Atypical Antipsychotics and Insulin Resistance in Acute Mental Illness: A Case Series
To the Editor: Kooy noted in 1919 that “it is a remarkable fact that some persons, who are in a state of agitation, especially those of a neuropathic condition, show a temporary excretion of sugar.”1(p215) This condition was referred to at the time as “nervous glycosuria.” It was carefully distinguished from diabetes, although an association was noted.1 Today, psychiatrists worry that the treatment of mental illness may cause diabetes. This is an important concern, as the prevalence of diabetes among the severely mentally ill is much higher than that of the general population. For example, one recent study found that 14.5% of patients with schizophrenia or schizoaffective disorder had diabetes, compared to only 1.5% of an age-matched control group.2 Atypical antipsychotic agents, the mainstay of treatment for psychosis, are thought by some to be a major cause of insulin resistance among the severely mentally ill.3
At the author’s institution, concern about the risk of inducing insulin resistance among the acutely mentally ill inspired monitoring of insulin levels on admission and on discharge. It turns out that using symptomatic response to the atypical antipsychotic, rather than an arbitrary length of time on the medication, is not often used as an endpoint for reassessment of insulin sensitivity. What was found is a tendency for acute mental illness to reduce insulin sensitivity and for effective treatment to improve it. Five cases are presented. The first 4 describe patients who do not carry diagnoses of diabetes. A fifth case is presented in which a patient diagnosed with, and treated for, type 2 diabetes was able to discontinue his diabetes treatment once his psychosis entered into sustained remission. DSM-IV-TR criteria were used throughout.
Case 1. Patient 1 was a 54-year-old white man with schizoaffective disorder admitted in 2007 for worsening depression. He was tearful on admission and endorsed sadness, decreased energy and appetite, poor concentration, and hypersomnia to as much as 14 hours a day. He had stopped his medications, and, so, on the first hospital day, he was restarted on his usual medication regimen of lithium carbonate 600 mg in the morning and 900 mg at bedtime, ziprasidone 80 mg twice a day, olanzapine 20 mg at bedtime, and trazodone 100 mg at bedtime as needed for insomnia. Other medical problems included hepatitis C and obesity. Patient 1 was 74 in tall and weighed 255 lb; his body mass index (BMI, kg/m2) was 32.8. Within 4 days, Patient 1 was much improved, with brightened mood and the sense that his clarity of thought was improved. Appetite and energy were improved, and Patient 1’s hypersomnia was resolved.
