Increased use of the opioid-related plant kratom as an alternative treatment for opioid withdrawal symptoms has raised concerns regarding its potential for abuse and severe adverse effects. A review of the literature was performed to characterize kratom’s pharmacology, clinical efficacy, and adverse effects to increase understanding and evaluate potential use as an alternative treatment for opioid dependence. Kratom use initiated as self-medication for an opioid use disorder or pain syndrome in the absence of effective alternatives is associated with a risk of kratom dependence, withdrawal, and life-threatening toxicity. The potential for a serious adverse reaction should discourage unregulated use of kratom products.
ABSTRACT
Increased use of the opioid-related plant kratom as an alternative treatment for opioid withdrawal symptoms has raised concerns regarding its potential for abuse and severe adverse effects. A review of the literature was performed to characterize kratom’s pharmacology, clinical efficacy, and adverse effects to increase understanding and evaluate potential use as an alternative treatment for opioid dependence. Kratom use initiated as self-medication for an opioid use disorder or pain syndrome in the absence of effective alternatives is associated with a risk of kratom dependence, withdrawal, and life-threatening toxicity. The potential for a serious adverse reaction should discourage unregulated use of kratom products.
Prim Care Companion CNS Disord 2020;22(1):19nr02507
To cite: Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22(1):19nr02507.
To share: https://doi.org/10.4088/PCC.19nr02507
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Addiction Psychiatry, University of Kansas Health System, Kansas City, Kansas
bDepartment of Psychiatry and Behavioral Sciences, University of Kansas Medical Center, Kansas City, Kansas
cSchool of Medicine, University of Kansas Medical Center, Kansas City, Kansas
*Corresponding author: Roopa Sethi, MD, Department of Psychiatry and Behavioral Sciences, University of Kansas Medical Center, 3901 Rainbow Blvd, Mail Stop 4015, Kansas City, KS 66160 ([email protected]).
Kratom is a psychoactive substance derived from the Mitragyna speciosa plant species native to Thailand and Southeast Asia,1 with stimulant-like effects at lower doses and opioid-like effects at higher doses.2–4 It has gained popularity in the United States as an inexpensive alternative for self-withdrawal treatment from opiates. Kratom is sold as capsules, tablets, loose raw leaves, gum, and powder through online vendors and at local smoke shops, bars, and gas stations. It is sold as kratom, mitragyna, and other street names such as ketum, thom, biak, and thang.2,3,5–7 Kratom is also available as green powder sold with the label “Not for human consumption.”7 Kratom has been used with O-desmethyltramadol, which is marketed as krypton,3,8 and may be added to synthetic cannabinoids such as K2 and spice.3,9 Kratom does not appear in regular urine drug screens and may be undetected during routine screening of acute drug intoxication, overdose, or withdrawal, as careful history is required for identification.2,3 There has been little scientific investigation to characterize kratom’s pharmacology, mechanism of action, side effects, or risk of use. Kratom has been designated as illegal in several countries. It remains unrestricted in the United States but is listed as a “drug of concern” by the US Drug
Enforcement Administration. This narrative review provides a survey of literature related to kratom’s clinical profile based on available empirical evidence and case reports.
METHODS
A literature search was performed using 4 databases (MEDLINE, PubMed, CINAHL, and Google Scholar) to identify English-language articles published from January 2000 to March 2019. Search terms were kratom, opioid withdrawal, opioid use disorder, opioid dependence, and mitragyna. Studies were selected on the basis of novel information related to kratom’s pharmacology, mechanism of action, clinical presentation, and adverse effects. Surveys and case reports were included given that kratom is not used clinically and the majority of kratom use is for recreational purposes. Commentaries were excluded. A total of 45 articles1–45 were included in the final review.
