The goals of schizophrenia treatment are to control symptoms, prevent relapse, and improve functioning and quality of life. For many patients, these goals are not being met. This report highlights information provided by experts on the reasons for, impact of, and means to reduce relapse in patients with schizophrenia; on patient-centered and patient-reported assessment; on the benefits and risks of medication options, including long-acting injectable (LAI) antipsychotics; and on psychosocial interventions that may improve adherence and help prevent relapse in individuals living with schizophrenia. Modifiable risk factors for poor outcomes in patients with schizophrenia include longer duration of untreated illness, comorbid substance abuse, early nonresponse to an antipsychotic, and the number of relapses that are related to nonadherence. Recommendations include 1) adopting a patient-centered approach that incorporates the use of patient-reported outcome measures; 2) selecting medications based on a balanced risk-benefit assessment, including a focus on addressing symptoms for which the agents were superior to placebo and/or active controls; 3) considering LAIs as an alternative to oral medications, as they offer benefits such as reliable drug delivery, uncovering nonadherence and pseudo-resistance, and reduced relapse risk and mortality; and 4) implementing psychosocial interventions that have been proven to be effective in improving adherence and overall outcomes.
This CME activity is expired. For more CME activities, visit cme.psychiatrist.com.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders

CME Background
Articles are selected for credit designation based on an assessment of the educational needs of CME participants, with the purpose of providing readers with a curriculum of CME articles on a variety of topics throughout each volume. Activities are planned using a process that links identified needs with desired results.
To obtain credit, read the article, correctly answer the questions in the Posttest, and complete the Evaluation.
This Academic Highlights section of The Journal of Clinical Psychiatry presents the highlights of the teleconference series “The Schizophrenia Remission Roller Coaster: Using Long-Acting Injectable Antipsychotics to Improve Adherence and Enhance Potential for Functional Recovery,” which was held in September and October 2019. This report was prepared and independently developed by the CME Institute of Physicians Postgraduate Press, Inc., and was supported by educational grants from Alkermes, Inc.; Otsuka America Pharmaceutical, Inc. and Lundbeck.
The teleconference was chaired by Christoph U. Correll, MD, Department of Psychiatry, The Zucker Hillside Hospital, Glen Oaks, and Department of Psychiatry and Molecular Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, New York, USA; and Department of Child and Adolescent Psychiatry, Charité Universitפtsmedizin, Berlin, Germany. The faculty was John Lauriello, MD, Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, Pennsylvania.
CME Objective
After studying this article, you should be able to:
• Select evidence-based treatment for patients with schizophrenia who often relapse
Accreditation Statement
The CME Institute of Physicians Postgraduate Press, Inc., is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.
Release, Expiration, and Review Dates
This educational activity was published in June 2020 and is eligible for AMA PRA Category 1 Creditâ„¢ through August 31, 2022. The latest review of this material was April 2020.
Financial Disclosure
All individuals in a position to influence the content of this activity were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. In the past year, Marlene P. Freeman, MD, Editor in Chief, has received research funding from JayMac and Sage; has been a member of the advisory boards for Otsuka, Alkermes, and Sunovion; has been a member of the Independent Data Safety and Monitoring Committee for Janssen; has been a member of the Steering Committee for Educational Activities for Medscape; and, as a Massachusetts General Hospital (MGH) employee, works with the MGH National Pregnancy Registry, which is sponsored by Teva, Alkermes, Otsuka, Actavis, and Sunovion, and works with the MGH Clinical Trials Network and Institute, which receives research funding from multiple pharmaceutical companies and the National Institute of Mental Health. No member of the CME Institute staff reported any relevant personal financial relationships.
Credit Designation
The CME Institute of Physicians Postgraduate Press, Inc., designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Creditâ„¢. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Note: The American Academy of Physician Assistants (AAPA) accepts certificates of participation for educational activities certified for AMA PRA Category 1 Creditâ„¢ from organizations accredited by ACCME or a recognized state medical society. Physician assistants may receive a maximum of 1 hour of Category I credit for completing this program.
Financial Disclosure
Dr Correll is a consultant for and has received honoraria from Acadia, Alkermes, Allergan, Angelini, Axsome, Boehringer-Ingeheim, Gedeon Richter, Gerson Lehrman Group, Indivior, IntraCellular Therapies, Janssen, LB Pharma, Lundbeck, MedAvante-ProPhase, Medscape, Merck, Neurocrine, Noven, Otsuka, Pfizer, Recordati, ROVI, Servier, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva; has received grant/research support from Janssen and Takeda; is a member of the speakers/advisory boards for Alkermes, Allergan, Angelini, Gedeon Richter, IntraCellular Therapies, Janssen, LB Pharma, Lundbeck, Neurocrine, Noven, Otsuka, Pfizer, Recordati, Sumitomo Dainippon, Sunovion, Supernus, Takeda, and Teva; is a stock option holder of LB Pharma; and has received other financial or material support for expert testimony from Bristol-Myers Squibb, Janssen, and Otsuka and royalties from UpToDate. Dr Lauriello is a consultant for Indivior and Alkermes, has received royalties from UptoDate, and has lectured on clinical trial training for Roche.
Review Process
The faculty member(s) agreed to provide a balanced and evidence-based presentation and discussed the topic(s) and CME objective(s) during the planning sessions. The faculty’s submitted content was validated by CME Institute staff, and the activity was evaluated for accuracy, use of evidence, and fair balance by the Chair and a peer reviewer who is without conflict of interest.
The opinions expressed herein are those of the faculty and do not necessarily reflect the opinions of the CME provider and publisher or the commercial supporter.
J Clin Psychiatry 2020;81(4):MS19053AH5C
To cite: Correll CU, Lauriello J. Using long-acting injectable antipsychotics to enhance potential for recovery from schizophrenia. J Clin Psychiatry. 2020;81(4):MS19053AH5C.