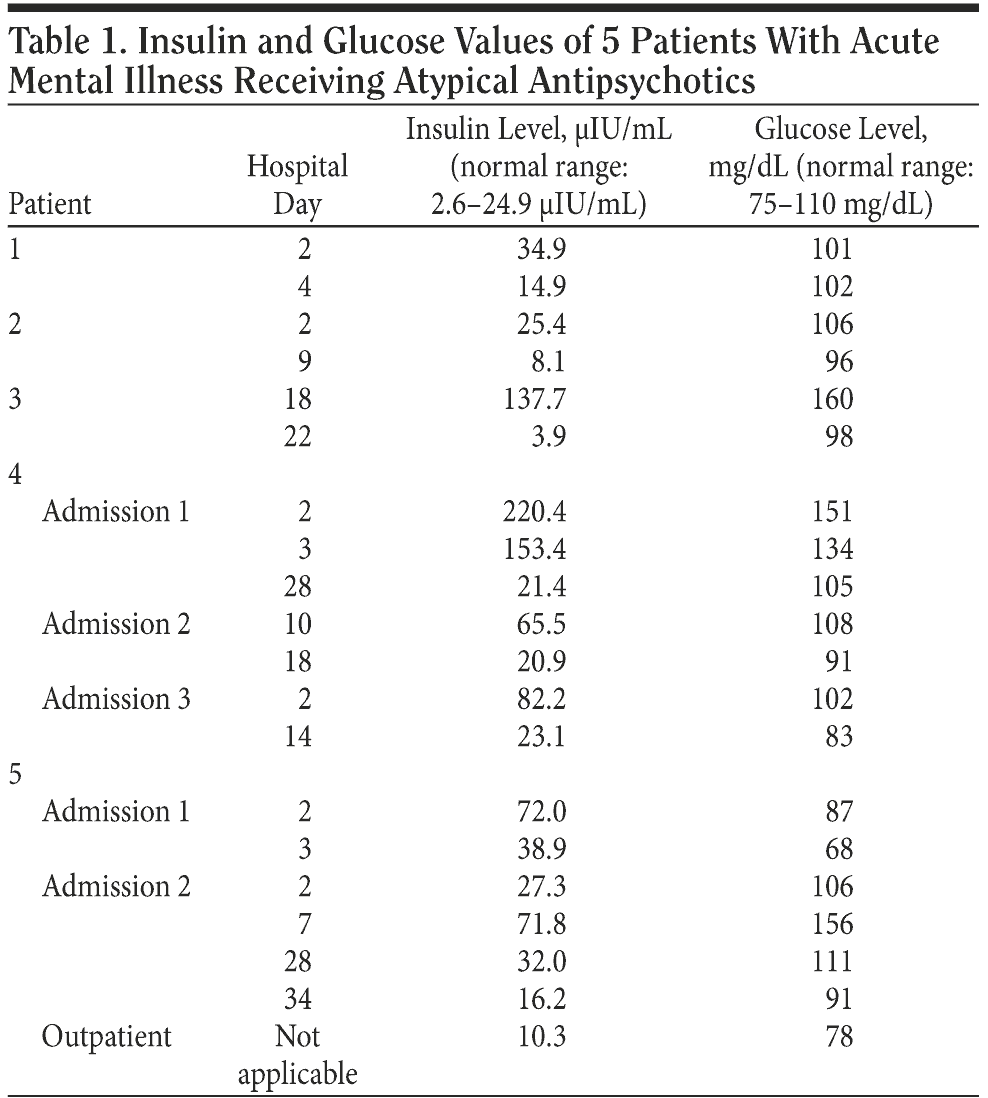
Table 1 outlines Patient 1’s serum insulin and glucose levels, as well as these values for the remaining patients.
Case 2. Patient 2 was a 43-year-old white man with a history of schizoaffective disorder, alcohol dependence in sustained full remission, and cocaine dependence in sustained full remission who was admitted in 2007 for severe depression with suicidal ideation. He had no other medical problems. He was 70 in tall and weighed 173 lb. His BMI was 24.9. He had avoided alcohol and cocaine for over 2 years. He had also stopped taking his outpatient medications. He was restarted on his usual outpatient medications: citalopram 40 mg/d, quetiapine 200 mg in the morning and 500 mg at bedtime, trazodone 50 mg at bedtime, and risperidone microspheres 50 mg every 2 weeks. By hospital day 8, his mood was much improved, and he was discharged soon thereafter.
Case 3. Patient 3 was a 55-year-old black man admitted in 2007 for a recurrence of severe depressive symptoms with suicidal ideation, auditory hallucinations, and a delusion that people were plotting to kill him; he received a DSM-IV-TR diagnosis of major depressive disorder, severe, recurrent, with psychosis and suicidality. Other medical problems included hypercholesterolemia, chronic obstructive pulmonary disease, chronic neuropathic pain, and gastroesophageal reflux disease. Patient 3 was 71 in tall and weighed 198 lb. His BMI was 27.7. Compliance with outpatient medications was unclear, but Patient 3 denied medication side effects. His medications were continued: bupropion 100 mg twice a day, citalopram 60 mg/d, risperidone 2 mg/d, trazodone 150 mg at bedtime as needed for insomnia, simvastatin 80 mg at bedtime, albuterol inhaler as needed every 2 hours, theophylline sustained-acting preparation 300 mg twice a day, montelukast 10 mg at bedtime, mometasone 220-μg inhaler 1 inhalation by mouth twice a day, levalbuterol 59-μg inhaler 2 puffs by mouth 4 times daily, ipratropium 0.02% solution 1 oral inhalation every 4 hours, and albuterol 0.5% solution every 6 hours. Patient 3’s improvement was slow, so on hospital day 14, olanzapine was added. This was increased to 20 mg at bedtime over several days. Within a week, Patient 3 began to show distinct improvement. Concern about the possibility of developing diabetes as a side effect of olanzapine led to a check of his serum insulin level on hospital day 18 and again on hospital day 22. By hospital day 26, his mood was stable, his auditory hallucinations and delusion had resolved, and his suicidal ideation was replaced by a sense of hopefulness.