results
Kratom Pharmacology
The psychoactive properties of kratom are mainly attributed to mitragynine and 7-hydroxymitragynine (7-OHMG).10–12 Both compounds act through g-protein–coupled signaling with partial agonist effects on μ-opioid receptors and antagonist effects on the κ- and δ-opioid receptors.10–12 The partial agonist activity at the μ-opioid receptor is believed to mitigate risks for respiratory depression in the event of overdose.12 According to the literature,2,3,13–16 kratom can prevent withdrawal symptoms from opioids but also has the capacity to induce its own withdrawal symptoms. Kratom has anti-inflammatory properties and has demonstrated parasympathetic-blocking effects.3 These effects were reversible by use of naloxone, suggesting that they are mediated by opioid agonist actions.3,17
Mitragynine stimulates α2-adrenergic receptors proposed to accentuate its sedative, hypnotic, and analgesic effects without increasing respiratory depression. Although this synergistic effect may be considered beneficial for opioid withdrawal therapy, the profile yields a potent central nervous system depressant effect that could be dangerous when combined with other sedative and hypnotic agents, such as opioids, alcohol, benzodiazepines, muscle relaxants, and anticonvulsants.2,3,12,18,19 Mitragynine has additional effects on adenosine 2, dopamine, and 5-HT7 receptors that are not fully characterized.12,18
Adverse Effects of Kratom
The Centers for Disease Control and Prevention reported 660 calls to US poison control centers related to kratom use from 2010 to 2015.20 The number of calls increased from 26 in 2010 to 263 in 2015, with 496 calls received from health care providers seeking guidance. Isolated kratom use was noted in 428 of these cases.20 Kratom use is reported in combination with other substances most commonly cited as benzodiazepines, narcotics, and acetaminophen.20,21
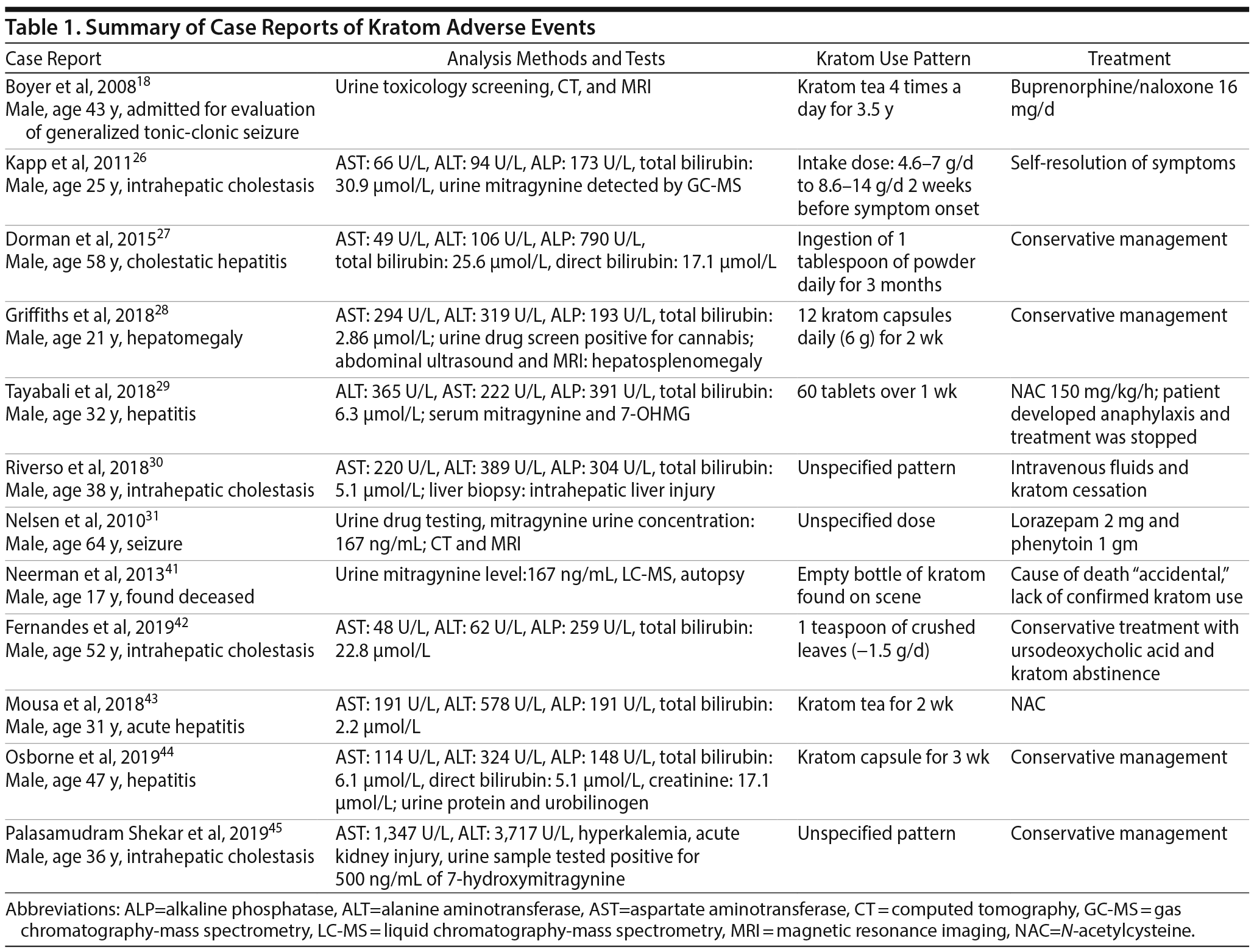