To share: https://doi.org/10.4088/JCP.MS19053AH5C
© Copyright 2020 Physicians Postgraduate Press, Inc.
The goals of schizophrenia treatment are to control symptoms, prevent relapse, and improve functioning and quality of life. For many patients, these goals are not being met.1 Evidence demonstrates that long-acting injectable (LAI) antipsychotics are one of the most effective ways to prevent relapse in patients with schizophrenia, yet they remain underused.2 Health care providers infrequently discuss LAI antipsychotic options with their patients with schizophrenia. Clinicians also may overestimate adherence or not even assess it.3 Patients with multiple relapses are often continued on oral medications, and few clinicians consider LAIs early in the course of illness.2
This report, based on a series of teleconferences given by Christoph U. Correll, MD, and John Lauriello, MD, will address the impact of relapse in patients with schizophrenia, patient-centered assessment, treatment options, and psychosocial interventions.
IMPACT OF RELAPSE
Schizophrenia is a complex disorder that is characterized by positive and negative symptoms.1,4 Positive symptoms include hallucinations and delusions. Negative symptoms consist of lack of motivation, reduction in feeling pleasure, affective blunting, alogia, avolition, and anhedonia.5 In addition to these symptoms, schizophrenia is associated with cognitive dysfunction, mood problems, and a high suicide rate.6-8
The onset of schizophrenia does not typically occur until late adolescence or early adulthood, stated Dr Lauriello.9 Throughout the course of the disease, patients tend to experience relapses and remissions, with each relapse potentially leading to further deterioration.3
Relapse: Definition, Impact, and Predictors
The definition of relapse in patients with schizophrenia varies among studies. In a large systematic literature review, Olivares et al10 found that hospitalization was the most widely used factor to define relapse across studies. Other factors included scores on rating scales that assess symptom severity (in particular, positive symptoms), such as the Positive and Negative Syndrome Scale (PANSS)11 and the Clinical Global Impression (CGI) scale.12 Exacerbation or re-emergence of symptoms was the next most common definition of relapse.10
Relapses are associated with long-term symptoms and disability,13 and with each relapse, patients have decline in brain function14 and are at a higher risk of attempting suicide.15 With multiple relapses, patients may have a less complete treatment response16,17 and a substantially longer time to remission.18
A representative from Schizophrenia and Related Disorders Alliance of America (SARDAA) interviewed a patient with schizophrenia (oral communication, December 2019), who talked about the impact of his last relapse:
 Patient Perspectives
Patient Perspectives
“I had my relapse and they put me back in the hospital. I was working at that time, and I had the relapse while I was still working and they put me in the hospital and of course, I lost my job.”
Predictors of relapse in patients with schizophrenia include poor social functioning at baseline,14 male sex, and nonadherence to antipsychotic medications.19 Patients with better social functioning at baseline have a more favorable course.20 Relapse is also associated with younger age and earlier onset of illness, more severe symptoms, and substance use disorder.21 Overall, modifiable risk factors for poor outcomes in patients with schizophrenia include longer duration of untreated illness, comorbid substance abuse, and early nonresponse to an antipsychotic, as well as the number of relapses that are related to nonadherence.22
The Role of Nonadherence in Relapse
Dr Lauriello explained that nonadherence is a major contributor to relapse in schizophrenia, and it often starts early in the course of treatment. In a 1-year follow-up study23 of 56 male patients with first-episode schizophrenia, 54% discontinued their medications, and 70% of these patients relapsed. A systematic review24 found that 77% of nonadherent patients relapsed within the first year of treatment, while only 3% of adherent patients relapsed within the first year of treatment.
