, respectively) is reassuring and gives credence to our findings.
CONCLUSIONS
Twice-weekly Swedish massage therapy was clinically effective in decreasing symptoms of anxiety and depression in otherwise treatment-free subjects with GAD. These findings suggest that a time-limited course of massage may be a reasonable treatment alternative for people with GAD. Further study to replicate and extend our findings by comparing Swedish massage therapy to accepted treatments for GAD is warranted.
Submitted: June 5, 2015; accepted August 11, 2015.
Author contributions: Conception or design: Drs Rapaport and Schettler; acquisition, analysis, or interpretation of data: Drs Rapaport, Schettler, Dunlop, Rakofsky, and Kinkead and Mss Edwards and Larson; drafting of the manuscript: Drs Rapaport, Schettler, and Kinkead; critical revision of the manuscript for important intellectual content: Drs Rapaport, Schettler, Dunlop, Rakofsky, and Kinkead and Mss Edwards and Larson; statistical analysis: Dr Schettler; obtaining funding: Dr Rapaport; administrative, technical, or material support: Mss Edwards and Larson and Drs Dunlop, Rakofsky, and Kinkead; supervision: Drs Rapaport, Dunlop, and Kinkead and Ms Edwards. Drs Rapaport and Schettler had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Potential conflicts of interest: Dr Rapaport has provided consulting services to PAX (unpaid) and has been funded by the National Institutes of Health (NIH). Dr Schettler works part-time both as Senior Research Associate in the Department of Psychiatry and Behavioral Sciences at Emory University School of Medicine, Atlanta, Georgia, as well as Principal Statistician in the Department of Psychiatry of the School of Medicine at the University of California, San Diego; has been funded by the NIH; and has no other direct or indirect affiliations or financial interests in connection with the contents of this article. Dr Dunlop has received grant support from AstraZeneca, Bristol-Myers Squibb, Forest, GlaxoSmithKline, National Institute of Mental Health, Otsuka and Pfizer; and has received honoraria for consulting from Genentech, Hoffman LaRoche, MedAvante, and Pfizer. Dr Rakofsky has received research funding from Takeda, AstraZeneca, and Novartis. Dr Kinkead has received research funding from NIH, PCORI, and the Brain and Behavior Research Foundation. Ms Larson is an employee of the Atlanta School of Massage. Ms Edwards reports no conflicts of interest.
Funding/support: The study was funded by grant R21AT004208 from the NIH to Dr Rapaport and received support from the National Center for Advancing Translational Sciences of the NIH under award number UL1TR000454.
Role of the sponsor: The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Disclaimer: The content of this study is solely the responsibility of the authors and does not necessarily represent the views of the NIH.
Previous presentation: Some of the preliminary analyses of these data were presented at the 53rd Annual Meeting of the American College of Neuropsychopharmacology; December 7-11, 2014; Phoenix, Arizona.
Acknowledgments: The authors would like to acknowledge and thank the following employees of the Atlanta School of Massage: staff members Leticia Allen, Lovelace Linares, and Aaron Gunn as well as the research massage therapists Dedric Carroll, Laureen Dietrick, Grace Prior, and Brittney Turner. This study could not have been performed without their partnership. The acknowledged individuals have no additional conflicts of interest to report.
Supplementary material: See accompanying pages.
REFERENCES
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. PubMed doi:10.1001/archpsyc.62.6.593
2. Ormel J, VonKorff M, Ustun TB, et al. Common mental disorders and disability across cultures: results from the WHO Collaborative Study on Psychological Problems in General Health Care. JAMA. 1994;272(22):1741-1748. PubMed doi:10.1001/jama.1994.03520220035028
3. Wittchen HU, Kessler RC, Beesdo K, et al. Generalized anxiety and depression in primary care: prevalence, recognition, and management. J Clin Psychiatry. 2002;63(suppl 8):24-34. PubMed
4. Lydiard RB, Fossey MD, Marsh W, et al. Prevalence of psychiatric disorders in patients with irritable bowel syndrome. Psychosomatics. 1993;34(3):229-234. PubMed doi:10.1016/S0033-3182(93)71884-8
5. Roy-Byrne PP, Wagner A. Primary care perspectives on generalized anxiety disorder. J Clin Psychiatry. 2004;65(suppl 13):20-26. PubMed
6. Wetherell JL, Ayers CR, Nuevo R, et al. Medical conditions and depressive, anxiety, and somatic symptoms in older adults with and without generalized anxiety disorder. Aging Ment Health. 2010;14(6):764-768. PubMed doi:10.1080/13607861003713240
7. Hoffman DL, Dukes EM, Wittchen HU. Human and economic burden of generalized anxiety disorder. Depress Anxiety. 2008;25(1):72-90. PubMed doi:10.1002/da.20257
8. Bystritsky A, Hovav S, Sherbourne C, et al. Use of complementary and alternative medicine in a large sample of anxiety patients. Psychosomatics. 2012;53(3):266-272. PubMed doi:10.1016/j.psym.2011.11.009
9. Barnes PM, Bloom B, Nahin RL. Complementary and Alternative Medicine Use Among Adults and Children: United States, 2007. Hyattsville, MD: National Center for Health Statistics; 2008.
10. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997: results of a follow-up national survey. JAMA. 1998;280(18):1569-1575. PubMed doi:10.1001/jama.280.18.1569
11. Eisenberg DM, Kessler RC, Foster C, et al. Unconventional medicine in the United States: prevalence, costs, and patterns of use. N Engl J Med. 1993;328(4):246-252. PubMed doi:10.1056/NEJM199301283280406
12. Cherkin DC, Deyo RA, Sherman KJ, et al. Characteristics of visits to licensed acupuncturists, chiropractors, massage therapists, and naturopathic physicians. J Am Board Fam Pract. 2002;15(6):463-472. PubMed
13. Moyer CA, Rounds J, Hannum JW. A meta-analysis of massage therapy research. Psychol Bull. 2004;130(1):3-18. PubMed doi:10.1037/0033-2909.130.1.3
14. Billhult A, Mפפttפ S. Light pressure massage for patients with severe anxiety. Complement Ther Clin Pract. 2009;15(2):96-101. PubMed doi:10.1016/j.ctcp.2008.10.003
15. Sherman KJ, Ludman EJ, Cook AJ, et al. Effectiveness of therapeutic massage for generalized anxiety disorder: a randomized controlled trial. Depress Anxiety. 2010;27(5):441-450. PubMed doi:10.1002/da.20671
16. Rapaport MH, Schettler P, Breese C. A preliminary study of the effects of a single session of Swedish massage on hypothalamic-pituitary-adrenal and immune function in normal individuals. J Altern Complement Med. 2010;16(10):1079-1088. PubMed doi:10.1089/acm.2009.0634
17. Rapaport MH, Schettler P, Bresee C. A preliminary study of the effects of repeated massage on hypothalamic-pituitary-adrenal and immune function in healthy individuals: a study of mechanisms of action and dosage. J Altern Complement Med. 2012;18(8):789-797. PubMed doi:10.1089/acm.2011.0071
18. Connor KM, Davidson JR. Generalized anxiety disorder: neurobiological and pharmacotherapeutic perspectives. Biol Psychiatry. 1998;44(12):1286-1294. PubMed doi:10.1016/S0006-3223(98)00285-6
19. Hoehn-Saric R, McLeod DR, Lee YB, et al. Cortisol levels in generalized anxiety disorder. Psychiatry Res. 1991;38(3):313-315. PubMed doi:10.1016/0165-1781(91)90021-G
20. Kim J, Gorman J. The psychobiology of anxiety. Clin Neurosci Res. 2005;4(5-6):335-347. doi:10.1016/j.cnr.2005.03.008
21. Vieira MM, Ferreira TB, Pacheco PA, et al. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol. 2010;229(1-2):212-218. PubMed doi:10.1016/j.jneuroim.2010.07.018
22. Sarris J, Moylan S, Camfield DA, et al. Complementary medicine, exercise, meditation, diet, and lifestyle modification for anxiety disorders: a review of current evidence. Evid Based Complement Alternat Med. 2012;2012:809653. PubMed doi:10.1155/2012/809653
23. First MB, Frances A, Pincus HA. DSM-IV Handbook of Differntial Diagnosis. 1st ed. Washington, DC: American Psychiatric Press; 1995.
24. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. PubMed doi:10.1111/j.2044-8341.1959.tb00467.x
25. Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Washington, DC: US Department of Health, Education, and Welfare; 1976.
26. Spielberger CD, Gorssuch RL, Lushene PR, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc; 1983.
27. Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. PubMed doi:10.1016/S0006-3223(02)01866-8
28. Pollock V, Cho DW, Reker D, et al. Profile of Mood States: the factors and their physiological correlates. J Nerv Ment Dis. 1979;167(10):612-614. PubMed doi:10.1097/00005053-197910000-00004
29. Endicott J, Nee J, Harrison W, et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321-326. PubMed
30. Devilly GJ, Borkovec TD. Psychometric properties of the Credibility/Expectancy Questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73-86. PubMed doi:10.1016/S0005-7916(00)00012-4
31. Patrick DL, Chiang YP. Measurement of health outcomes in treatment effectiveness evaluations: conceptual and methodological challenges. Med Care. 2000;38(9 suppl):II14-II25. PubMed doi:10.1097/00005650-200009002-00005
32. Rapaport MH, Clary C, Fayyad R, et al. Quality-of-life impairment in depressive and anxiety disorders. Am J Psychiatry. 2005;162(6):1171-1178. PubMed doi:10.1176/appi.ajp.162.6.1171
33. Poland RE, Gertsik L, Favreau JT, et al. Open-label, randomized, parallel-group controlled clinical trial of massage for treatment of depression in HIV-infected subjects. J Altern Complement Med. 2013;19(4):334-340. PubMed doi:10.1089/acm.2012.0058
34. Perlman AI, Ali A, Njike VY, et al. Massage therapy for osteoarthritis of the knee: a randomized dose-finding trial. PLoS ONE. 2012;7(2):e30248. PubMed doi:10.1371/journal.pone.0030248
35. Furlan AD, Imamura M, Dryden T, et al. Massage for low back pain: an updated systematic review within the framework of the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009;34(16):1669-1684. PubMed doi:10.1097/BRS.0b013e3181ad7bd6
36. Crane JD, Ogborn DI, Cupido C, et al. Massage therapy attenuates inflammatory signaling after exercise-induced muscle damage. Sci Transl Med. 2012;4(119):19ra13. PubMed
37. Crawford SK, Haas C, Butterfield TA, et al. Effects of immediate vs delayed massage-like loading on skeletal muscle viscoelastic properties following eccentric exercise. Clin Biomech (Bristol, Avon). 2014;29(6):671-678. PubMed doi:10.1016/j.clinbiomech.2014.04.007
38. Haas C, Best TM, Wang Q, et al. In vivo passive mechanical properties of skeletal muscle improve with massage-like loading following eccentric exercise. J Biomech. 2012;45(15):2630-2636. PubMed doi:10.1016/j.jbiomech.2012.08.008
39. Haas C, Butterfield TA, Abshire S, et al. Massage timing affects postexercise muscle recovery and inflammation in a rabbit model. Med Sci Sports Exerc. 2013;45(6):1105-1112. PubMed doi:10.1249/MSS.0b013e31827fdf18
Enjoy this premium PDF as part of your membership benefits!