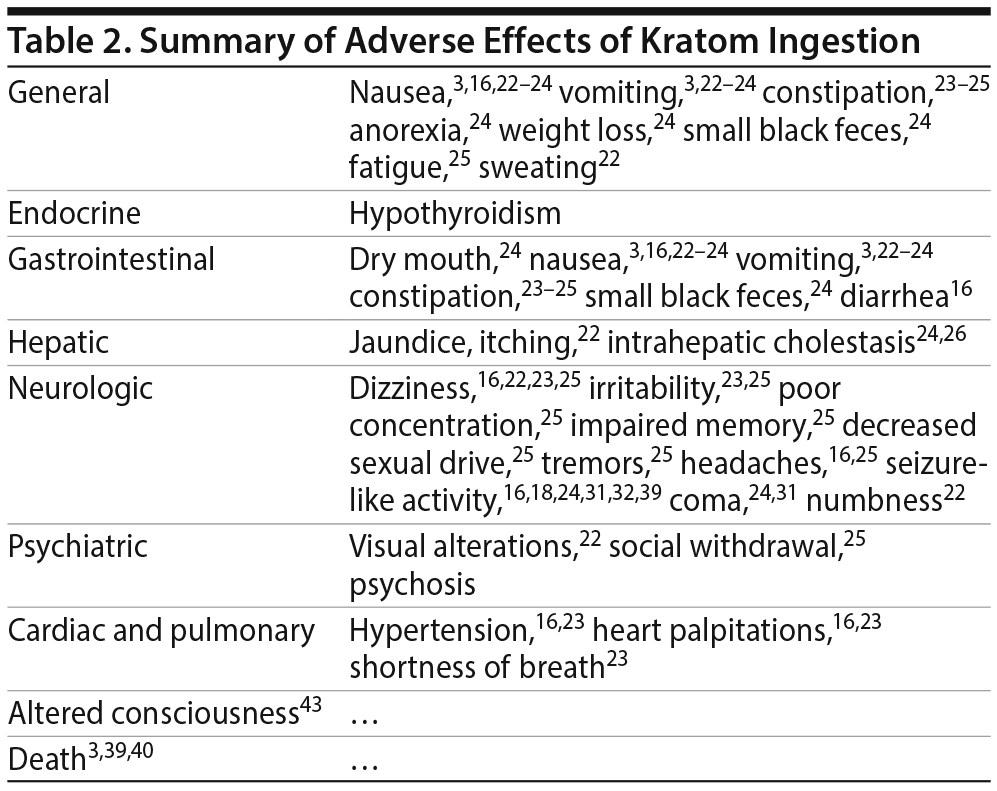
There are several published case reports18,26–31,41–45 about the side effects and withdrawal symptoms associated with cessation of kratom use and how to successfully treat those withdrawal symptoms (Table 1). These case reports are of interest, as they can be helpful in clinical scenarios for which there is no guidance on how to treat kratom overdose and withdrawal. The most commonly reported adverse effects include nausea, vomiting, tremor, diaphoresis, tachycardia, hypertension, and elevated creatinine phosphokinase concentrations3,16,22–25 (Table 2). Treatment for adverse effects includes use of intravenous fluids, sedatives, benzodiazepines, antiemetics, and, in rare cases, antihypertensives and oxygen.3 A total of 36 deaths have been reported with illicit use of kratom-containing products. Nine deaths have been linked to krypton use (kratom and O-desmethyltramadol) and 1 death to kratom combined with propylhexedrine.3
Several reports26–30 have identified severe liver injury and intrahepatic cholestasis with kratom. The first reported case26 of kratom-induced hepatoxicity was in 2011 describing a 25-year-old man who developed abdominal pain and jaundice 10 days after use of kratom. His symptoms resolved after cessation of kratom intake.26 Swogger et al22 reported 2 separate cases: a female who developed acute jaundice following consumption of kratom and a male who presented with acute hepatitis after 2 weeks of kratom use. Other examples include a 58-year-old chronic kratom user who developed jaundice and hepatotoxicity,27 a 21-year-old man with 1-month history of kratom use who presented with hepatosplenomegaly,28 a 32-year-old man with cholestatic hepatitis after 2 weeks of kratom use,29 and a 38-year-old man with an acute onset of kratom-induced hepatitis.30 There are also case reports31,32 that document seizures as an adverse effect of kratom use.