Influences on and obstacles to adherence. Several factors influence treatment adherence, including patient-related, illness-related, medication-related, clinician-related, and environmental factors.3 Illness factors that affect whether patients take their medications include positive symptoms, disorganization, and lack of insight.25,26 Other factors that influence adherence include the effectiveness and side effects of the treatment, the patient’s relationship with the treating clinician, the treatment setting (inpatient or outpatient), and caregiver and social support.25,26
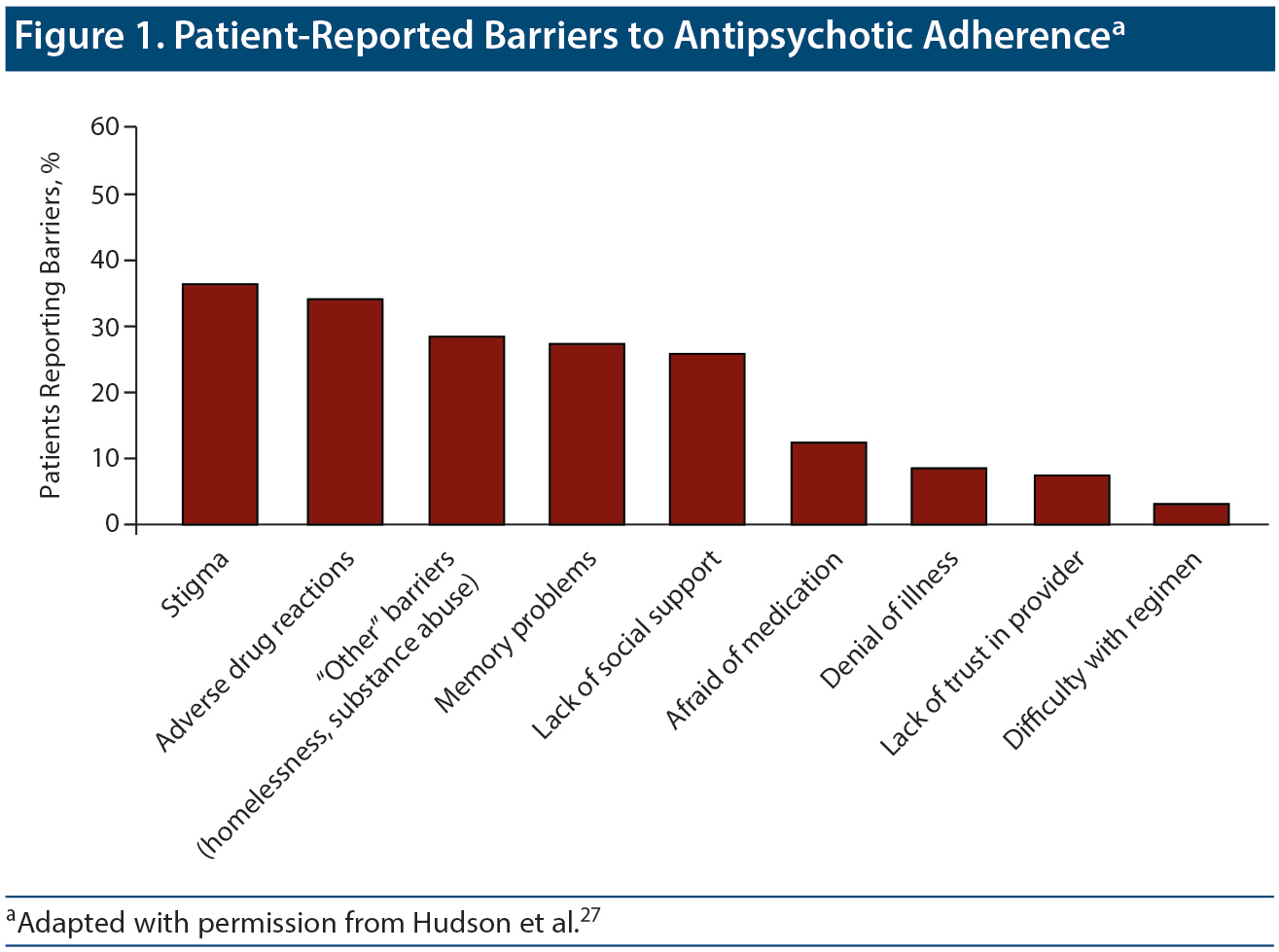
Hudson et al27 examined self-reported barriers to adherence in a cohort of inpatients and outpatients with schizophrenia (Figure 1). The most common barrier reported by patients was stigma related to having psychosis and having to take antipsychotic medications. In a review28 of 36 articles on nonadherence, problems with illness insight (56%), substance abuse (36%), attitudes toward medications (31%), and medication side effects (28%) were the top cited reasons for antipsychotic nonadherence.
Measuring nonadherence is difficult. Asking the patient or a caregiver if the patient has taken the medications as prescribed is quite imprecise.3 Even measuring blood antipsychotic levels as part of therapeutic drug monitoring29 does not adequately capture partial or intermittent nonadherence. Treating a patient with a long-acting antipsychotic, on the other hand, marks the beginning of nonadherence at the moment a person does not return for the injection visit
A SARDAA representative interviewed the mother of a patient with schizophrenia (oral communication, December 2019), and she noted that lack of illness insight led to her son’s longtime nonadherence.
 Family Perspectives
Family Perspectives
“My son was diagnosed with schizophrenia at age 24. From the get-go he was resistant to medication, and he did not believe he was ill. So over the years, he must have been hospitalized at least 12 times. He started on oral medications and eventually ended up on a long-acting antipsychotic.”
For more information, see the activity “Prevalence and Impact of Relapse in Patients With Schizophrenia” in this CME series.
PATIENT-CENTERED ASSESSMENT
Dr Correll addressed proposed definitions for response, remission, and recovery; the need for assessing patient-reported outcomes in the clinical setting; and the treatment challenges posed by negative, cognitive, and affective symptoms and adverse effects.
Definitions of Clinical Outcomes
Treatment outcomes in schizophrenia can be divided into categories, including response, remission, and recovery. Response is generally defined as a certain percentage decrease in symptoms compared to baseline, usually measured with the Brief Psychiatric Rating Scale (BPRS)30 or the PANSS.31,32 Using any of the appropriate scales, remission is defined as a rating of mild or less on 4 positive and 4 negative symptom items, sustained for at least 6 months.33 Recovery is the most desired treatment outcome. Liberman et al34 defined recovery as a period of at least 2 years in which the patient has maintained symptom remission, vocational functioning, independent living, and peer relationships (at least 1 interaction with someone outside the family per week).
Patient-Centered Outcomes and Decision-Making
A comprehensive assessment of the efficacy and impact of care in schizophrenia requires a patient-centered approach to treatment evaluation. In addition to clinical outcomes, clinicians should consider patient-centered treatment aims, including quality of life, health-related quality of life, and functioning.
A patient-centered framework, constructed by Kikkert and Dekker26 and based on the Health Belief Model, provides a first-person perspective on key factors related to patient decision-making. This model suggests that, for patients, taking their medication—or not taking it—is a means to achieving the best possible outcome.26
Positive treatment decisions are tied to patient-defined goals.35,36 To help patients make positive treatment decisions, using a combined shared decision-making and motivational interviewing technique is recommended.35,37
Patient-Reported Outcomes
Self-reporting can enhance a patient’s feeling of involvement in the treatment process, thereby improving self-esteem while also providing the clinician with knowledge of the patient’s insight into the illness.31 A patient-reported outcome (PRO) is a measurement that is directly reported by the patient about his or her health, quality of life, or functional status that is not amended or interpreted by the clinician or anyone else.