Case 4. Patient 4 was a 50-year-old black man with chronic paranoid schizophrenia admitted in 2007 for psychosis with depressed mood and suicidal ideation. Patient 4’s other medical problems were hepatitis C, hypertension, obesity, and obstructive sleep apnea. Patient 4 was 65 in tall and weighed 209 lb, for a BMI of 34.9. Patient 4 said he had stopped his outpatient medications, and, so, he was restarted on his usual outpatient regimen of citalopram 60 mg/d, clonazepam 0.5 mg 3 times a day, lisinopril 30 mg/d, prazosin 4 mg at bedtime, hydrochlorothiazide 12.5 mg/d, docusate 240 mg/d, methadone 10 mg 3 times a day, tizanidine 4 mg 3 times a day as needed for muscle spasm, and risperidone microspheres 25 mg intramuscularly every 2 weeks. Quetiapine was started and gradually escalated to 50 mg every morning and at noon and 300 mg at bedime. Patient 4 slowly improved, but by hospital day 28, was enjoying sustained stability.
Patient 4 was readmitted 14 months later with the same problems. He weighed 217 lb. Risperidone had been discontinued, and Patient 4 was receiving only quetiapine. His other medications were unchanged. On hospital day 10, he began a taper off quetiapine and was started on clozapine treatment at the request of his outpatient provider. The author encountered Patient 4 on-call on hospital day 18, and because Patient 4 was still psychotic, ordered an increase in his clozapine from a total of 150 mg/d to 200 mg/d. He was discharged on hospital day 28 much improved on a total clozapine dose of 250 mg/d.
Patient 4 was admitted again 2 months later for psychosis associated with bereavement. His weight had increased to 226 lb. His total daily clozapine dose was 300 mg on admission. It was increased to 350 mg at night. Patient 4 was discharged much improved on hospital day 14. Serum insulin levels were measured the morning after admission and the morning of discharge.
Case 5. Patient 5 was a 66-year-old Hispanic man with bipolar 1 disorder admitted in 2006 for mania after an attempt was made to treat his parkinsonism by lowering by half his risperidone dosage of 2 mg by mouth twice a day. Within days, Patient 5 was frankly manic. He was agitated, spending excessive amounts of money, sexually preoccupied, and not sleeping. Other illnesses included type 2 diabetes, hypothyroidism, and nephrogenic diabetes insipidus. His outpatient medications were risperidone 1 mg orally twice a day, lithium carbonate 600 mg at bedtime, divalproex 500 mg at bedtime, amiloride 5 mg twice daily, levothyroxine 0.137 mg/d, and pramipexole 0.5 mg twice daily. Patient 5’s type 2 diabetes was managed through diet at home, with the addition of sliding-scale insulin in the hospital. His height was 64 in and weight 168 lb, for a BMI of 28.9. Patient 5 did not use alcohol or illicit agents. Compliance with outpatient medications was good: Patient 5 lived with his healthy brother, who carefully monitored medication compliance. On admission, risperidone was increased back to 2 mg twice daily. Within days, Patient 5 was calmer, able to focus on his condition and medication effects, and able to make reasonable plans for discharge. Patient 5 was discharged on hospital day 10 much improved.
