Cognitive-Behavioral Therapy for the Treatment of Depression and Adherence in Patients With Type 1 Diabetes: Pilot Data and Feasibility

ABSTRACT
Objective: Depression is one of the most common psychological problems affecting individuals with type 1 diabetes, and it is associated with treatment nonadherence and worse clinical outcomes. The research on treating depression or nonadherence in adults with type 1 diabetes is limited. We adapted an evidence-supported treatment, individual cognitive-behavioral therapy for adherence and depression (CBT-AD), for type 1 diabetes and examined its feasibility, acceptability, and potential for an effect.
Method: The pilot study included 9 patients with a DSM-IV diagnosis of major depression, dysthymia, or residual depressive symptoms despite treatment with an antidepressant; a diagnosis of type 1 diabetes per patient self-report; and a glycosylated hemoglobin A1c (HbA1c) level of 8.0% or greater. Patients were referred by their diabetes care providers to a behavioral medicine specialty setting and received 10 to 12 sessions of CBT-AD. Main outcome measures included percent of eligible participants who enrolled in the study, session attendance, independently-rated Montgomery-Asberg Depression Rating Scale (MADRS) score, self-reported adherence to diabetes care activities, and adherence to self-monitoring of blood glucose levels. Data were collected from June 27, 2008, through March 31, 2010.
Results: There was a clinically meaningful decrease in depression severity (mean [SD] MADRS score decrease from 26.0 [4.73] to 12.3 [7.37], Cohen d = 2.90), demonstrated improvements in diabetes self-care (increase in blood glucose monitoring from 65.0 [26.72] to 82.7 [22.75], Cohen d = -0.66, and a difference in self-reported percent insulin doses in the past 2 weeks from 77.1 [29.84] to 87.1 [23.6], Cohen d = -0.34), and possible improvement in glycemic control (decrease in HbA1c levels from 9.6 [1.32] to 9.0 [1.04], Cohen d = 0.45).
Conclusions: These preliminary results provide evidence for the acceptability, feasibility, and potential utility of CBT-AD for patients with type 1 diabetes and depression.
Trial Registration: clinicaltrials.gov Identifier: NCT01527981
Prim Care Companion CNS Disord 2012;14(2):doi:10.4088/PCC.11m01220
© Copyright 2012 Physicians Postgraduate Press, Inc.
Submitted: May 23, 2011; accepted August 5, 2011.
Published online: March 15, 2012.
Corresponding author: Sarah M. Markowitz, PhD, Wells College, 170 Main St, Aurora, NY 13026 ([email protected]).
Depression is a serious and prevalent condition that is especially common in patients with chronic disease in general1 and with diabetes in particular.2 Meta-analyses indicate that the rate of depression in patients with type 2 diabetes (formerly known as adult-onset diabetes) is 1.6 to 2.0 times higher than that in the general population.2,3 Data suggest, but are insufficient to be conclusive, that patients with type 1 diabetes (formerly known as childhood-onset diabetes) have similarly elevated rates of depression.4
Depression is associated with poorer adherence to medical regimens among patients with chronic disease.5,6 Poor adherence is particularly problematic for patients with type 1 diabetes, as self-care in type 1 diabetes is extremely important in achieving the glycemic control necessary to prevent diabetes-related complications. Adherence with treatment guidelines decreases rates of mortality and morbidity in diabetes.7 Meta-analysis indicates that among youth with type 1 diabetes, adherence is significantly associated with better glycemic control.8 Patients with diabetes who are depressed have increased rates of mortality, diabetes-related complications, health care costs, and medical symptom burden, as well as a decreased quality of life relative to patients with diabetes who are not depressed.9-14 Furthermore, meta-analysis indicates that depression is associated with hyperglycemia in patients with diabetes15; some evidence suggests that this relationship may be stronger in type 1 diabetes than in type 2 diabetes.16 Among patients with type 1 diabetes, depressive symptoms are associated with increased diabetes symptom reporting, poorer physical functioning, decreased adherence to exercise regimens and diet, higher glycosylated hemoglobin A1c (HbA1c) levels, and increased complications.17,18
Although depression is associated with a number of adverse outcomes in patients with diabetes, there is limited evidence that treating depression alone will result in improved diabetes outcomes.19 Furthermore, there is currently a paucity of research on treating depression in patients with type 1 diabetes. The single study published to date is a pilot study of group cognitive-behavioral therapy (CBT) for adolescents with type 1 diabetes and depressive symptoms that showed improvement in depressive symptoms, but not in HbA1c levels, from preintervention to postintervention.20 Similarly, another uncontrolled pilot study comparing group CBT for depression and diabetes between patients with type 1 and type 2 diabetes found improvement in depression but not in HbA1c levels, with no difference between type 1 and type 2 patients.21 Treatment that integrates adherence counseling with depression treatment may be required to improve both depressive symptoms and diabetes outcomes among depressed patients with diabetes. Cognitive-behavioral therapy for adherence and depression (CBT-AD) has been successful at improving adherence to antiretroviral medications and depression in patients with human immunodeficiency virus (HIV).22 Pilot data indicate that this treatment can be successfully adapted to address adherence to diabetes self-care regimens and could hold promise in improving both depression and glycemic control in depressed patients with type 2 diabetes.23 The current study tests the feasibility and potential clinical utility of adapting this intervention for depressed patients with type 1 diabetes.

