“I Can Hear the Noise, But Not the Voice”—A Case of Bilateral Sylvian Fissure Infarct in a 52-Year-Old Man
To the Editor: Bilateral Sylvian fissure infarct is rare, and only a small percentage of the population with such infarct present with pure expressive and receptive aphasia without any focal neurologic deficits. We report a rare case of bilateral Sylvian fissure infarct in an adult male patient.
Case report. Mr A, a 52-year-old white man with known history of type 2 diabetes mellitus, smoking, and noncompliance with medications, was brought to the emergency department in 2009 by his wife, who described him as unresponsive to verbal commands. The patient was found on the floor after, probably, cutting the grass with a manual lawnmower. While asleep, he was crying intermittently while speaking loudly, “It will be alright! You take care of the kids.”
On examination at the emergency department, he was disoriented to time and place. Confusion with global aphasia was noted first and was later found to be pure word deafness with expressive and receptive aphasia. He also was apyrexial. Findings of cardiovascular, respiratory, and gastrointestinal system examinations were unremarkable. Neurologic examination showed normal power and tone in both arms and legs as well as normal symmetrical deep tendon reflexes. His gait and coordination were normal, and cranial nerve and fundus examinations revealed no abnormalities with the exception of optic atrophy.
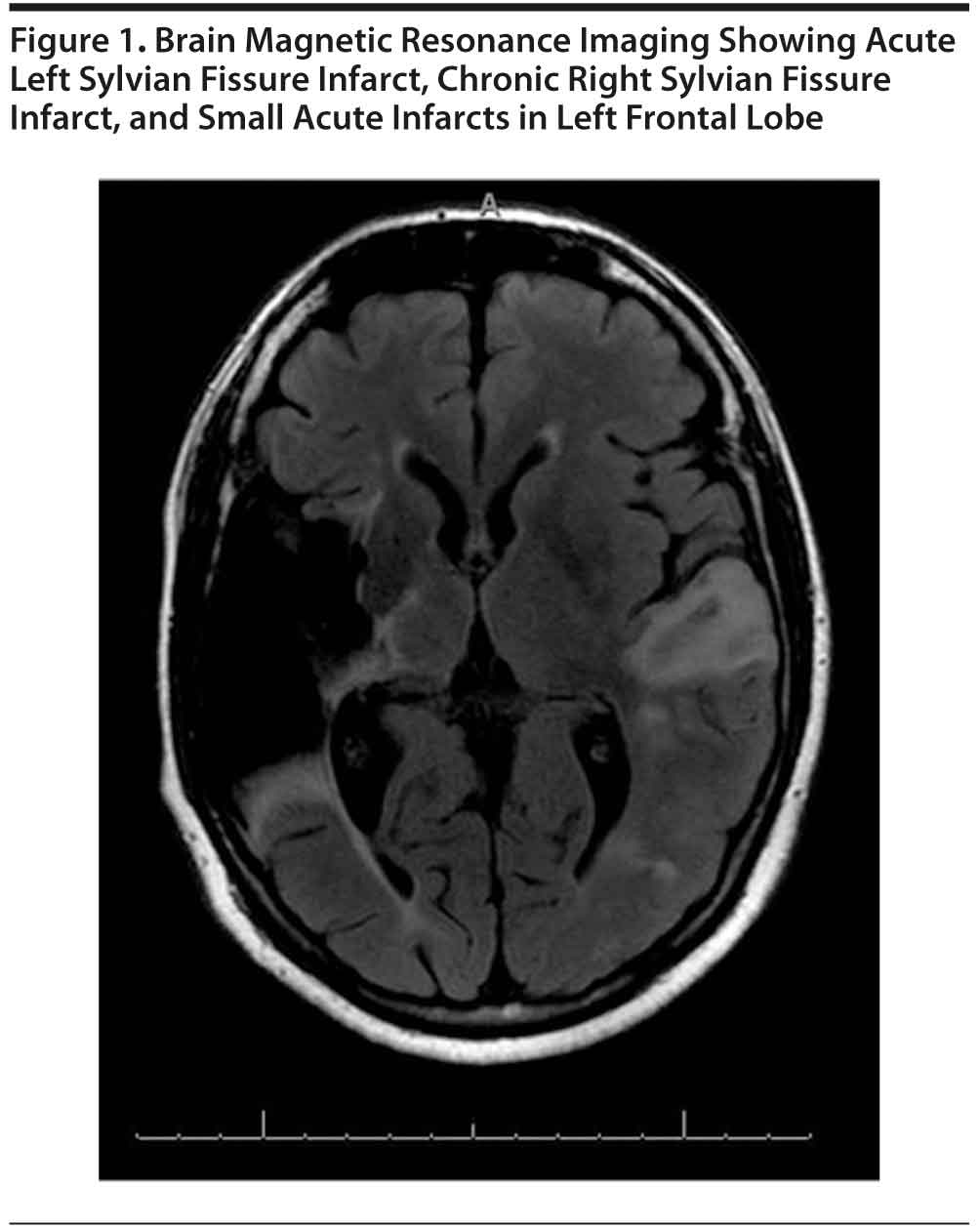
Investigations revealed a complete blood cell count within normal limits and unremarkable inflammatory markers, with routine biochemical tests showing a clinically significantly high blood glucose level. Findings of laboratory tests generally ordered for complete stroke evaluation in young adults, including measurements of erythrocyte sedimentation rate, plasma homocysteine, blood peripheral antineutrophil cytoplasmic antibodies, serum creatine kinase-MB fraction, serum cardiolipin levels, antinuclear antibodies, and lipid profile, were not clinically significant. Contrast-enhanced computed tomography of the brain was negative for acute intracranial hemorrhage, cortical infarct, or mass effect, but extensive encephalomalacia