Kratom Withdrawal Symptoms After Cessation of Use
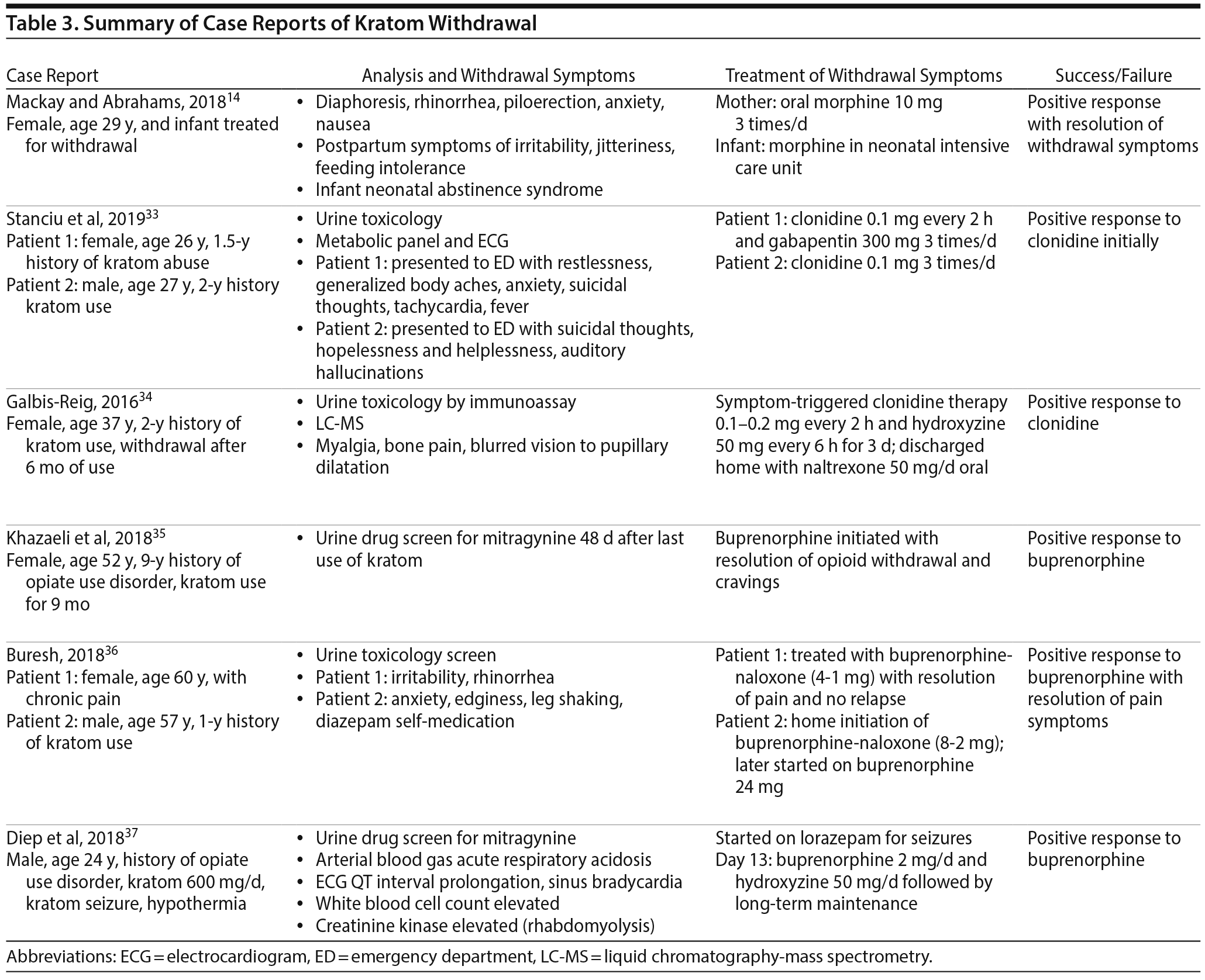
Prolonged use of kratom is associated with physiologic dependence capable of precipitating withdrawal symptoms with decreased use or abstinence. The literature suggests that withdrawal symptoms from cessation of kratom use are similar to opioid-like withdrawal symptoms (Table 3).14,33–37 These opioid-like withdrawal symptoms were mitigated with naloxone,3 clonidine,33,34 and buprenorphine.35,36
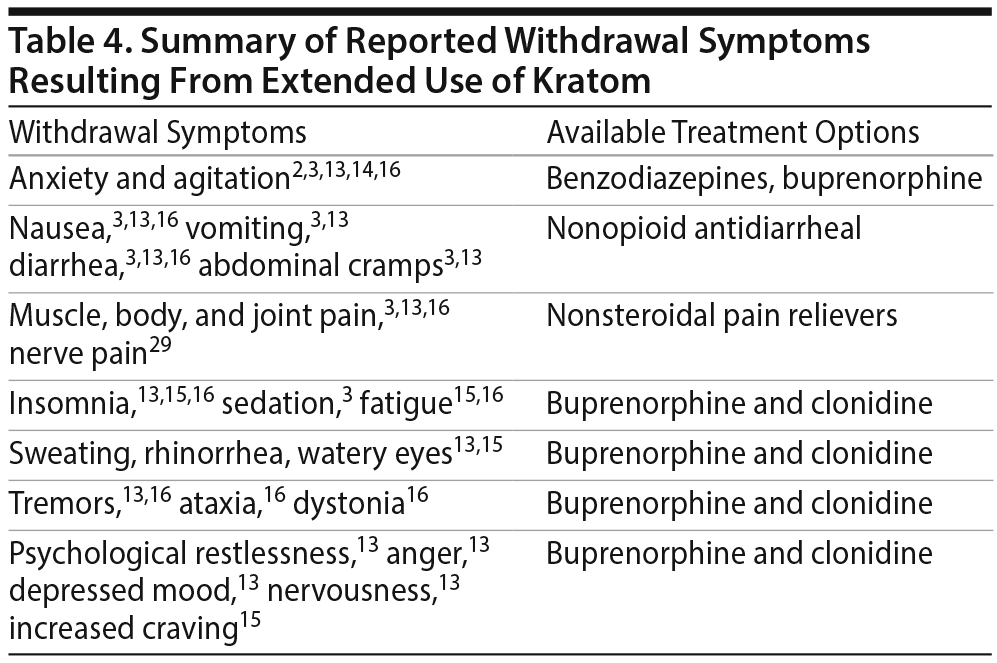
Several case reports document withdrawal symptoms after kratom cessation such as severe muscle spasms, myalgia, chronic pain, sleeping difficulty, and intense cravings (Table 4). Many individuals reported that these symptoms and the intense craving made them unable to discontinue use of kratom.13,15 Some case reports3,33–36 describe successful treatment with different medications. Buresh36 presented a case series featuring successful treatment of kratom dependence in 2 patients with the initiation of buprenorphine treatment. Khazaeli et al35 reported another case of a patient with opioid use disorder who self-medicated with kratom and became addicted. Her kratom withdrawal symptoms were successfully treated with buprenorphine. The authors35 recommend divided dosing of buprenorphine to maximize analgesia for patients who have used kratom to manage pain. Diep et al37 reported successful treatment of kratom withdrawal with associated seizures using buprenorphine. Mackay and Abrahams14 illustrated the case of a mother and neonate whose kratom withdrawal symptoms were successfully treated with morphine. Galbis-Reig34 described successful treatment of kratom withdrawal symptoms in a female patient with clonidine and hydroxyzine. Stanciu et al33 presented a case series of 2 patients successfully treated for kratom withdrawal with clonidine treatment.
Ecologic Factors Influencing Kratom Use
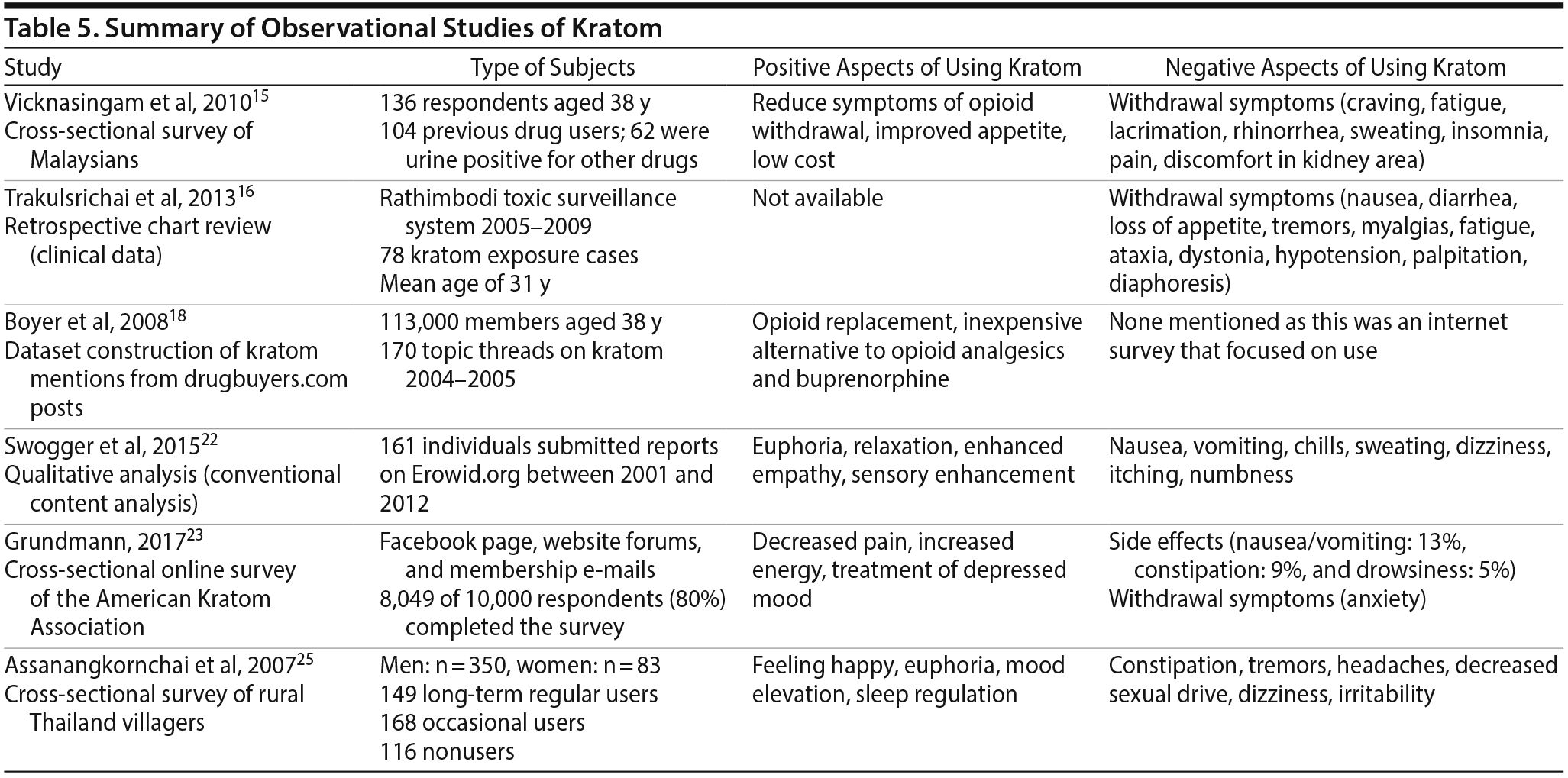
Most human studies15,16,18,22,23,25 on kratom utilize observational survey designs and originate in Malaysia and Thailand, with very few surveys from the United States (Table 5). Vicknasingam et al15 conducted an anonymous survey to assess patterns of ketum (kratom) use in Malaysia and effects reported by current users. A total of 136 respondents with a mean