One year later, on the same medications, Patient 5 was admitted to the physical rehabilitation wing of the same hospital to build his strength and pursue long-term placement. However, his parkinsonism interfered with treatment. Risperidone was therefore stopped, and electroconvulsive therapy planned, in the hope that both interventions would reduce his parkinsonism without precipitating manic decompensation. However, after stopping risperidone, Patient 5 refused electroconvulsive therapy. For 7 days, he received only lithium. Patient 5 rapidly deteriorated. He believed he was being accused of homosexuality. This led to a physical altercation between Patient 5 and hospital staff, in which he attacked several staff with a fire extinguisher. As noted in Table 1, his insulin sensitivity by hospital day 7 had plummeted. Quetiapine was started and rapidly increased to 500 mg at bedtime. By day 28, Patient 5’s insulin sensitivity had improved greatly, though it was still not at baseline level. By day 34, Patient 5 was clinically much improved, as was his insulin sensitivity.
Two months after discharge, Patient 5 did not require treatment for type 2 diabetes. On his discharge psychotropic regimen, his mental illness and insulin sensitivity remained stable.
Discussion. Although the laboratory work reported in these cases was ordered due to concern that atypical antipsychotics may cause insulin resistance, this was not seen. Furthermore, in the cases described, insulin sensitivity and mental state improved within about a week of admission, before any significant weight change could occur. In the author’s institution, patients have ad lib access to sweetened soft drinks and other foods rich in fat or high-fructose corn syrup; diet was unlikely to be responsible for improved glucose control or insulin sensitivity. Of course, other medications were used in each case. Whether intended to improve glucose control or not, some of these other agents may have helped. Lithium, for example, has insulin-like activity.4 From a psychiatrist’s perspective, it is important that treatment with atypical antipsychotic agents did not acutely worsen insulin sensitivity in any case. This may be related to the fact that mental illness can itself provoke insulin resistance.5
Psychosis is associated with impaired pancreatic β-cell function and a decline in insulin sensitivity, both of which reverse as the psychosis resolves.6 Depression is strongly associated with the development of type 2 diabetes and inversely associated with insulin sensitivity.7,8 Mania, too, provokes insulin resistance.9 Lawlor and colleagues suggested that peripheral insulin resistance may be a mechanism by which the brain attempts to protect itself from depression.7 This is not a new idea: some authors argue that physiologic stress may trigger a state of peripheral insulin resistance, in part to ensure the brain a steady stream of both insulin and glucose.10
Going back further, insulin coma therapy was once employed by psychiatrists to treat depression, mania, and psychosis. As coma does not routinely relieve mental illness, and indeed may increase the risk of depression, the coma of insulin therapy was probably a marker of central penetration of insulin and the benefits due to the insulin itself. Insulin is neuroprotective, in addition to being neurotrophic.11,12 The brain makes neither insulin nor glucose, but requires a continuous supply of both for normal function and to prevent neuronal death. During significant stress, the brain may export insulin from the pancreas, past the periphery, and into the brain preferentially. Viewed from the periphery, this is insulin resistance. Viewed centrally, this is neuroprotection.13
The association of atypical antipsychotic medications with insulin sensitivity is surely complex. Acute as well as chronic factors may play distinct roles. Acutely, mental illness may reduce insulin sensitivity, and treatment of psychiatric symptoms may tend to improve it. In some studies, certain atypical antipsychotics alter insulin release or action in a manner that seems to worsen insulin resistance. But perhaps transient hyperinsulinemia reflects an amplification of an endogenous neuroprotective mechanism. To sort through this issue, it may be necessary to correlate changes in insulin sensitivity with symptomatic response to medication: perhaps patients who respond well to an antipsychotic have a more favorable change in insulin sensitivity than those patients whose mental illness does not respond well to the antipsychotic. Or perhaps the reverse is true: perhaps patients who respond robustly to an atypical antipsychotic have a period of increased peripheral insulin resistance. The latter seemed true for lithium in one of the rare studies to link changes in insulin sensitivity with response to a psychotropic medication.14
Conclusion. The value of these cases is that they serve as reminders, in a natural clinical setting, that improved insulin sensitivity may accompany effective treatment of acute mental illness. Because all of the patients’ medical problems were addressed simultaneously, it is impossible to isolate the effects of the atypical antipsychotics on insulin sensitivity. But the temporal link between symptom relief and improvement of insulin sensitivity suggests that atypical antipsychotic medications may in some cases improve the insulin resistance associated with acute mental illness. Prospective studies are much needed to quantify the strength of the link between symptomatic response to an atypical antipsychotic and improvement in insulin sensitivity.