within the right middle cerebral artery territory was found. Brain magnetic resonance imaging (Figure 1) showed acute left Sylvian fissure infarct, chronic right Sylvian fissure infarct, and small acute infarcts in the left frontal lobe. Findings of transthoracic echocardiogram and carotid Doppler were negative except for age-related changes.
Clinically, the patient continued to show confusion in the first 48 hours of hospitalization, and on the second day he had an episode of agitation and was given haloperidol 2 mg every 4 hours that was later tapered. We also found that the patient had once had a similar poststroke agitation episode after a previous stroke. After 3 days, the patient’s only disability associated with stroke was expressive and receptive aphasia. Consults for speech pathology, occupational therapy, and physical therapy were placed as a part of stroke rehabilitation. Physical therapy and occupational therapy were not recommended due to patient’s visible functionality to perform day-to-day activities. The speech pathologists were at first confused regarding the diagnosis of global aphasia with receptive and expressive aphasia because the patient could understand most written communication but could not comprehend and understand verbal instructions. The patient also did not meet the full criteria for global or Wernicke’s aphasia. The patient experienced further difficulty understanding verbal as well as written instructions as the days passed and with increased length of speech therapy sessions. His performance was deteriorating with longer sentences, so the treatment team decided to keep written messages short and succinct and to not use verbal messages.
The patient was transferred from acute medicine to a long-term care facility for further rehabilitation, where he continued to receive speech therapy and also recreation therapy. There was some disagreement about the patient’s short-term memory, but later it was found to be intact, evidenced by his quick learning of written communication. He successfully communicated with written messages and replied verbally. The patient’s paraphasia and neologism improved with time, but he continued to show some paraphasic errors with neologism.