age of 38 years were stratified into short-term (53%) and long-term (47%) kratom users. Both groups reported using kratom to reduce addiction to other drugs and use of more expensive opioids (90.4%) and to treat symptoms of opioid withdrawal (83.8%). They reported benefits including increased propensity for hard work, heightened sexual desire, increased appetite, and reduced heroin withdrawal symptoms. Side effects from kratom included weight loss, dehydration, constipation, tiredness, and hyperpigmentation of cheeks. Seventy-eight percent of respondents reported symptoms of fatigue, lacrimation, rhinorrhea, insomnia, diaphoresis, and nerve pain when they stopped taking kratom.15 This finding contrasts that of a prior study25 in Thailand of an adult (mean age = 45 years) population with no evidence of previous drug use that identified increased physical endurance as a key motivational influence for use. A second survey13 in Malaysia evaluated the frequency of dependence, cravings, and withdrawal symptoms in males taking kratom for longer than 6 months. Of the 293 respondents, clinically meaningful dependence was identified in 100% of respondents, with 55% reporting severe and 45% reporting moderate dependence. Sixty-five percent of respondents experienced mild withdrawal symptoms, while 35% reported moderate-to-severe symptoms. Further, significant cravings for kratom were reported in 77% of kratom users, while 23% had only mild cravings.13 The consensus findings identified high rates of dependence associated with kratom use but limited impact on social functioning in this setting.13
A qualitative analysis by Swogger et al22 described 161 individuals who submitted their own experience with kratom on the website Erowid.org. Erowid is a nonprofit organization that provides online informational resources about psychoactive substances.22 Positive experiences reported by kratom users included relaxation, euphoria, state of well-being, pain relief, and enhanced empathy. Seventeen individuals reported successful use of kratom as replacement for unwanted substance use, especially opioids. Common reported adverse effects were nausea (16%) and withdrawal symptoms (10%), though the symptoms were milder compared to opiates. Although uncommon, visual alterations and sedation were noted, but many individuals reported