- Patients with type 1 diabetes and depression have poorer adherence and worse clinical outcomes than patients without depression.
- Cognitive-behavioral therapy (CBT) for adherence and depression has shown promise in improving depression and adherence in type 2 diabetes.
- Integration of the treatment of depression with CBT-informed strategies to improve self-care and treatment adherence may improve health outcomes for adult patients with type 1 diabetes and depression.
METHOD
Participants
Nine participants (mean ± SD age = 51.2 ± 5.88 years) met criteria for the pilot study and were enrolled. All participants were white and had a mean of 16 years of education (SD = 2.40).
Participants were eligible for the study if they had a diagnosis of type 1 diabetes per patient self-report, met DSM-IV-TR24 criteria for major depressive disorder (MDD) or dysthymia or had residual depressive symptoms (eg, Clinical Global Impressions of Illness scale (CGI-I) score of 2 or “minimally ill, depressive symptoms but able to function in all areas”) despite treatment with an antidepressant medication, were between the ages of 18 and 80 years, and had a HbA1c level ≥ 8.0%. The American Diabetes Association recommends a target HbA1c level of < 7%7; on the basis of clinical experience, the diabetes care providers and Massachusetts General Hospital (MGH) Diabetes Treatment Center (Boston, Massachusetts) suggested that an HbA1c level ≥ 8.0% would be indicative of problems with adherence in this patient population.
Participants were excluded if they were currently on dialysis, had a diagnosis of bipolar disorder (I or II), had active or untreated major mental illness that would interfere with study participation (eg, untreated psychosis, eating disorder, mental retardation), or had engaged in CBT during the last 3 years.
Of the 14 participants who presented for a screening visit, 3 (21%) were excluded because they did not meet a diagnosis of MDD or dysthymia and did not have residual depressive symptoms despite treatment with an antidepressant medication. One participant (7%) was excluded due to major mental illness that would interfere with study participation, and 1 participant (7%) dropped out of the study after his baseline evaluation, but prior to beginning treatment. Ten patients qualified for the study, and 9 (90%) participated. All participants provided informed consent, and all study procedures were approved by the Institutional Review Board at MGH (clinicaltrials.gov Identifier: NCT01527981). Data were collected from June 27, 2008, through March 31, 2010.
Measures
Physiological. HbA1c. Participants had blood drawn at baseline and postassessment to measure their HbA1c levels. HbA1c levels were measured using the glycosylated hemoglobin assay, which provides the most objective and reliable information about long-term glucose control.25,26
Adherence. Self-reported adherence. Adherence to insulin was assessed using a questionnaire that instructed participants to report how often they took their insulin on a 10-point scale from 0% to 100% of the time. This questionnaire was adapted from a questionnaire used by Lu and colleagues27 to assess adherence to antiretroviral medications.
Self-monitoring of blood glucose. OneTouch Ultra 2 (LifeScan, Inc, Milpitas, California) was used in this study to provide a measure of frequency of self-monitoring of blood glucose. Adherence to self-monitoring of blood glucose was assessed by downloading readings from participants’ glucometers and dividing the number of times each participant tested over the past 2 weeks by the number of tests prescribed over the same time period. These percentages were corrected if participants reported that they used another glucometer to test during certain days but did not bring that glucometer to the study visit.
Depression
For all clinician-rated measures, a trained PhD or master’s -level rater other than the therapist completed the assessment at posttreatment.
Mini-International Neuropsychiatric Inventory. The Mini-International Neuropsychiatric Inventory (MINI)28 was used to establish baseline diagnoses for all patients. The MINI is a valid, structured psychiatric diagnostic tool that has been shown to reliably detect DSM-IV psychiatric disorders through the administration of questions regarding specific symptoms of various diagnoses.