Patient-reported outcome measures (PROMs) are the tools or instruments used to measure PROs.38 PROMS can be general in nature or disease-specific and are usually self-completed questionnaires. Multiple PROMS can be used for patients to report on domains relevant to schizophrenia.31 (For more information on PROM scales, see the activity “Using Patient-Centered Assessment in Schizophrenia Care: Defining Recovery and Discussing Concerns and Preferences” in this CME series.)
 Case Practice Question
Case Practice Question
Discussion of the best response can be found at the end of the activity.
Case 1. Karl is a 24-year-old patient with schizophrenia since he was 19 years old; he lives with his parents. Karl had been stable on oral risperidone 3 mg/d. He stopped this medication unbeknownst to his psychiatrist and parents. He has started to hear voices again and has become very suspicious of two neighbors. Karl’s parents bring him to your psychiatric office seeking help to avoid a full relapse and rehospitalization. You discover that he stopped the medication due to sexual side effects and “brain fog.”
Which is NOT an evidence-based argument that you could offer Karl and his parents that he should consider trying a long-acting injectable (LAI) antipsychotic as his next treatment step?
a. LAIs significantly reduce the risk for relapse.
b. LAIs are helpful for treatment-resistant patients.
c. LAIs reduce the risk of premature death.
d. LAIs reduce nonadherence rates.
Challenges in Relapse Prevention
Dr Correll stated that relapse prevention and recovery require treatment that (ideally) successfully targets all of the clinical domains of schizophrenia and minimizes adverse effects.5 Unfortunately, most pharmacotherapeutic options with demonstrated efficacy address mainly the positive symptoms of the disease and produce adverse events that might contribute to nonadherence.
Positive and negative symptoms. Antipsychotic medications indicated for the treatment of schizophrenia are generally effective at controlling positive symptoms but have limited efficacy against the remaining clinical domains.7 Negative symptoms are relatively common and impair functionality.5 In an analysis of 1,447 antipsychotic-treated outpatients, approximately 1 in 5 had predominant negative symptoms.39
Patients who exhibit negative symptoms in their first episode of psychosis have lower rates of response, higher rates of delayed response, higher rates of nonresponse, and higher rates of relapse compared with patients exhibiting positive symptoms.40
Cognition. Cognitive dysfunction in schizophrenia affects all cognitive domains6 and has an impact on real-world functioning.41-43 Although currently no medications are approved for the treatment of cognitive deficits in schizophrenia, clinicians can address secondary cognitive symptoms to improve patients’ functioning and quality of life.5
Affective symptoms. Patients with schizophrenia commonly experience affective symptoms, including depression, anxiety, and suicidality, but it can be difficult for clinicians to differentiate negative symptoms from depressive symptoms. An analysis44 of patients presenting with symptoms that could be categorized as depressive or negative found that certain symptoms indicated the likelihood of either depression or negative symptoms. Depression in people with schizophrenia is relevant because low mood is a strong predictor of low quality of life45 and suicidality.26,35
Adverse events. Side effects of antipsychotics46 impact adherence levels,47 poor adherence is associated with increased rates of relapse,24,48 and relapse prevents recovery.22 Therefore, medication tolerability is the link between effective symptom control, recovery, quality of life, and functional capacity.49 The clinician’s objective assessment and the patient’s subjective perspective are both important when assessing side effects. In a large, nationwide study47 of adults with a self-reported diagnosis of schizophrenia, approximately 86% of participants reported experiencing at least 1 side effect.
TREATMENT OPTIONS
Dr Correll reviewed existing antipsychotic treatments. (For information on emerging agents, see the activity “Current Treatment Options and Emerging Agents for Schizophrenia” in this CME series.)
A recent network meta-analysis by Huhn et al50 analyzed data from 402 randomized, placebo-controlled trials including 53,463 participants to make direct and indirect efficacy and safety comparisons among 32 oral antipsychotics in acute schizophrenia. Seventeen outcomes—8 efficacy and 9 major adverse effect outcomes—were included. Results indicated that clozapine, amisulpride, zotepine, olanzapine, and risperidone may have slightly better total symptom and positive symptom efficacy than others, but these differences were relatively small.50 On the other hand, side effect differences were much larger and considered clinically relevant.50
Similarly, a meta-analysis51 of maintenance oral antipsychotic treatment indicated that some antipsychotics have slight symptom advantages compared with others, but the same antipsychotics have clinically relevant side effect burdens. Side effects can impact adherence levels,47 and poor adherence is associated with increased rates of relapse.48 Novel agents, therefore, are needed to provide broader symptom improvement with fewer tolerability issues.
An example of a novel agent is lumateperone, which is approved by the US Food and Drug Administration (FDA) for the treatment of schizophrenia; it is a partial D2 agonist presynaptically and a full D2 antagonist postsynaptically.52 Other antipsychotics are either partial agonists or full antagonists on the dopamine receptors both pre- and postsynaptically. Additionally, lumateperone has selective serotonin reuptake inhibition and D1 receptor modulation-related, proglutamatergic activity.52 In 2 controlled studies, lumateperone was superior to placebo as measured by the PANSS total score.53,54 One study found similar efficacy for lumateperone and risperidone in total symptom reduction.54 However, in a third study with a high placebo response, lumateperone was not superior to placebo on the PANSS total score, while risperidone was.55 Risperidone was associated with more adverse effects, which could have partially uncovered treatment assignment. Risperidone also had the highest dropout rate, calling into question whether more poorly responsive patients dropped out early on risperidone.56 Across the 3 trials, lumateperone had minimal adverse effects, with sedation/somnolence and dry mouth being present in at least 5% and at twice the rate as with placebo.