benefits from milder withdrawal symptoms and stimulant effects, which appeared to drive the motivational attributes associated with use. Swogger and colleagues22 concluded that kratom could potentially be used as adjunctive therapy for pain relief with less cognitive impairment and less sedation, but further research on safety and administration guidelines is needed.
A large cross-sectional online survey23 (N = 8,049 respondents) of members of the American Kratom Association found kratom use was primarily associated with the self-treatment of acute and chronic pain and symptoms of opioid withdrawal. More than 80% of respondents reported decreased pain, increased energy, and less depressive mood with kratom. Furthermore, 49% of users stopped or reduced their use of opiates. The adverse effects most commonly reported with kratom use were nausea (13%), constipation (9%), and dizziness or drowsiness (5%). The study23 also identified a dose-dependent relationship with both beneficial and adverse effects of kratom.
Other reports3,38 have documented therapeutic use of kratom to prevent opioid withdrawal. One report38 presented the case of a 53-year-old man with a history of opioid use disorder recently tapered off methadone after 6 years of maintenance therapy who started using kratom to control his withdrawal symptoms and prevent a relapse. Another published report3 described use of kratom to treat 2 individuals with a long history of heroin use. One patient did not have access to methadone and the other patient had persistent withdrawal symptoms despite methadone treatment. Both patients reported improvement of their symptoms after they started using kratom.3
CONCLUSION
The increasing popularity of kratom has been accompanied by dependence, adverse effects, and withdrawal symptoms following cessation of use. Use may be partly attributed to increasing costs of opioids, the era of the current opioid epidemic and reduction in prescriptions of opiates, online availability, and insufficient public awareness of potential side effects and propensity to experience withdrawal symptoms on cessation. One weakness of our analysis is the inclusion of anecdotal reports of kratom obtained through survey and analysis of online forums, which put the findings at risk for potential bias and other confounding factors.