Montgomery-Asberg Depression Rating Scale. The Montgomery-Asberg Depression Rating Scale (MADRS),29 a structured 10-item interview, was utilized to measure specific symptoms of depression and to provide a rating of depression severity over the past 7 days. The scale has acceptable reliability, validity, and sensitivity to change. The MADRS was chosen over other clinical assessments of depression such as the Hamilton Depression Rating Scale30 because of its emphasis on symptoms of depression that are not somatic in nature.
Center for Epidemiologic Studies Depression Scale. The Center for Epidemiologic Studies Depression Scale (CES-D)31 is a 20-item self-report questionnaire designed to measure the severity of depressive symptomatology. The CES-D has been found to have acceptable construct validity and test-retest reliability as well as high internal consistency.31
Global Assessment of Functioning. The Global Assessment of Functioning (GAF)24 is the Axis V rating from the DSM-IV and was used to rate participants’ current functioning on a scale of 0 to 100. The GAF is a reliable and valid means of rating current functioning.
Clinical Global Impressions of Illness. The CGI-I32 (1 = not ill to 7 = extremely ill) was utilized to provide a global rating of depression. The CGI is a valid and reliable measure of the severity of global impairment and distress related to depression, with a score ≥ 3 signifying that the patient meets criteria for the disorder in question.
Feasibility. Feasibility was operationalized as the percentage of eligible participants who enrolled in the study.
Acceptability. Acceptability was operationalized as session attendance.
Procedure
Screening. Potential participants were referred to the study at the Behavioral Medicine Service of the Department of Psychiatry at MGH by providers from the MGH Diabetes Treatment Center. They completed a brief telephone screen, followed by an assessment with a master’s -level or PhD-level clinician. After obtaining informed consent, the clinician completed the MINI, MADRS, CGI, and GAF. Participants also completed a battery of self-report questionnaires and a blood draw to determine their HbA1c levels.
Intervention. All participants were offered 2 visits with a certified diabetes nurse educator, 3 visits with a registered dietitian, and 10 to 12 sessions of CBT-AD.33,34 The nurse educator worked with each participant to review current medications and to establish goals for the treatment regimen, including those related to medication, self-monitoring of blood glucose, and foot care. At the second nurse educator visit, the nurse was available to answer any questions that participants had and to follow up on the goals established at the first visit. At the initial dietitian visit, the dietitian conducted a comprehensive evaluation of the participant’s nutrition history. The dietitian then used this information to work with the participant to set 2 dietary goals and 1 exercise goal that were individually tailored on the basis of the nutrition and lifestyle assessment. These goals were selected on the basis of their potential to significantly lower HbA1c levels and on the participant’s self-report of at least 80% confidence in ability to achieve the goals. At the second and third dietitian visits, the dietitian worked with the participant to reevaluate the goals set at the previous visit and to continue with, build on, or change the existing goals using the same criteria. A letter was sent to each participant’s medical provider of choice, most frequently the primary care physician, describing the individual’s participation in the study and any diagnoses that the participant met criteria for at the baseline evaluation.
Following the initial sessions with the nurse educator and dietitian, participants completed 10 to 12 sessions of CBT-AD. A comprehensive description of CBT-AD can be found elsewhere.33,34 The treatment is organized into a 1-session intervention focused on adherence,35 followed by 6 modules focused on adherence and depression (CBT-AD).33,34 Although the modules are presented in a specific sequence, the intervention is designed to provide the clinician with the flexibility to adapt the treatment to the patient’s needs. The number of sessions spent on each module is designed to be flexible as well in order to address areas that are particularly salient to the patient or difficult for the patient to implement. The first session, referred to as “Life-Steps,”35 is a stand-alone, 1-session intervention designed to improve adherence to medical recommendations for the effective management of chronic illness. Life-Steps was adapted to patients with type 1 diabetes by emphasizing self-monitoring of blood glucose levels and insulin adherence as important adherence goals and by problem-solving as to how to use reminder cues to achieve improved adherence. After Life-Steps, 9 to 11 sessions of CBT-AD focus on addressing deficits in self-care and teaching specific cognitive-behavioral skills to treat symptoms of depression. The number of sessions was also designed to be flexible to meet the needs of the patient. The clinician and the patient collaboratively determined whether to use all 12 sessions or stop after 10 or 11.
