Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: The impact of thyroid disease on psychiatric symptoms is well known but barely understood. Thyroid hormone dysregulation is closely associated with psychiatric symptomatology. Patients with hyperthyroidism frequently have depressive or anxiety disorders and in rare cases also psychotic symptoms.
Graves’ Disease and Psychosis in a Young Woman: Pathophysiologic Considerations
To the Editor: The impact of thyroid disease on psychiatric symptoms is well known but barely understood. Thyroid hormone dysregulation is closely associated with psychiatric symptomatology.1 Patients with hyperthyroidism frequently have depressive or anxiety disorders2-5 and in rare cases also psychotic symptoms.6-8 A rare condition attracting increasing scientific interest is steroid responsive encephalopathy associated with autoimmune thyroiditis (SREAT). The clinical presentation includes amnesia, seizures, focal neurologic deficits, depression, mania, psychosis, and hallucinations,9,10 making it an interdisciplinary challenge. We report the case of a woman who experienced psychotic symptomatology in the course of hyperthyroidism due to Graves’ disease. Possible pathomechanisms are discussed.
Case report. A 29-year-old woman with no personal or family history of psychiatric illness was admitted to our inpatient treatment facility in February 2015 reporting delusional ideation and perception (predominantly persecutory delusions), auditory hallucinations, and slightly disorganized thinking for the past 2 weeks.
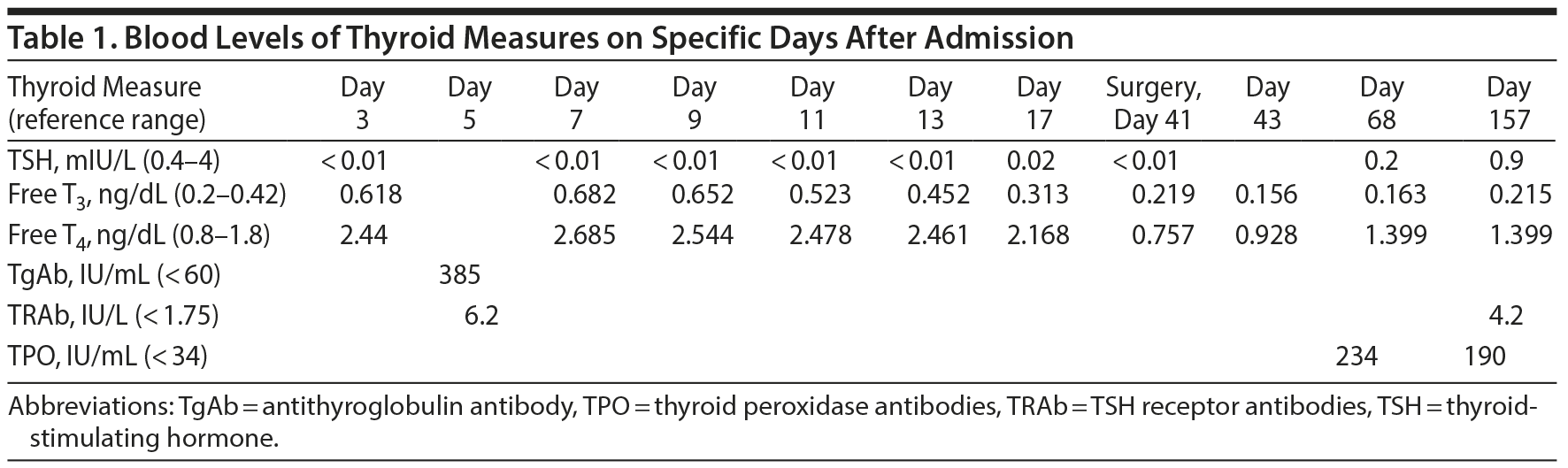
The clinical and neurologic examination, computed tomography, electroencephalogram, analysis of cerebrospinal fluid, and drug use screening revealed no pathological findings, but blood analysis (Table 1) showed elevated levels of thyroid hormones (free T3 = 0.618 ng/dL, normal range, 0.2-0.42 ng/dL; free T4 = 2.44 ng/dL, normal range, 0.8-1.8 ng/dL) and suppressed levels of thyroid-stimulating hormone (TSH < 0.1 mIU/L). Thyroid ultrasound, scintigraphy, and thyroid-stimulating antibodies confirmed the diagnosis of Graves’ disease (ICD-10) (antithyroglobulin antibody = 385 IU/mL, normal range, < 60 IU/mL; TSH receptor antibody = 6.2 IU/L, normal range, < 1.75 IU/L). Graves’ disease had been diagnosed 4 years ago, but medical treatment had been replaced by traditional Chinese medicine techniques after approximately 12 weeks because of side effects. Since then, the patient had involuntarily lost 15 lb (7 kg).
We treated the patient with olanzapine 5 mg/d, methimazole 10 mg/d, prednisolone 25 mg/d, and propranolol 10 mg/d. Within a few days, there was a clear reduction of symptoms. Thyroid hormone levels decreased slowly with methimazole 10 mg and prednisolone 20 mg. Prednisolone was discontinued on the 28th day of her hospitalization.
Despite normalization of thyroid hormone levels, the patient continued to experience paranoia and felt hampered in her normal thinking, although to a lesser extent than previously. The patient refused higher doses of olanzapine. On day 41, the patient underwent total thyroidectomy. Within 2 days, all psychiatric symptoms ceased. Methimazole and propranolol were discontinued, and levothyroxine 100 µg was used to treat the hormone deficiency. Three weeks later, olanzapine was stopped. The patient has had no recurrence of symptoms (last interview in February 2016).