Kratom’s unique dual property as an opioid with stimulant properties makes it a potential alternative in the management of opioid withdrawal symptoms. Review of the literature suggests a high potential for abuse and dependence with kratom that may outweigh the potential benefits. The risk of increased central nervous system depression with concomitant sedative hypnotics and alcohol makes it a drug of concern. Also, reports26–30,42,43 have shown an association between liver injury and kratom ingestion, supporting the need for controlled trials to further evaluate the safety and efficacy of kratom.
Because of the rise in kratom use in Western countries, medical providers should be aware of the effects of kratom. Physicians should also be aware of the withdrawal symptoms and need for treatment. Although data on kratom are of low quality, the findings from this study may provide a better understanding of the effects of kratom consumption in humans. More susceptible populations including those with compromised liver function or history of prior addiction as well as young adults and adolescents should be cautious when using this unregulated substance.
Clinical Points
- Kratom has gained popularity as an inexpensive and accessible alternative to manage symptoms of opioid use disorder and pain.
- Kratom use can lead to physical dependence and precipitate withdrawal symptoms with abstinence.
- Even short-term kratom use has been associated with serious medical comorbidities including hepatitis, seizures, and death.
Submitted: June 28, 2019; accepted September 30, 2019.
Published online: January 30, 2020.
Potential conflicts of interest: None.
Funding/support: None.
REFERENCES
1. Grewal KS. The effect of mitragynine on man. Br J Med Psychol. 1932;12(1):41–58. CrossRef
2. Prozialeck WC, Jivan JK, Andurkar SV. Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792–799. PubMed
3. White CM. Pharmacologic and clinical assessment of kratom. Am J Health Syst Pharm. 2018;75(5):261–267. PubMed
4. Babu KM, McCurdy CR, Boyer EW. Opioid receptors and legal highs: salvia divinorum and kratom. Clin Toxicol (Phila). 2008;46(2):146–152. PubMed CrossRef
5. MacLaren E. The effects of kratom use. drugabuse.com website. www.drugabuse.com/library/ the-effects-of-kratom-use/. Accessed December 17, 2019.
6. Kratom. US Drug Enforcement Administration website. https://www.dea.gov/factsheets/kratom. Accessed December 17, 2019.
7. National Institute on Drug Abuse. Monitoring the future study: trends in prevalence of various drugs. https://www.drugabuse.gov/trends-statistics/monitoring-future/monitoring-future-study-trends-in-prevalence-various-drugs. Accessed December 17, 2019.
8. Arndt T, Claussen U, Güssregen B, et al. Kratom alkaloids and O-desmethyltramadol in urine of a “Krypton” herbal mixture consumer. Forensic Sci Int. 2011;208(1–3):47–52. PubMed CrossRef
9. Logan BK, Reinhold LE, Xu A, et al. Identification of synthetic cannabinoids in herbal incense blends in the United States. J Forensic Sci. 2012;57(5):1168–1180. PubMed CrossRef
10. Farrell M. Opiate withdrawal. Addiction. 1994;89(11):1471–1475. PubMed CrossRef
11. Matsumoto K, Horie S, Takayama H, et al. Antinociception, tolerance and withdrawal symptoms induced by 7-hydroxymitragynine, an alkaloid from the Thai medicinal herb Mitragyna speciosa. Life Sci. 2005;78(1):2–7. PubMed CrossRef
12. Kruegel AC, Grundmann O. The medicinal chemistry and neuropharmacology of kratom: a preliminary discussion of a promising medicinal plant and analysis of its potential for abuse. Neuropharmacology. 2018;134(pt A):108–120. PubMed CrossRef
13. Singh D, Müller CP, Vicknasingam BK. Kratom (Mitragyna speciosa) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132–137. PubMed CrossRef
14. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121–122. PubMed
15. Vicknasingam B, Narayanan S, Beng GT, et al. The informal use of ketum (Mitragyna speciosa) for opioid withdrawal in the northern states of peninsular Malaysia and implications for drug substitution therapy. Int J Drug Policy. 2010;21(4):283–288. PubMed CrossRef
16. Trakulsrichai S, Tongpo A, Sriapha C, et al. Kratom abuse in Ramathibodi Poison Center, Thailand: a five-year experience. J Psychoactive Drugs. 2013;45(5):404–408. PubMed CrossRef
17. Hassan Z, Muzaimi M, Navaratnam V, et al. From kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neurosci Biobehav Rev. 2013;37(2):138–151. PubMed CrossRef
18. Boyer EW, Babu KM, Adkins JE, et al. Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth). Addiction. 2008;103(6):1048–1050. PubMed CrossRef