At the beginning of each treatment session, the patient completed the CES-D and adherence questionnaires, including adherence to the patient’s goals set with the dietitian and nurse educator, and the patient’s glucometer was read electronically. The therapist then addressed remaining deficits in self-care and established new goals as necessary. The remaining time was spent on CBT skills for managing depression, including (1) psychoeducation and motivational interviewing, (2) activity scheduling, (3) cognitive restructuring, (4) problem-solving, (5) relaxation training and diaphragmatic breathing, and (6) maintenance and relapse prevention.
During psychoeducation, patients were provided information about the importance of adherence to self-monitoring of blood glucose, insulin, medications, diet, and exercise and on how tight glycemic control is associated with reduced risk of complications. This information was linked back to the goals that the patients had already set with the dietitian and nurse educator. Throughout treatment, therapists encouraged patients to use self-monitoring of blood glucose data to notice patterns and learn information, rather than focusing on “good” or “bad” blood glucose readings. Patients were encouraged to think about their blood glucose numbers “like a scientist,” rather than from a judgmental perspective.
RESULTS
Feasibility
Of the 10 patients who met criteria to participate in the study, 9 (90%) participated in the treatment and 7 (70%) completed treatment and posttreatment assessment. One participant did not begin treatment despite eligibility. Two participants dropped out partway through treatment and did not complete posttreatment assessment. One of these 2 participants dropped out after 3 sessions, stating it was due to his work and travel schedule. The other dropped out after 6 sessions because she was not comfortable working with a therapist of a different religion from her own. Only the data from the 7 participants who completed the posttreatment assessment are analyzed here. Given the small sample size, Cohen d was calculated using the difference in means between baseline and posttreatment divided by the standard deviation of the baseline scores to provide a measure of the magnitude of the therapeutic effect.