Maintenance treatment is strongly suggested in the management of schizophrenia,57,58 but a considerable proportion of patients have suboptimal outcomes despite continued treatment.22 However, what appears to be treatment resistance in some patients may actually be the result of poor adherence with the medication regimen. The Treatment Response and Resistance in Psychosis (TRRIP) consensus guidelines59 suggest the use of LAI antipsychotics to help ensure adherence and differentiate pseudoresistance from true antipsychotic treatment resistance. Although some LAIs must be initiated with a few weeks of oral cotreatment to establish therapeutic plasma levels,2 several new LAIs reduce or remove this requirement.60
Certain adverse effects are especially problematic for patients. For example, weight gain is often associated with some antipsychotics, particularly olanzapine.46 Tardive dyskinesia (TD) is a potentially persistent side effect that can impair function and quality of life.61 Incidence rates with SGAs are much lower compared with first-generation antipsychotics,61 but TD can still occur. Two novel vesicular monoamine transporter-2 (VMAT-2) inhibitors—deutetrabenazine and valbenazine—have demonstrated efficacy in (and have received FDA approval for) reducing TD.62
 Case Practice Question
Case Practice Question
Discussion of the best response can be found at the end of the activity.
Case 2. Joseph is a 28-year-old single man who was diagnosed with schizophrenia at the end of his senior year in college. His symptoms were mainly delusions and intermittent hallucinations, which appeared not to respond well to a number of different oral antipsychotics. It was unclear if Joseph took his medications consistently. Prior to his illness, he had been doing well and had been accepted to law school. In the first year of law school, he continued to have symptoms and was unable to complete the year. He has now brought to a new psychiatrist for a second opinion. Which intervention would be the best next step?
a. Prescribe Joseph clozapine because he is clearly treatment resistant.
b. Consider that the diagnosis might not be schizophrenia because the symptoms have not responded well to antipsychotics.
c. Give Joseph an LAI antipsychotic to determine if he is truly resistant or if the lack of response is due to nonadherence.
d. Consider transcranial magnetic stimulation (TMS).
PATIENT-CENTERED INTERVENTIONS
Dr Lauriello described how clinicians can greatly reduce the risk of relapse in patients with schizophrenia and keep them on the course to recovery using patient-centered care.61 The collaborative model of care comprises a team-based approach to improve patient care via a partnership between the clinician and the patient.62 The clinician’s expertise is recognized and valued, but patients ultimately decide which treatment course is best for them.63
Pharmacologic Interventions
Although adherence rates are commonly thought to be higher with atypical than typical antipsychotics, patients are still nonadherent to atypical medications. Dolder and colleagues72 used pharmacy refill records to compare medication adherence rates in veterans receiving typical versus atypical antipsychotics over a 12-month period. Cumulative mean gap ratios were 23.2% for typical and 14.1% for atypical antipsychotics at 12 months. Patients who received typical antipsychotics did not have medication for an average of 7 days per month, and patients who received atypical antipsychotics did not have medication for an average of 4 days per month. Interventions are needed to improve adherence in patients who are receiving atypical antipsychotics.
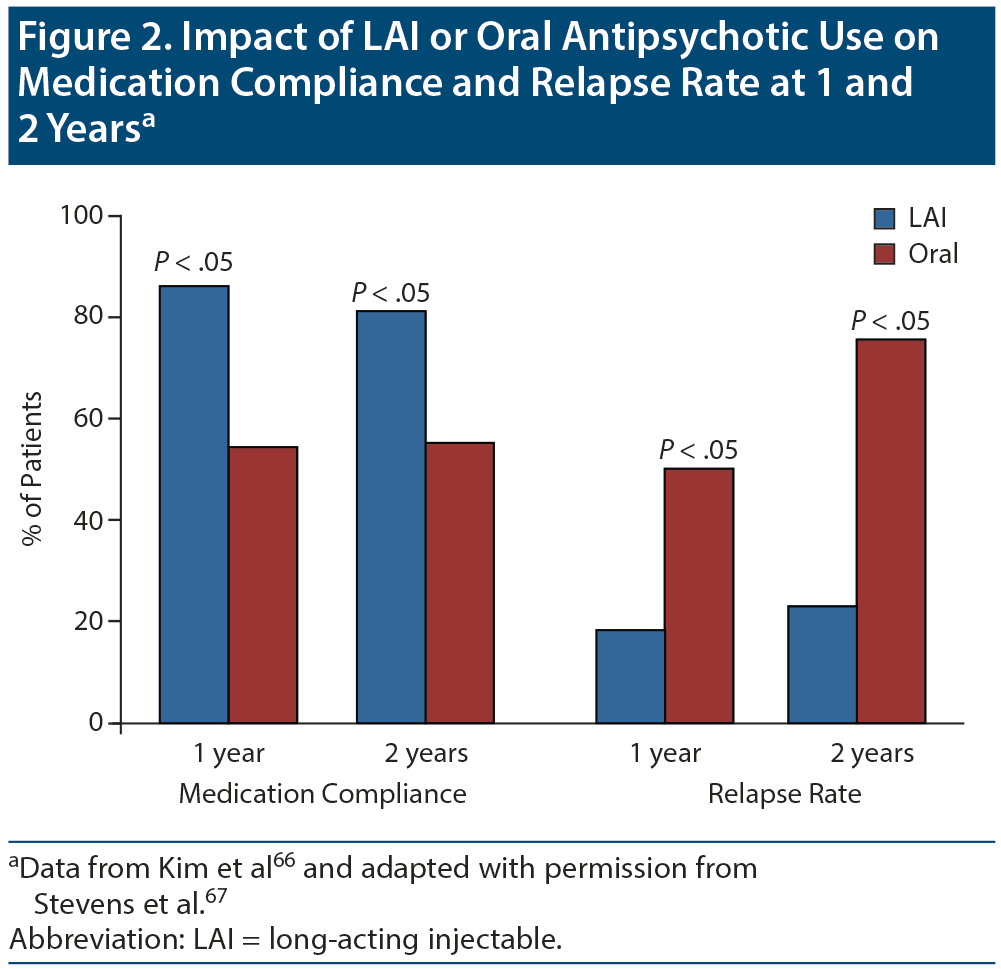
Long-acting injectable antipsychotics. Guidelines63,64 recommend that LAIs should be considered as an alternative to oral formulations of antipsychotics because they provide more reliable drug delivery, reduce peak-trough level differences, and offer greater dosing precision.65 Researchers have reported significantly higher medication adherence rates and lower relapse rates with LAIs compared to oral antipsychotics (Figure 2).66-69 Additionally, LAIs have been shown to reduce the risk of mortality more than oral antipsychotics compared to no antipsychotic use at all in people with schizophrenia.70
Barriers to using LAIs. Unfortunately, despite the many benefits of LAIs, clinicians rarely discuss this option with their patients. One reason for this is that clinicians believe that patients will not be open to treatment with LAIs. Jaeger and Rossler71 examined the attitudes to LAIs of psychiatrists, patients, and their relatives using a semistructured questionnaire. They found that 67% of patients in the study did not receive information about LAIs from their psychiatrist. Thus, patients’ negative attitudes toward LAIs might, at least in part, be related to the low level of information that clinicians provide them.
