A Case of Drug Overdose-Induced, Severe Electrocardiogram Abnormalities: Investigation Through Plasma Drug Concentrations
To the Editor: We report a case of a patient with concomitant overdose of antidepressants and antianxiety agents, and we also discuss the need for measurement of drug concentrations in plasma.
Case report. Ms A, a 46-year-old woman with recurrent DSM-IV depressive disorder, was transported in 2011 to our emergency center. On the day prior to examination, the patient overdosed on nortriptyline 3,750 mg, paroxetine 250 mg, mirtazapine 50 mg, estazolam 375 mg, and bromazepam 50 mg.
Her status at the time of transport included a Glasgow Coma Scale (GCS)1 score of 6 (best eye response, 1; best verbal response, 1; best motor response, 4), blood pressure of 90/60 mm Hg, heart rate of 90 bpm, body temperature of 37.4°C, and SpO2 of 95% (with administration of 6 L of O2). Electrocardiogram (ECG) demonstrated sinus rhythm, wide QRS, and elevated T wave. Blood collection showed only elevated white blood cell count. QT extension increased, left bundle-branch block appeared, blood pressure decreased, and convulsions occurred 2 hours after hospital consultation. Following tracheal intubation and connection to an artificial respirator, gastric and intestinal lavage were performed, and arrhythmia improved. On the morning following admission, Ms A’s GCS score had improved to 7 (1-1-5), and no ECG abnormalities were observed. On hospital day 3, she was nearly lucid.
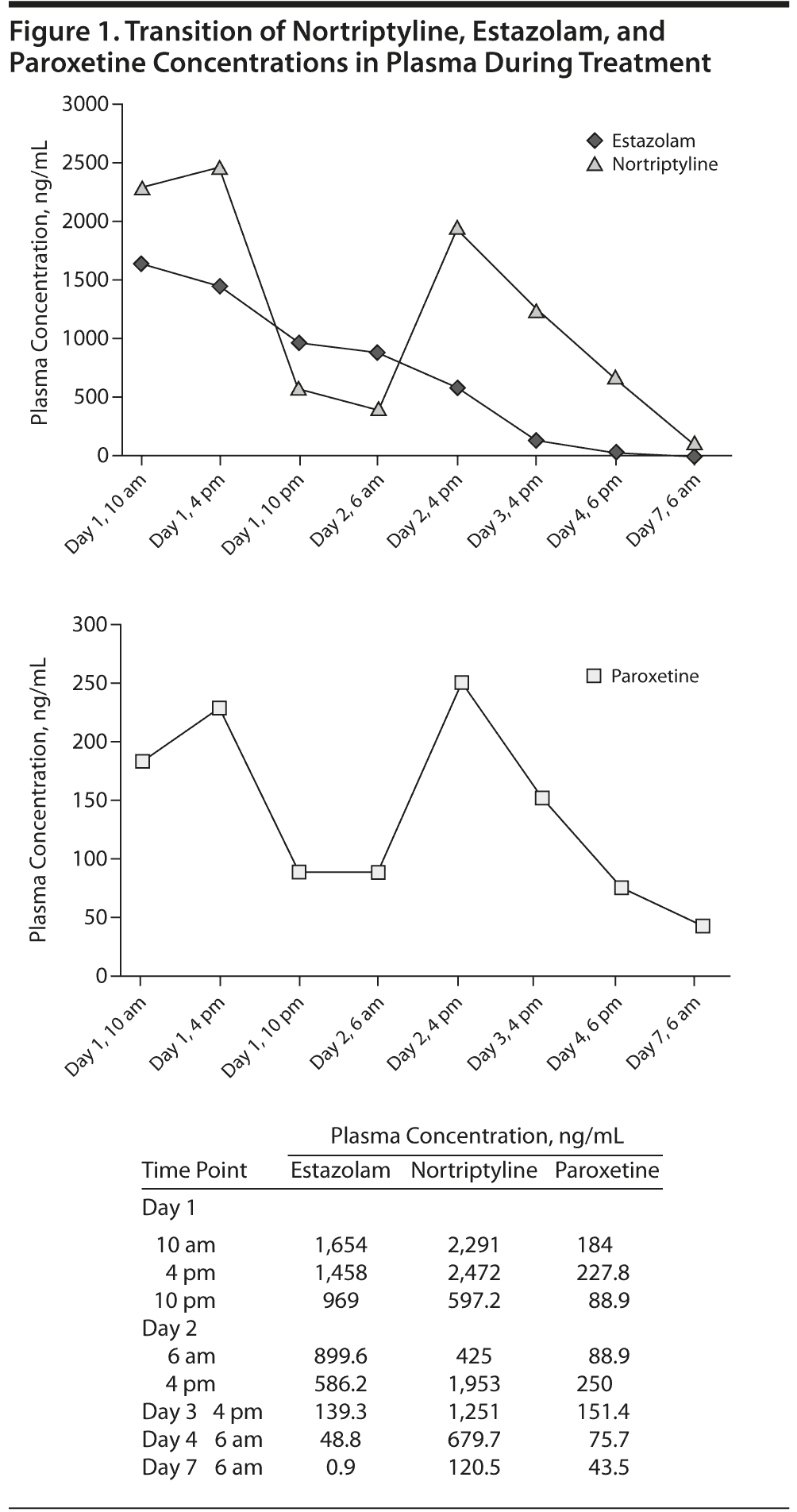
Nortriptyline, paroxetine, and estazolam concentrations in plasma were measured continually by column-switching high-pressure liquid chromatography with ultraviolet detector (Figure 1). Nortriptyline showed the highest drug concentration, and its concentration in plasma changed in conjunction with that of paroxetine. Estazolam concentration decreased linearly throughout the treatment period. The measurements of plasma concentration were performed with approval by the Institutional Review Board of Iwate Medical University and the consent of the family. Additional confirmation was provided by the patient personally after consciousness was regained, and approval for case reporting was granted.
There is a noted association between extension of QRS interval and onset of convulsions during tricyclic antidepressant (TCA) overdose.2 Comparison of the plasma concentration levels of each drug in our case shows a clear association between nortriptyline and paroxetine. Coadministration of a TCA and paroxetine, a metabolic inhibitor of cytochrome P450 (CYP) 2D6, is known to cause an increase in the plasma concentration of TCAs,3 and we believe that the high dosage of nortriptyline by itself, coupled with coadministration of paroxetine, increased the plasma concentration of the former.