We found 638 articles in MEDLINE with full-text search with the search term Sylvian fissure1; these articles mostly involved Sylvian fissure hemorrhage due to middle cerebral artery aneurysm, arachnoid cyst of Sylvian fissure, and metastatic injury to Sylvian fissure. We found another 6 cases of pure word deafness using the search terms pure word deafness and bilateral primary auditory cortex. The initial presentation is extensive like global aphasia or Wernicke’s aphasia, but later the patient will be left with pure word deafness. Pure word deafness without elements of other focal neurologic deficits is a rare disorder and was first articulated by Kussmaul in 1877.2 It is also described as “verbal auditory agnosis” or “auditory agnosis for linguistic material.” It is even rarer in its pure form3 and is usually explained by other deafness. Etiologic factors are mostly vascular, but it can be caused by tumor, trauma, or infections.4 In our case, etiologic factors could be poorly controlled diabetes mellitus and hypertension without evidence of infarct. Pathogenesis involved dissociation of Broca’s and Wernicke’s areas.5
There are 2 different types of pure word deafness according to Auerbach et al6: type 1, with bilateral temporal lobe lesions, in which the deficit is prephonemic and related to a temporal auditory acuity disorder; and type 2, with only left unilateral lesions, a form that is independent of temporal auditory acuity disorder and has a deficit in linguistic discrimination that does not adhere to a prephonemic pattern. The first form is an apperceptive disorder, whereas the second form represents a higher disorder in phonemic discrimination and may be considered a fragment of Wernicke’s aphasia. Our patient demonstrated symptoms related to type 1 pure word deafness.
In our case, comprehension and repetition of speech were impaired, but reading, writing, and parts of spontaneous speech were preserved. Environmental sounds differentiate pure word deafness from global aphasia; still, pure word deafness in its “pure” form is rare. Some authors claim that their patients could recognize music and musical instruments.7 Our patient did not recognize environmental sounds or music. Speech comprehension is done by the primary auditory cortex, but comprehension of affective prosody and nonverbal sounds may be done by the auditory association cortex.4
Although rare, Sylvian fissure infarct should be suspected in patients with expressive and receptive aphasia as well as pure word deafness.
References
1. Ulrich G. Interhemispheric functional relationships in auditory agnosia: an analysis of the preconditions and a conceptual model. Brain Lang. 1978;5(3):286-300. PubMed doi:10.1016/0093-934X(78)90027-5
2. Bauer RM, Rubens AB. Pure word deafness. In: Heilman KM, Valenstein E, eds. Clinical Neuropsychology. 2nd ed. New York, NY: Oxford University Press; 1985:210-213.
3. Geschwind N. Disconnexion syndromes in animals and man. Brain. 1965;88(2):237-294, 585-644. PubMed doi:10.1093/brain/88.2.237
4. Coslett HB, Brashear HR, Heilman KM. Pure word deafness after bilateral primary auditory cortex infarcts. Neurology. 1984;34(3):347-352. PubMed
5. Pinard M, Chertkow H, Black S, et al. A case study of pure word deafness: modularity in auditory processing? Neurocase. 2002;8(1-2):40-55. PubMed doi:10.1093/neucas/8.1.40
6. Auerbach SH, Allard T, Naeser M, et al. Pure word deafness: analysis of a case with bilateral lesions and a defect at the prephonemic level. Brain. 1982;105(pt 2):271-300. PubMed doi:10.1093/brain/105.2.271
7. Yaqub BA, Gascon GG, Al-Nosha M, et al. Pure word deafness (acquired verbal auditory agnosia) in an Arabic speaking patient. Brain. 1988;111(pt 2):457-466. PubMed doi:10.1093/brain/111.2.457
Author affiliations: Carilion Clinic, Virginia Tech-Carilion School of Medicine Psychiatry Residency Program, Roanoke (Dr Sharma); and Veterans Affairs Medical Center, Salem (Ms Riddle), Virginia.
Potential conflicts of interest: None reported.
Funding/support: None reported.
Previous presentation: Presented at the Medical Grand Rounds at the Veterans Affairs Medical Center; October 23, 2009; Salem, Virginia.
Published online: January 5, 2012.
© Copyright 2012 Physicians Postgraduate Press, Inc
Prim Care Companion CNS Disord 2012;14(1):doi:10.4088/PCC.11l01159.