Another barrier to the use of LAIs is clinicians’ misconception that LAIs are only for chronically ill patients. To the contrary, studies suggest that LAI formulations are effective early in the course of treatment or for a first episode of schizophrenia.67
Patient-related barriers to using LAIs include fear of injection site pain and a general dislike of receiving injections.71 Patients may also be concerned that using LAIs will mean that they lose control over taking their medications.
Psychosocial Interventions
When working with patients with schizophrenia, clinicians need to be nonjudgmental, empathic, and proactive in taking a therapeutic stance. Clinicians may be able to change patients’ attitudes toward adherence with psychosocial strategies and counseling.
Psychosocial strategies that may improve adherence can be divided into 3 major categories—educational, behavioral, and affective.72 Psychoeducation is the education of an individual with mental illness in areas that serve the goals of treatment and rehabilitation. Behavioral strategies address unfavorable patient attitudes toward taking or staying on medications through routine assessment, emphasis on the patient-clinician alliance, and the use of a patient-centered approach to care. Behavioral strategies that have been proven to be effective in improving adherence include motivational interviewing, cognitive-behavioral therapy, and compliance therapy. Affective strategies address factors such as family support. For more information on psychosocial strategies that may improve adherence, see the activity “Patient-Centered Psychopharmacology and Psychosocial Interventions: Treatment Selection and Shared Decision-Making to Enhance Chances for Recovery” in this CME series.
 Clinical Points
Clinical Points
- Adopt a patient-centered approach that incorporates the use of patient-reported outcome measures.
- Select medications based on a balanced risk-benefit assessment including the focus on addressing symptoms for which the agents were superior to placebo and/or active controls.
- Consider LAI antipsychotics as an alternative to oral medications, as they offer benefits such as reliable drug delivery, uncovering nonadherence and pseudoresistance, and reduced risk of relapse and mortality.
- Implement psychosocial interventions that have been proven to be effective in improving adherence and overall outcomes.
 Discussion of Case Practice Questions
Discussion of Case Practice Questions
Case 1. Preferred response is b. LAIs are helpful for treatment-resistant patients.
In meta-analyses, LAIs have been shown to reduce the risk of relapse, nonadherence, and mortality in people with schizophrenia compared with placebo and oral antipsychotic treatment. However, while an LAI treatment trial has been suggested by the TRRIP consensus group to rule out pseudoresistance (lack of response due to insufficient medication adherence), the evidence base is lacking that in patients with established treatment resistance, LAIs are the treatment of choice (here, clozapine has the best evidence). Moreover, Karl has not yet failed two adequate trials with an antipsychotic, which would define treatment resistance. He is in the process of relapsing after having stopped his oral antipsychotic medication.
Case 2. Preferred response is c. Give Joseph an LAI antipsychotic to determine if he is truly resistant or if the lack of response is due to nonadherence.
Not responding to antipsychotics does not eliminate the diagnosis; many patients do not respond to specific antipsychotics. Starting Joseph on clozapine is a reasonable option if you are sure he has taken his previous oral antipsychotics. His questionable adherence raises the question of whether he has had adequate trials of any medications. Clozapine is the only proven antipsychotic for treatment resistance, but it has a number of serious side effects and requires blood monitoring. While TMS is an approved treatment for refractory depression and OCD, it is only used off-label for schizophrenia and has mixed results. A trial of an LAI antipsychotic is a good option here because it assures that Joseph is receiving medication, and then you can evaluate whether the medication has any effect.
Published online: June 30, 2020.
Disclosure of off-label usage: Dr Correll has determined that, to the best of his knowledge, no investigational information about pharmaceutical agents or device therapies that is outside US Food and Drug Administration-approved labeling has been presented in this activity.