19. Giovannitti JA Jr, Thoms SM, Crawford JJ. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog. 2015;62(1):31–39. PubMed CrossRef
20. Anwar M, Law R, Schier J. Notes from the field: kratom (Mitragyna speciosa) exposures reported to poison centers—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65(29):748–749. PubMed CrossRef
21. Griffin OH, Webb ME. The scheduling of kratom and selective use of data. J Psychoactive Drugs. 2018;50(2):114–120. PubMed CrossRef
22. Swogger MT, Hart E, Erowid F, et al. Experiences of kratom users: a qualitative analysis. J Psychoactive Drugs. 2015;47(5):360–367. PubMed CrossRef
23. Grundmann O. Patterns of kratom use and health impact in the US: results from an online survey. Drug Alcohol Depend. 2017;176:63–70. PubMed CrossRef
24. Ulbricht C, Costa D, Dao J, et al. An evidence-based systematic review of kratom (Mitragyna speciosa) by the Natural Standard Research Collaboration. J Diet suppl. 2013;10(2):152–170. PubMed CrossRef
25. Assanangkornchai S, Muekthong A, Sam-Angsri N, et al. The use of Mitragynine speciosa (“krathom”), an addictive plant, in Thailand. Subst Use Misuse. 2007;42(14):2145–2157. PubMed CrossRef
26. Kapp FG, Maurer HH, Auwärter V, et al. Intrahepatic cholestasis following abuse of powdered kratom (Mitragyna speciosa). J Med Toxicol. 2011;7(3):227–231. PubMed CrossRef
27. Dorman C, Wong M, Khan A. Cholestatic hepatitis from prolonged kratom use: a case report. Hepatology. 2015;61(3):1086–1087. PubMed CrossRef
28. Griffiths CL, Gandhi N, Olin JL. Possible kratom-induced hepatomegaly: a case report. J Am Pharm Assoc (2003). 2018;58(5):561–563. PubMed CrossRef
29. Tayabali K, Bolzon C, Foster P, et al. Kratom: a dangerous player in the opioid crisis. J Community Hosp Intern Med Perspect. 2018;8(3):107–110. PubMed CrossRef
30. Riverso M, Chang M, Soldevila-Pico C, et al. Histologic characterization of kratom use-associated liver injury. Gastroenterol Res. 2018;11(1):79–82. PubMed CrossRef
31. Nelsen JL, Lapoint J, Hodgman MJ, et al. Seizure and coma following kratom (Mitragyna speciosa korth) exposure. J Med Toxicol. 2010;6(4):424–426. PubMed CrossRef
32. Roche KM, Hart K, Sangalli B, et al. Kratom: a case of a legal high. Clinical Toxicology. 2008;46(7):598.
33. Stanciu CN, Gnanasegaram SA, Ahmed S, et al. Kratom withdrawal: a systematic review with case series. J Psychoactive Drugs. 2019;51(1):12–18. PubMed CrossRef
34. Galbis-Reig D. A case report of kratom addiction and withdrawal. WMJ. 2016;115(1):49–52, quiz 53. PubMed
35. Khazaeli A, Jerry JM, Vazirian M. Treatment of kratom withdrawal and addiction with buprenorphine. J Addict Med. 2018;12(6):493–495. PubMed CrossRef
36. Buresh M. Treatment of kratom dependence with buprenorphine-naloxone maintenance. J Addict Med. 2018;12(6):481–483. PubMed CrossRef
37. Diep J, Chin DT, Gupta S, et al. Kratom, an emerging drug of abuse: a case report of overdose and management of withdrawal. A A Pract. 2018;10(8):192–194. PubMed CrossRef
38. Sethi R, Miller KA, McAllister R. Kratom: is it the new illicit opiate on the market? Prim Care Companion CNS Disord. 2018;20(4):15l01895. PubMed CrossRef
39. Bäckstrom BG, Classon G, Löwenhielm P, et al. Krypton—new, deadly internet drug: since October 2009 have nine young persons died in Sweden [article in Swedish]. Lakartidningen. 2010;107(50):3196–3197. PubMed
40. Kronstrand R, Roman M, Thelander G, et al. Unintentional fatal intoxications with mitragynine and O-desmethyltramadol from the herbal blend krypton. J Anal Toxicol. 2011;35(4):242–247. PubMed CrossRef
41. Neerman MF, Frost RE, Deking J. A drug fatality involving kratom. J Forensic Sci. 2013;58(suppl 1):S278–S279. PubMed CrossRef
42. Fernandes CT, Iqbal U, Tighe SP, et al. Kratom-induced cholestatic liver injury and its conservative management. J Investig Med High Impact Case Rep. 2019;7:2324709619836138. PubMed
43. Mousa MS, Sephien A, Gutierrez J, et al. N-acetylcysteine for acute hepatitis induced by kratom herbal tea. Am J Ther. 2018;25(5):e550–e551. PubMed CrossRef
44. Osborne CS, Overstreet AN, Rockey DC, et al. Drug-induced liver injury caused by kratom use as an alternative pain treatment amid an ongoing opioid epidemic. J Investig Med High Impact Case Rep. 2019;7:2324709619826167. PubMed
45. Palasamudram Shekar S, Rojas EE, D’Angelo CC, et al. Legally lethal kratom: a herbal supplement with overdose potential. J Psychoactive Drugs. 2019;51(1):28–30. PubMed CrossRef
Enjoy this premium PDF as part of your membership benefits!