References
1. Kooy FH. Hyperglycaemia in mental disorders. Brain. 1919;42(3):214-290. doi:10.1093/brain/42.3.214
2. Cohen D, Stolk RP, Grobbee DE, et al. Hyperglycemia and diabetes in patients with schizophrenia or schizoaffective disorders. Diabetes Care. 2006;29(4):786-791. PubMed doi:10.2337/diacare.29.04.06.dc05-1261
3. Guo JJ, Keck PE Jr, Corey-Lisle PK, et al. Risk of diabetes mellitus associated with atypical antipsychotic use among Medicaid patients with bipolar disorder: a nested case-control study. Pharmacotherapy. 2007;27(1):27-35. PubMed doi:10.1592/phco.27.1.27
4. Okosieme OE, Campbell A, Patton K, et al. Transient diabetes associated with withdrawal of lithium therapy. Diabetes Care. 2006;29(5):1181. PubMed doi:10.2337/dc06-0291
5. Toalson P, Ahmed S, Hardy T, et al. The metabolic syndrome in patients with severe mental illness. Prim Care Companion J Clin Psychiatry. 2004;6(4):152-158. PubMed doi:10.4088/PCC.v06n0402
6. Shiloah E, Witz S, Abramovitch Y, et al. Effect of acute psychotic stress in nondiabetic subjects on beta-cell function and insulin sensitivity. Diabetes Care. 2003;26(5):1462-1467. PubMed doi:10.2337/diacare.26.5.1462
7. Lawlor DA, Ben-Shlomo Y, Ebrahim S, et al. Insulin resistance and depressive symptoms in middle aged men: findings from the Caerphilly prospective cohort study. BMJ. 2005;330(7493):705-706. PubMed doi:10.1136/bmj.38377.616921.F7
8. Timonen M, Laakso M, Jokelainen J, et al. Insulin resistance and depression: cross sectional study. BMJ. 2005;330(7481):17-18. PubMed doi:10.1136/bmj.38313.513310.F71
9. Taylor V, MacQueen G. Associations between bipolar disorder and metabolic syndrome: a review. J Clin Psychiatry. 2006;67(7):1034-1041. PubMed doi:10.4088/JCP.v67n0704
10. Turner RC, Levy JC, Clark A. Complex genetics of type 2 diabetes: thrifty genes and previously neutral polymorphisms. Q J Med. 1993;86(7):413-417. PubMed
11. Wada A, Yokoo H, Yanagita T, et al. New twist on neuronal insulin receptor signaling in health, disease, and therapeutics. J Pharmacol Sci. 2005;99(2):128-143. PubMed doi:10.1254/jphs.CRJ05006X
12. Nelson TJ, Sun MK, Hongpaisan J, et al. Insulin, PKC signaling pathways and synaptic remodeling during memory storage and neuronal repair. Eur J Pharmacol. 2008;585(1):76-87. PubMed doi:10.1016/j.ejphar.2008.01.051
13. Moosavi M, Naghdi N, Maghsoudi N, et al. Insulin protects against stress-induced impairments in water maze performance. Behav Brain Res. 2007;176(2):230-236. PubMed doi:10.1016/j.bbr.2006.10.011
14. Heninger GR, Mueller PS. Carbohydrate metabolism in mania before and after lithium carbonate treatment. Arch Gen Psychiatry. 1970;23(4):310-319. PubMed
Author affiliations: Psychosomatic Medicine, Department of Psychiatry, Audie L. Murphy Memorial VAMC, San Antonio, Texas.
Potential conflicts of interest: Dr Brown has served on the speakers or advisory boards for AstraZeneca and Janssen.
Funding/support: None reported.
Published online: January 13, 2011 (doi:10.4088/PCC.10l00985yel).
Prim Care Companion CNS Disord 2011;13(1):e1-e3
© Copyright 2011 Physicians Postgraduate Press, Inc.