REFERENCES
1.Kahn RS, Sommer IE, Murray RM, et al. Schizophrenia. Nat Rev Dis Primers. 2015;1(1):15067. PubMed CrossRef
2.Correll CU, Citrome L, Haddad PM, et al. The use of long-acting injectable antipsychotics in schizophrenia: evaluating the evidence. J Clin Psychiatry. 2016;77(suppl 3):1-24. PubMed CrossRef
3.Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216-226. PubMed CrossRef
4.Ebert A, Bär K-J. Emil Kraepelin: a pioneer of scientific understanding of psychiatry and psychopharmacology. Indian J Psychiatry. 2010;52(2):191–192. PubMed CrossRef
5.Correll CU, Schooler NR. Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatr Dis Treat. 2020;16:519-534. PubMed CrossRef
6.Millan MJ, Agid Y, Brüne M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11(2):141-168. PubMed CrossRef
7.Millan MJ, Fone K, Steckler T, et al. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol. 2014;24(5):645-692. PubMed CrossRef
8.Berardelli I, Sarubbi S, Rogante E, et al. The role of demoralization and hopelessness in suicide risk in schizophrenia: a review of the literature. Medicina (Kaunas). 2019;55(5):E200. PubMed CrossRef
9.Gogtay N, Vyas NS, Testa R, et al. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull. 2011;37(3):504-513. PubMed CrossRef
10.Olivares JM, Sermon J, Hemels M, et al. Definitions and drivers of relapse in patients with schizophrenia: a systematic literature review. Ann Gen Psychiatry. 2013;12(1):32. PubMed CrossRef
11.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261-276. PubMed CrossRef
12.Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Washington, DC: US Department of Health, Education, and Welfare; 1976.
13.Harrison G, Hopper K, Craig T, et al. Recovery from psychotic illness: a 15- and 25-year international follow-up study. Br J Psychiatry. 2001;178(6):506-517. PubMed CrossRef
14.Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609-615. PubMed CrossRef
15.Herings RM, Erkens JA. Increased suicide attempt rate among patients interrupting use of atypical antipsychotics. Pharmacoepidemiol Drug Saf. 2003;12(5):423-424. PubMed CrossRef
16.Emsley R, Nuamah I, Hough D, et al. Treatment response after relapse in a placebo-controlled maintenance trial in schizophrenia. Schizophr Res. 2012;138(1):29-34. PubMed CrossRef
17.Takeuchi H, Siu C, Remington G, et al. Does relapse contribute to treatment resistance? antipsychotic response in first- vs second-episode schizophrenia. Neuropsychopharmacology. 2019;44(6):1036-1042. PubMed CrossRef
18.Lieberman JA, Drake RE, Sederer LI, et al. Science and recovery in schizophrenia. Psychiatr Serv. 2008;59(5):487-496. PubMed CrossRef
19.Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247. PubMed CrossRef
20.Haro JM, Novick D, Suarez D, et al. Predictors of the course of illness in outpatients with schizophrenia: a prospective three year study. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1287-1292. PubMed CrossRef
21.Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10(1):2. PubMed CrossRef
22.Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16(4):505-524. PubMed
23.Novak-Grubic V, Tavcar R. Predictors of noncompliance in males with first-episode schizophrenia, schizophreniform and schizoaffective disorder. Eur Psychiatry. 2002;17(3):148-154. PubMed CrossRef
24.Zipursky RB, Menezes NM, Streiner DL. Risk of symptom recurrence with medication discontinuation in first-episode psychosis: a systematic review. Schizophr Res. 2014;152(2-3):408-414. PubMed CrossRef
25.Chaudhari B, Saldanha D, Kadiani A, et al. Evaluation of treatment adherence in outpatients with schizophrenia. Ind Psychiatry J. 2017;26(2):215-222. PubMed CrossRef
26.Kikkert MJ, Dekker J. Medication adherence decisions in patients with schizophrenia. Prim Care Companion CNS Disord. 2017;19(6):17n02182. PubMed CrossRef
27.Hudson TJ, Owen RR, Thrush CR, et al. A pilot study of barriers to medication adherence in schizophrenia. J Clin Psychiatry. 2004;65(2):211-216. PubMed CrossRef
28.Velligan DI, Sajatovic M, Hatch A, et al. Why do psychiatric patients stop antipsychotic medication? a systematic review of reasons for nonadherence to medication in patients with serious mental illness. Patient Prefer Adherence. 2017;11:449-468. PubMed CrossRef
29.Schoretsanitis G, Kane JM, Correll CU, et al; American Society of Clinical Psychopharmacology. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81(3):19cs13169. PubMed CrossRef
30.Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10(3):799-812. CrossRef
31.Correll CU, Kishimoto T, Nielsen J, et al. Quantifying clinical relevance in the treatment of schizophrenia. Clin Ther. 2011;33(12):B16-B39. PubMed CrossRef
32.Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res. 2005;79(2-3):231-238. PubMed CrossRef
33.Andreasen NC, Carpenter WT Jr, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441-449. PubMed CrossRef
34.Liberman RP, Kopelowicz A, Ventura J, et al. Operational criteria and factors related to recovery from schizophrenia. Int Rev Psychiatry. 2002;14(4):256-272. CrossRef
35.Lasser RA, Schooler NR, Kujawa M, et al. A new psychosocial tool for gaining patient understanding and acceptance of long-acting injectable antipsychotic therapy. Psychiatry (Edgmont). 2009;6(4):22-27. PubMed
36.Magill M, Gaume J, Apodaca TR, et al. The technical hypothesis of motivational interviewing: a meta-analysis of MI’s key causal model. J Consult Clin Psychol. 2014;82(6):973-983. PubMed CrossRef
37.Stovell D, Morrison AP, Panayiotou M, et al. Shared treatment decision-making and empowerment-related outcomes in psychosis: systematic review and meta-analysis. Br J Psychiatry. 2016;209(1):23-28. PubMed CrossRef
38.Weldring T, Smith SMS. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv Insights. 2013;6:61-68. PubMed