To encourage regular session attendance, participants received reminder telephone calls or e-mails the day before their appointments. They received reimbursement for use of public transit or parking to attend sessions.
Acceptability
Seven of 9 participants (78%) who began treatment completed the study. These 7 participants attended a mean of 11.71 sessions. Of the 9 participants who began treatment, the mean number of sessions attended was 10.22. It took a mean of 5.14 months from baseline evaluation to treatment completion among the 7 completers.
Depression
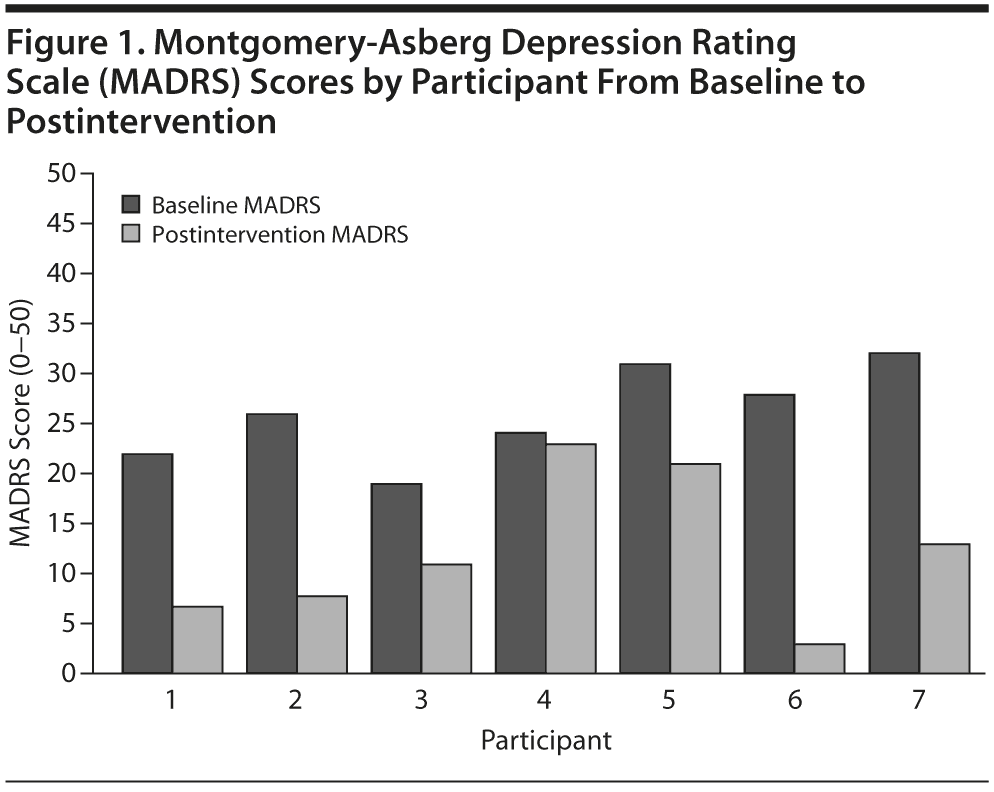
Among the 7 participants who completed treatment, the independently-rated depression severity as assessed by the MADRS decreased from baseline (mean = 26.0, SD = 4.73) to postintervention (mean = 12.3, SD = 7.37), which represents a large effect (Cohen d = 2.90) (Figure 1). The baseline MADRS mean score represents a moderate level of clinician-rated depressive symptoms, and the postintervention mean score represents clinician-rated depressive symptoms below clinical significance.32
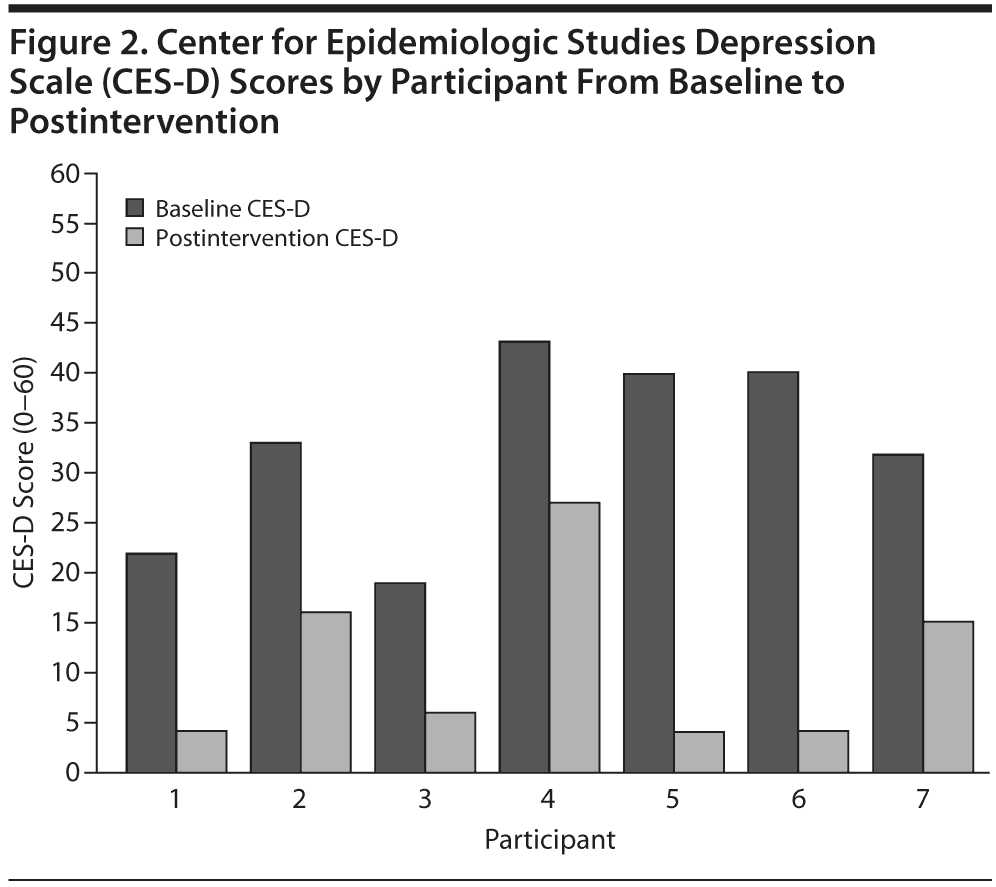
There was also a change in self-reported depression severity as measured by the CES-D from baseline (mean = 32.7, SD = 9.27) to postintervention (mean = 10.9, SD = 8.84), which also represents a large effect (Cohen d = 2.35) (Figure 2). The baseline CES-D mean score represents participant-rated depressive symptoms in the moderate to severe range, and the postintervention CES-D mean score represents participant-rated depressive symptoms in the nonclinical range.31 Participants also rated themselves as a mean of 55.7% improved on depression at their posttreatment assessment.
There was also a change in independently-rated CGI scores from baseline (mean = 4.0, SD = 1.00) to postintervention (mean = 2.0, SD = 1.00), which is equivalent to a change in the clinician’s overall impression of depressive symptoms from “moderately ill” (daily difficulties in functioning, thoughts of life being not worth living) to “minimally ill” (depressive symptoms, but able to function in all areas). The effect size of the reduction in participants’ CGI scores was 2.0.
