Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Since Kraepelin’s description, negative symptoms have been considered central to schizophrenia. Negative symptoms cause serious personal, social, and occupational functioning disability in schizophrenia patients. As pharmacologic strategies are frequently insufficient in the treatment of negative symptoms, alternative treatment approaches are required.
Bright Light Therapy for Negative Symptoms
To the Editor: Since Kraepelin’s description,1 negative symptoms have been considered central to schizophrenia. Negative symptoms cause serious personal, social, and occupational functioning disability in schizophrenia patients. As pharmacologic strategies are frequently insufficient in the treatment of negative symptoms, alternative treatment approaches are required. Aichhorn et al2 showed that bright light therapy improved negative symptoms in residual schizophrenia. In contrast, a recent study3 revealed that bright light therapy did not improve negative symptoms but exacerbated general psychopathology. To date, the effect of bright light therapy on negative symptoms has been rarely studied, and no definite conclusion regarding its efficacy has been determined. We describe a case of residual schizophrenia in which negative symptoms were improved by bright light therapy.
Case report. A 66-year-old male inpatient with schizophrenia demonstrated severe negative symptoms. He had suffered from schizophrenia since the age of 19 years and had several hospitalizations at local psychiatric hospitals. His auditory hallucinations were exacerbated after his mother’s death, and he was referred to our hospital at the age of 56 years. After admission, his auditory hallucinations improved, but his negative symptoms persisted. He spent most of his time sitting in his room in an inert state. His persistent negative symptoms did not improve with administration of aripiprazole 6 mg/d. He was diagnosed with residual schizophrenia (ICD-10 code F295.6).
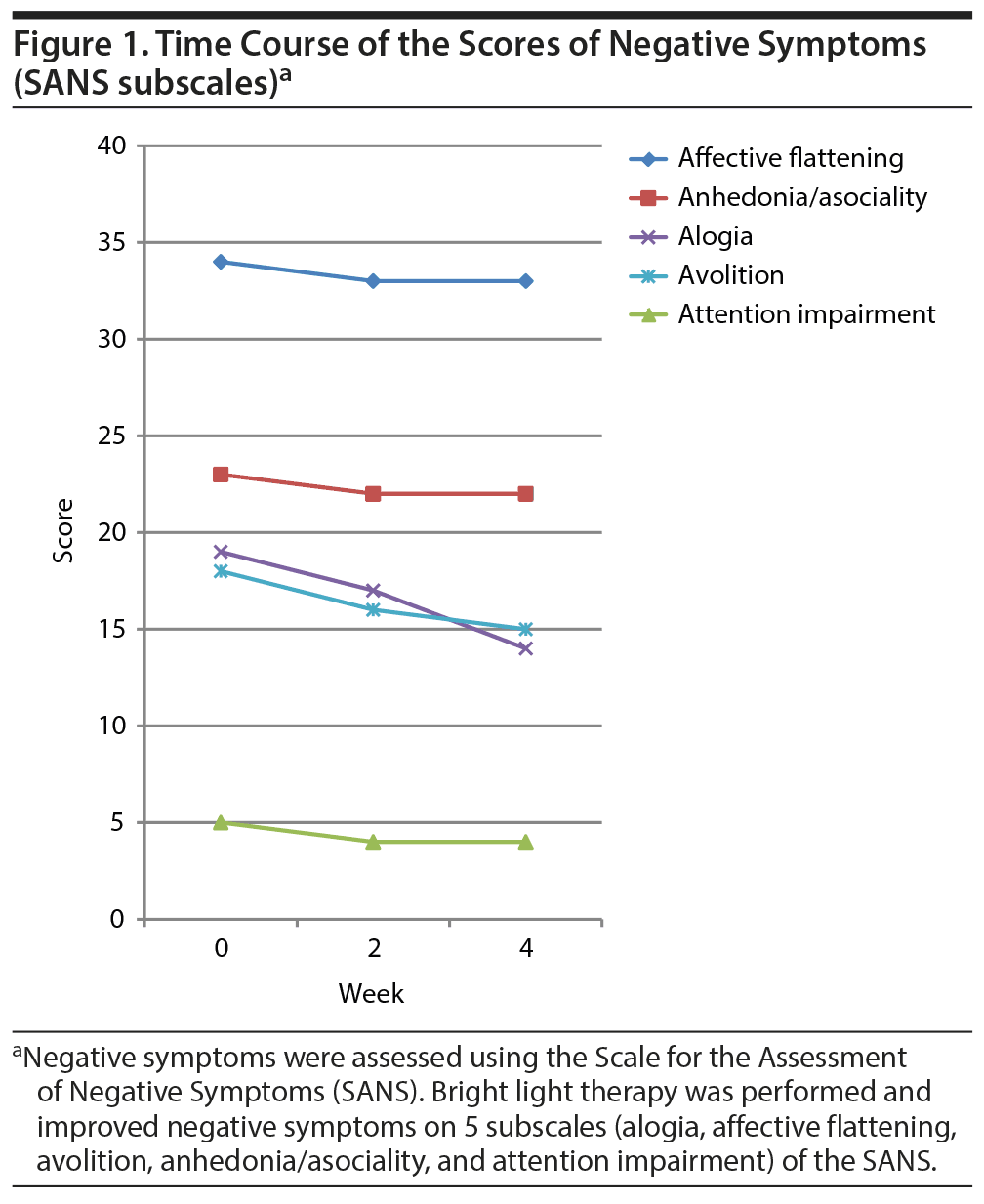
We decided to start bright light therapy in the hope of improving his negative symptoms. Negative and depressive symptoms were assessed with the Scale for the Assessment of Negative Symptoms (SANS)4 and the Hamilton Depression Rating Scale (HDRS), respectively.5 Before the first bright light therapy session, his SANS score was 99 and HDRS score was 12. According to the report of Aichhorn et al,2 bright light therapy with 10,000 lux was administered in the hospital for 1 hour from 7:30 am to 8:30 am, 5 days per week, for 4 weeks. After starting bright light therapy, he began to respond more quickly to our questions, and impoverishment of thought mildly improved. While he still spent most of his time sitting in his room, he also began to watch television programs in the living room. His medication was not changed throughout the 4-week bright light therapy period. Following the 4-week treatment period, his SANS score decreased to 88 and HDRS score to 8. The SANS consists of 5 subscales (alogia, affective flattening, avolition, anhedonia/asociality, and attention impairment), and all subscale scores decreased with bright light therapy (Figure 1). Bright light therapy was safe in our patient and did not cause psychotic exacerbation. Follow-up assessment was performed 16 weeks after the completion of bright light therapy. His SANS score did not change, and negative symptoms were not exacerbated during follow-up periods.