39.Rabinowitz J, Berardo CG, Bugarski-Kirola D, et al. Association of prominent positive and prominent negative symptoms and functional health, well-being, healthcare-related quality of life and family burden: a CATIE analysis. Schizophr Res. 2013;150(2-3):339-342. PubMed CrossRef
40.Austin SF, Mors O, Budtz-J׸rgensen E, et al. Long-term trajectories of positive and negative symptoms in first episode psychosis: a 10-year follow-up study in the OPUS cohort. Schizophr Res. 2015;168(1-2):84-91. PubMed CrossRef
41.Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67(suppl 9):3-8, discussion 36-42. PubMed CrossRef
42.Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev. 2009;19(3):365-384. PubMed CrossRef
43.Bowie CR, Leung WW, Reichenberg A, et al. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatry. 2008;63(5):505-511. PubMed CrossRef
44.Krynicki CR, Upthegrove R, Deakin JFW, et al. The relationship between negative symptoms and depression in schizophrenia: a systematic review. Acta Psychiatr Scand. 2018;137(5):380-390. PubMed CrossRef
45.Siu CO, Harvey PD, Agid O, et al. Insight and subjective measures of quality of life in chronic schizophrenia. Schizophr Res Cogn. 2015;2(3):127-132. PubMed CrossRef
46.Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag. 2017;13:757-777. PubMed CrossRef
47.Dibonaventura M, Gabriel S, Dupclay L, et al. A patient perspective of the impact of medication side effects on adherence: results of a cross-sectional nationwide survey of patients with schizophrenia. BMC Psychiatry. 2012;12(1):20. PubMed CrossRef
48.Sun SX, Liu GG, Christensen DB, et al. Review and analysis of hospitalization costs associated with antipsychotic nonadherence in the treatment of schizophrenia in the United States. Curr Med Res Opin. 2007;23(10):2305-2312. PubMed CrossRef
49.Correll CU. What are we looking for in new antipsychotics? J Clin Psychiatry. 2011;72(suppl 1):9-13. PubMed CrossRef
50.Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939-951. PubMed CrossRef
51.Kishimoto T, Hagi K, Nitta M, et al. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry. 2019;18(2):208-224. PubMed CrossRef
52.Davis RE, Correll CU. ITI-007 in the treatment of schizophrenia: from novel pharmacology to clinical outcomes. Expert Rev Neurother. 2016;16(6):601-614. PubMed CrossRef
53.Lieberman JA, Davis RE, Correll CU, et al. ITI-007 for the treatment of schizophrenia: a 4-week randomized, double-blind, controlled trial. Biol Psychiatry. 2016;79(12):952-961. PubMed CrossRef
54.Correll CU, Davis RE, Weingart M, et al. Efficacy and safety of lumateperone for treatment of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2020;77(4):349-358. PubMed CrossRef
55.Krogmann A, Peters L, von Hardenberg L, et al. Keeping up with the therapeutic advances in schizophrenia: a review of novel and emerging pharmacological entities. CNS Spectr. 2019;24(S1):38-69. PubMed CrossRef
56.Vanover K, Dmitrienko A, Glass S, et al. S44. Lumateperone (ITI-007) for the treatment of schizophrenia: placebo-controlled clinical trials and an open-label safety switching study. Schizophr Bull. 2018;44(suppl 1):S341. CrossRef
57.Correll CU, Rubio JM, Kane JM. What is the risk-benefit ratio of long-term antipsychotic treatment in people with schizophrenia? World Psychiatry. 2018;17(2):149-160. PubMed CrossRef
58.Taipale H, Tanskanen A, Mehtפlפ J, et al. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry. 2020;19(1):61-68. PubMed CrossRef
59.Howes OD, McCutcheon R, Agid O, et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am J Psychiatry. 2017;174(3):216-229. PubMed CrossRef
60.Kane JM, Correll CU. Optimizing treatment choices to improve adherence and outcomes in schizophrenia. J Clin Psychiatry. 2019;80(5):IN18031AH1C. PubMed CrossRef
61.Carbon M, Hsieh C-H, Kane JM, et al. Tardive dyskinesia prevalence in the period of second-generation antipsychotic use: a meta-analysis. J Clin Psychiatry. 2017;78(3):e264-e278. PubMed CrossRef
62.Solmi M, Pigato G, Kane JM, et al. Treatment of tardive dyskinesia with VMAT-2 inhibitors: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther. 2018;12:1215-1238. PubMed CrossRef
63.Galletly C, Castle D, Dark F, et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the management of schizophrenia and related disorders. Aust N Z J Psychiatry. 2016;50(5):410-472. PubMed CrossRef
64.Llorca PM, Abbar M, Courtet P, et al. Guidelines for the use and management of long-acting injectable antipsychotics in serious mental illness. BMC Psychiatry. 2013;13(1):340. PubMed CrossRef
65.Ereshefsky L, Mascarenas CA. Comparison of the effects of different routes of antipsychotic administration on pharmacokinetics and pharmacodynamics. J Clin Psychiatry. 2003;64(suppl 16):18-23. PubMed
66.Kim B, Lee S-H, Choi TK, et al. Effectiveness of risperidone long-acting injection in first-episode schizophrenia: in naturalistic setting. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1231-1235. PubMed CrossRef
67.Stevens GL, Dawson G, Zummo J. Clinical benefits and impact of early use of long-acting injectable antipsychotics for schizophrenia. Early Interv Psychiatry. 2016;10(5):365-377. PubMed CrossRef
68.Kishimoto T, Hagi K, Nitta M, et al. Effectiveness of long-acting injectable vs oral antipsychotics in patients with schizophrenia: a meta-analysis of prospective and retrospective cohort studies. Schizophr Bull. 2018;44(3):603-619. PubMed CrossRef
69.Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965. PubMed CrossRef
70.Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280. PubMed CrossRef
71.Jaeger M, Rossler W. Attitudes towards long-acting depot antipsychotics: a survey of patients, relatives and psychiatrists. Psychiatry Res. 2010;175(1-2):58-62. PubMed CrossRef
72.Dolder CR, Lacro JP, Leckband S, et al. Interventions to improve antipsychotic medication adherence: review of recent literature. J Clin Psychopharmacol. 2003;23(4):389-399. PubMed CrossRef
This PDF is free for all visitors!