Finally, the independently-rated GAF score increased from baseline (mean = 53.0, SD = 3.56) to postintervention (mean = 68.6, SD = 6.60), indicating a change from the lower end of “moderate symptoms or moderate difficulty in functioning” to the upper end of “some mild symptoms or some difficulty in functioning.” Cohen d for the change in GAF scores was −4.38, indicating a large effect size.
Adherence
There was an increase in percent of recommended blood glucose monitoring tests performed in the past 2 weeks from baseline (mean = 65.0, SD = 26.72) to postintervention (mean = 82.7, SD = 22.75) among the 6 participants who provided glucometer data at postintervention assessment (Cohen d = −0.66). There was also a difference in self-reported percent insulin doses administered in the past 2 weeks from baseline (mean = 77.1, SD = 29.84) to postintervention (mean = 87.1, SD = 23.6), which represents a medium effect (Cohen d = −0.34). Participants also rated themselves as a mean of 54.3% improved on overall diabetes adherence at their posttreatment assessment.
HbA1c Levels
There was a trend toward a decrease in HbA1c levels from baseline (mean = 9.6, SD = 1.32) to postintervention (mean = 9.0, SD = 1.04), which also represents a medium effect (Cohen d = 0.45), and more importantly, a clinically meaningful difference.
DISCUSSION
This study provides preliminary evidence for a successful adaptation of CBT-AD, originally developed for patients with HIV, for type 1 diabetes. CBT-AD appears to have been acceptable and feasible and may have been helpful in producing improvements in diabetes self-care and depression. Many patients expressed both appreciation for the skills they gained and the need for more attention to the psychological health of adults with type 1 diabetes. Results from the MADRS, CES-D, CGI, and GAF indicate that the intervention may be effective in reducing depressive symptoms. Participants improved in the self-rated measure of depression and independently assessed clinical severity of depression and overall functioning. On average, they went from the moderate clinically significant range of symptoms to the mild or nonclinical range of symptoms, suggesting that this change is clinically meaningful. Objectively measured adherence to glucose self-monitoring via downloaded glucometer data suggests that the intervention also had a powerful effect on increasing the frequency of this important self-care activity. Moreover, the improvement in insulin adherence (a medium effect size) is clinically meaningful given the importance of insulin adherence in achieving HbA1c targets in type 1 diabetes. Finally, the improvements in HbA1c levels suggest that the intervention could potentially be effective at improving glycemic control. As HbA1c represents a summary of blood glucose values in the past 3 months, it is noteworthy that there was some indication that participants’ values may have decreased during the intervention, which took approximately 3 months. If such improvement continues or is maintained, a change of 0.6% could be clinically significant in reducing the risk for future diabetes complications.
This study is limited by the small sample size of homogenous (white, well-educated) participants, which may reduce generalizabilty, and lack of randomization. Therefore, we cannot conclude that the intervention was responsible for the improvements in depressive symptoms or adherence experienced by participants. However, because this is a small demonstration study, it is noteworthy that significant improvements were seen from baseline to postintervention on all measures of depression and on important indicators of diabetes self-management.
Self-monitoring of blood glucose is a particularly important adherence behavior in type 1 diabetes given its impact on improved glycemic control,36 so, it is encouraging that participants showed significant improvement in this aspect of adherence. Participants were on average 77% adherent to insulin before the study and 87% adherent postintervention. This increase was in the small (d = 0.2) to medium (d = 0.5) range.37 Furthermore, participants showed a trend toward decreased HbA1c levels. This difference is clinically meaningful and, with a larger sample, could be significant. We might even expect that further significant changes in HbA1c levels could continue postintervention, as HbA1c level is a summary of blood glucose control over the past 3 months, so participants may not show evidence of their improved adherence through improved glycemic control until a couple of months after the intervention is complete.