Three pathophysiologic pathways for psychiatric symptoms in Graves’ disease should be considered:
- Thyroid hormones. Hyperthyroidism induces overactivity of the adrenergic system associated with an increased level of arousal and symptoms of vulnerability to stress such as insomnia, anxiety, irritability, emotional instability, and exaggerated fear or aggressiveness.11 Polymorphisms in deiodinase genes and in transporter genes have been suggested to influence the presentation of psychiatric symptomatology.12
- Autoimmunity. Neuropsychiatric symptoms in Graves’ disease might develop due to SREAT. The following criteria define SREAT: (1) acute or subacute onset of altered mental status, (2) elevated antithyroid antibodies, and (3) rapid response in mental status with corticosteroids and absence of other factors that could explain the symptoms or treatment response to steroids.9 Prednisolone might have improved our patient’s condition, although it is not possible to distinguish the effect of prednisolone from the effects of the other medications.
- Thyroid gland. Interestingly, in our case, psychiatric symptomatology ceased completely only after total thyroidectomy, when thyroid hormone levels were already normalized, but antithyroid antibodies were still increased. This clinical course resembles the clinical course of Graves’ orbitopathy.13,14 Studies13,14 showed that thyroidectomy is the most effective treatment in Graves’ orbitopathy independent of thyroid hormone status and antithyroid autoantibodies.
This phenomenon suggests that there might be a third pathomechanism causing psychiatric symptoms in Graves’ disease as well as in Graves’ orbitopathy beyond the effects of thyroid hormones and autoantibody levels. This phenomenon should be the objective of future studies.
References
1. Smith CK, Barish J, Correa J, et al. Psychiatric disturbance in endocrinologic disease. Psychosom Med. 1972;34(1):69-86. PubMed doi:10.1097/00006842-197201000-00008
2. Kathol RG, Delahunt JW. The relationship of anxiety and depression to symptoms of hyperthyroidism using operational criteria. Gen Hosp Psychiatry. 1986;8(1):23-28. PubMed doi:10.1016/0163-8343(86)90060-5
3. Stern RA, Robinson B, Thorner AR, et al. A survey study of neuropsychiatric complaints in patients with Graves’ disease. J Neuropsychiatry Clin Neurosci. 1996;8(2):181-185. PubMed doi:10.1176/jnp.8.2.181
4. Bunevicius R, Velickiene D, Prange AJ Jr. Mood and anxiety disorders in women with treated hyperthyroidism and ophthalmopathy caused by Graves’ disease. Gen Hosp Psychiatry. 2005;27(2):133-139. PubMed doi:10.1016/j.genhosppsych.2004.10.002
5. Bunevicius R, Prange AJ Jr. Psychiatric manifestations of Graves’ hyperthyroidism: pathophysiology and treatment options. CNS Drugs. 2006;20(11):897-909. PubMed doi:10.2165/00023210-200620110-00003
6. Katsigiannopoulos K, Georgiadou E, Pazarlis P, et al. Psychotic disorder as a manifestation of Graves’ disease. Psychosomatics. 2010;51(5):449-450. PubMed doi:10.1016/S0033-3182(10)70732-5
7. Hazen EP, Sherry NA, Parangi S, et al. Case records of the Massachusetts General Hospital. Case 10-2015: a 15-year-old girl with Graves’ disease and psychotic symptoms. N Engl J Med. 2015;372(13):1250-1258. PubMed doi:10.1056/NEJMcpc1314239
8. Brownlie BEW, Rae AM, Walshe JWB, et al. Psychoses associated with thyrotoxicosis: ‘ thyrotoxic psychosis:’ a report of 18 cases, with statistical analysis of incidence. Eur J Endocrinol. 2000;142(5):438-444. PubMed doi:10.1530/eje.0.1420438
9. Olmez I, Moses H, Sriram S, et al. Diagnostic and therapeutic aspects of Hashimoto’s encephalopathy. J Neurol Sci. 2013;331(1-2):67-71. PubMed doi:10.1016/j.jns.2013.05.009
10. Kirshner HS. Hashimoto’s encephalopathy: a brief review. Curr Neurol Neurosci Rep. 2014;14(9):476. PubMed doi:10.1007/s11910-014-0476-2
11. Yamamoto K, Shinba T, Yoshii M. Psychiatric symptoms of noradrenergic dysfunction: a pathophysiological view. Psychiatry Clin Neurosci. 2014;68(1):1-20. PubMed doi:10.1111/pcn.12126
12. Bunevicius R, Prange AJ Jr. Thyroid disease and mental disorders: cause and effect or only comorbidity? Curr Opin Psychiatry. 2010;23(4):363-368. PubMed doi:10.1097/YCO.0b013e3283387b50
13. De Bellis A, Conzo G, Cennamo G, et al. Time course of Graves’ ophthalmopathy after total thyroidectomy alone or followed by radioiodine therapy: a 2-year longitudinal study. Endocrine. 2012;41(2):320-326. PubMed doi:10.1007/s12020-011-9559-x
14. Menconi F, Leo M, Vitti P, et al. Total thyroid ablation in Graves’ orbitopathy. J Endocrinol Invest. 2015;38(8):809-815. PubMed doi:10.1007/s40618-015-0255-1
aDepartment of Psychiatry and Psychotherapy, Heidelberg University Hospital, Germany
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Permission was obtained from the patient to present the case, and the information was de-identified to protect anonymity.
Published online: August 31, 2017.
Prim Care Companion CNS Disord 2017;19(4):16l02081
https://doi.org/10.4088/PCC.16l02081
© Copyright 2017 Physicians Postgraduate Press, Inc.
Enjoy this premium PDF as part of your membership benefits!