TCAs in general are fat-soluble and have a high protein-binding ratio and diffuse rapidly into the organs.4 In our case, a temporary decrease in plasma concentration was observed, but the following day, plasma concentrations of nortriptyline and paroxetine rose again. This rise in concentration is attributed to reappearance in the blood of drug agents previously bound to tissues.5
The poor association observed between changes in clinical symptoms and trends in plasma concentration means that a latent high risk persists even when somatic effects are not severe. Clinically, we believe that patients should be treated with this risk kept in mind. Measurement of plasma concentration during emergency treatment is a highly objective biological index that represents a criterion for treatment planning and resumption of psychotropic therapy. Our case also has an implication that prescription drugs should be selected with reference to dangers in the event of overdose.
References
1. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;(7872):81-84. PubMed doi:10.1016/S0140-6736(74)91639-0
2. Hultén BA, Heath A. Clinical aspects of tricyclic antidepressant poisoning. Acta Med Scand. 1983;213(4):275-278. PubMed doi:10.1111/j.0954-6820.1983.tb03733.x
3. Sindrup SH, Br׸sen K, Gram LF, et al. The relationship between paroxetine and the sparteine oxidation polymorphism. Clin Pharmacol Ther. 1992;51(3):278-287. PubMed doi:10.1038/clpt.1992.23
4. Manoguerra AS. Poisoning with tricyclic antidepressant drugs. Clin Toxicol. 1977;10(2):149-158. PubMed doi:10.3109/15563657708987964
5. Spiker DG, Biggs JT. Tricyclic antidepressants: prolonged plasma levels after overdose. JAMA. 1976;236(15):1711-1712. PubMed doi:10.1001/jama.1976.03270160033025
Author affiliations: Department of Neuropsychiatry (Drs Sanjo, Koeda, Koizumi, Onuma, Saga, Tomizawa, Sato, Fukumoto, Takeuchi, and Sakai and Ms Kikuchi), Department of Critical Care Medicine, (Drs Koeda, Koizumi, Fujino, Inoue, and Endo), and Department of Internal Medicine, Neurology and Geriatrics (Dr Natori), School of Medicine, Iwate Medical University, Morioka; and Mitsubishi Tanabe Pharma Factory Ltd, Fukuoka (Mr Inoue), Japan.
Financial disclosure: None reported.
Funding/support: None reported.
Published online: June 7, 2012.
Prim Care Companion CNS Disord 2012;14(3):doi:10.4088/PCC.11l01321
© Copyright 2012 Physicians Postgraduate Press, Inc.