Despite these limitations, the results of this preliminary study suggest that the treatment was feasible and acceptable to patients. Most patients successfully completed all study visits and procedures, and unstructured feedback from patients suggested that they appreciated the opportunity to participate. Anecdotal evidence suggests that diabetes treatment providers (who were often in contact with the study investigators) noted improvements in their patients and were grateful for the opportunity to refer patients for care. Preliminary evidence suggests that CBT-AD was useful in helping patients improve both adherence to diabetes self-care and depression. These findings are particularly promising given the seemingly high rates of depression in patients with type 1 diabetes,4 the association of depression with worse diabetes outcomes,17,18 and the lack of treatment studies on adult patients with type 1 diabetes and depression.19
The integration of adherence training with cognitive-behavioral techniques in CBT-AD is based on the belief that the strategies employed in CBT for depression (eg, activity scheduling and mood monitoring, cognitive restructuring) have important applications in facilitating successful treatment adherence in patients with chronic illness (eg, increasing physical activity, correcting maladaptive beliefs about the illness and treatment). Our intervention addresses diabetes and depression as related conditions that may have a bidirectional influence on each other, which is novel in the field of diabetes and depression. For example, negative thinking, which is common in depression, would lead to worse self-care behaviors, making the medical illness worse and allowing for more negative thinking because one’s illness is worse. Each session of the treatment focuses on the difficulties that the patient is having with diabetes management, the symptoms of depression that the patient is experiencing, and how these 2 problems influence each other. The strategies employed in this modular treatment are presented to the patient as equally applicable to the difficulties of diabetes management and to the symptoms of depression. The results of our study provide support for the hypothesis that integrating the treatment of depression with CBT-informed strategies to improve skills and motivation for self-care and treatment adherence may maximize the effects of psychological treatments on health outcomes for adult patients with type 1 diabetes struggling with depression.
Author affiliations: Department of Psychology, Wells College, Aurora, New York (Dr Markowitz); Ferkauf Graduate School of Psychology and Albert Einstein College of Medicine, Yeshiva University, New York, NewYork (Dr Gonzalez); and Diabetes Research Center (Ms Delahanty) and Behavioral Medicine Service (Mr Carper and Dr Safren), Massachusetts General Hospital and Harvard Medical School, Boston.
Potential conflicts of interest: None reported.
Funding/support: The project was supported by a grant from the National Institute of Mental Health (R01MH078571) and was partially supported by the Harvard Catalyst-Harvard Clinical and Translational Science Center through a grant from the National Institutes of Health (1 UL1 RR025758-03) for a portion of the nurse and dietician study visits. The research was also conducted with support from the Investigator-Initiated Study Program of LifeScan, Inc, Milpitas, California, in the form of donated glucometers and glucose test strips.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health.
Previous presentation: Data from this article were presented at the Annual Convention for the Association of Behavioral and Cognitive Therapies; November 19-22, 2009; New York, New York.
REFERENCES
1. Evans DL, Charney DS. Mood disorders and medical illness: a major public health problem. Biol Psychiatry. 2003;54(3):177-180. PubMed doi:10.1016/S0006-3223(03)00639-5
2. Anderson RJ, Freedland KE, Clouse RE, et al. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24(6):1069-1078. PubMed doi:10.2337/diacare.24.6.1069
3. Ali S, Stone MA, Peters JL, et al. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23(11):1165-1173. PubMed doi:10.1111/j.1464-5491.2006.01943.x
4. Barnard KD, Skinner TC, Peveler R. The prevalence of co-morbid depression in adults with type 1 diabetes: systematic literature review. Diabet Med. 2006;23(4):445-448. PubMed doi:10.1111/j.1464-5491.2006.01814.x
5. Dunbar-Jacob J, Burke LE, Pyczynski S. Clinical assessment and management of adherence to medical regiments. In: Nicassio PM, Smith TW, eds. Managing Chronic Illness: A Biopsychosocial Perspective. Washington, DC: American Psychological Association; 1995. doi:10.1037/10511-009
6. DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107. PubMed doi:10.1001/archinte.160.14.2101
7. American Diabetes Association. Standards of medical care in diabetes—2009. Diabetes Care. 2009;32(suppl 1):S13-S61. PubMed doi:10.2337/dc09-S013