Bright light therapy is known to improve depressive symptoms of several disorders such as seasonal affective disorder, nonseasonal depression, and bipolar depression.6 Negative symptoms such as alogia and avolition have been reported to correlate with depression.7 In our patient, SANS subscale scores, which correlated with depression (alogia, avolition), decreased with bright light therapy. Interestingly, negative symptoms that do not overlap with depression (affective flattening, anhedonia/asociality, and attention impairment) were also improved by bright light therapy (see Figure 1). It is probable that while the improvement of negative symptoms can be attributed to improvement in depressive symptoms, bright light therapy directly improved negative symptoms as well. The effect of bright light therapy for negative symptoms is still controversial.2,3 Further studies are needed to clarify the effect of bright light therapy for negative symptoms.
In conclusion, this case suggests that bright light therapy improves negative symptoms in some patients with residual schizophrenia.
References
1. Kraepelin E. Lecture III, Dementia Praecox: Lectures on Clinical Psychiatry. New York, NY: Wiliam Wood; 1917:21-29.
2. Aichhorn W, Stelzig-Schoeler R, Geretsegger C, et al. Bright light therapy for negative symptoms in schizophrenia: a pilot study. J Clin Psychiatry. 2007;68(7):1146. PubMed doi:10.4088/JCP.v68n0726a
3. Roopram SM, Burger AM, van Dijk DA, et al. A pilot study of bright light therapy in schizophrenia. Psychiatry Res. 2016;245:317-320. PubMed doi:10.1016/j.psychres.2016.07.034
4. Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry suppl. 1989;155(7):49-58. PubMed
5. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. PubMed doi:10.1136/jnnp.23.1.56
6. Prasko J. Bright light therapy. Neuroendocrinol Lett. 2008;29(suppl 1):33-64. PubMed
7. Brébion G, Amador X, Smith M, et al. Depression, psychomotor retardation, negative symptoms, and memory in schizophrenia. Neuropsychiatry Neuropsychol Behav Neurol. 2000;13(3):177-183. PubMed
aDepartment of Neuropsychiatry, Oita University Faculty of Medicine, Yufu, Oita, Japan
bOosada Hospital, Nakatsu, Oita, Japan
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Permission was received from the patient to present the case, and the information was de-identified to protect anonymity.
Published online: September 7, 2017.
Prim Care Companion CNS Disord 2017;19(5):17l02117
https://doi.org/10.4088/PCC.17l02117
© Copyright 2017 Physicians Postgraduate Press, Inc.
Enjoy this premium PDF as part of your membership benefits!