8. Hood KK, Peterson CM, Rohan JM, et al. Association between adherence and glycemic control in pediatric type 1 diabetes: a meta-analysis. Pediatrics. 2009;124(6):e1171-e1179. PubMed doi:10.1542/peds.2009-0207
9. Zhang X, Norris SL, Gregg EW, et al. Depressive symptoms and mortality among persons with and without diabetes. Am J Epidemiol. 2005;161(7):652-660. PubMed doi:10.1093/aje/kwi089
10. Egede LE, Nietert PJ, Zheng D. Depression and all-cause and coronary heart disease mortality among adults with and without diabetes. Diabetes Care. 2005;28(6):1339-1345. PubMed doi:10.2337/diacare.28.6.1339
11. de Groot M, Anderson RJ, Freedland KE, et al. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63(4):619-630. PubMed
12. Simon GE, Katon WJ, Lin EH, et al. Diabetes complications and depression as predictors of health service costs. Gen Hosp Psychiatry. 2005;27(5):344-351. PubMed doi:10.1016/j.genhosppsych.2005.04.008
13. Ludman EJ, Katon WJ, Russo JE, et al. Depression and diabetes symptom burden. Gen Hosp Psychiatry. 2004;26(6):430-436. PubMed doi:10.1016/j.genhosppsych.2004.08.010
14. Kohen D, Burgess AP, Catalסn J, et al. The role of anxiety and depression in quality of life and symptom reporting in people with diabetes mellitus. Qual Life Res. 1998;7(3):197-204. PubMed doi:10.1023/A:1008817812520
15. Lustman PJ, Anderson RJ, Freedland KE, et al. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care. 2000;23(7):934-942. PubMed doi:10.2337/diacare.23.7.934
16. Van Tilburg MAL, McCaskill CC, Lane JD, et al. Depressed mood is a factor in glycemic control in type 1 diabetes. Psychosom Med. 2001;63(4):551-555. PubMed
17. Ciechanowski PS, Katon WJ, Russo JE, et al. The relationship of depressive symptoms to symptom reporting, self-care and glucose control in diabetes. Gen Hosp Psychiatry. 2003;25(4):246-252. PubMed doi:10.1016/S0163-8343(03)00055-0
18. Gendelman N, Snell-Bergeon JK, McFann K, et al. Prevalence and correlates of depression in individuals with and without type 1 diabetes. Diabetes Care. 2009;32(4):575-579. PubMed doi:10.2337/dc08-1835
19. Markowitz SM, Gonzalez JS, Wilkinson JL, et al. A review of treating depression in diabetes: emerging findings. Psychosomatics. 2011;52(1):1-18. PubMed
20. Rosello JM, Jimenez-Chaffey MI. Cognitive-behavioral group therapy for depression in adolescents with diabetes: a pilot study. Rev Interam Psicol. 2006;40:219-226.
21. Georgiades A, Zucker N, Friedman KE, et al. Changes in depressive symptoms and glycemic control in diabetes mellitus. Psychosom Med. 2007;69(3):235-241. PubMed doi:10.1097/PSY.0b013e318042588d
22. Safren SA, O’ Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1-10. PubMed doi:10.1037/a0012715
23. Gonzalez JS, McCarl LA, Wexler DD, et al. Cognitive-Behavioral Therapy for Adherence and Depression (CBT-AD) in type 2 diabetes. J Cogn Psychother. 2010;24(4):329-343. doi:10.1891/0889-8391.24.4.329
24. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
25. Nathan DM, Singer DE, Hurxthal K, et al. The clinical information value of the glycosylated hemoglobin assay. N Engl J Med. 1984;310(6):341-346. PubMed doi:10.1056/NEJM198402093100602
26. Singer DE, Coley CM, Samet JH, et al. Tests of glycemia in diabetes mellitus: their use in establishing a diagnosis and in treatment. Ann Intern Med. 1989;110(2):125-137. PubMed
27. Lu M, Safren SA, Skolnik PR, et al. Optimal recall period and response task for self-reported HIV medication adherence. AIDS Behav. 2008;12(1):86-94. PubMed doi:10.1007/s10461-007-9261-4
28. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33, quiz 34-57. PubMed
29. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed doi:10.1192/bjp.134.4.382
30. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
31. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401. doi:10.1177/014662167700100306
32. Guy W. Clinical global impressions. In: New Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental health, Psychopharmacology Research Brand, Division of Extramural Research Programs; 1976: 218-222.
33. Safren SA, Gonzalez JS, Soroudi N. Coping With Chronic Illness: A Cognitive-Behavioral Therapy Approach for Adherence and Depression: Therapist Guide. New York, NY: Oxford University Press; 2007.
34. Safren SA, Gonzalez JS, Soroudi N. Coping With Chronic Illness: A Cognitive-Behavioral Therapy Approach for Adherence and Depression: Workbook. New York, NY: Oxford University Press; 2007.
35. Safren SA, Otto MW, Worth JL, et al. Two strategies to increase adherence to HIV antiretroviral medication: life-steps and medication monitoring. Behav Res Ther. 2001;39(10):1151-1162. PubMed doi:10.1016/S0005-7967(00)00091-7
36. Helgeson VS, Honcharuk E, Becker D, et al. A focus on blood glucose monitoring: relation to glycemic control and determinants of frequency. Pediatr Diabetes. 2011;12(1):25-30. PubMed doi:10.1111/j.1399-5448.2010.00663.x
37. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988.