Meta-Analysis of Suicidality in Placebo-Controlled Clinical Trials of Adults Taking Bupropion
Objective: To assess a possible relationship between treatment with bupropion (vs placebo) and expressed suicidal ideation and behavior.
Data Sources: This analysis, based on the US Food and Drug Administration’s (FDA’s ) analysis of antidepressant suicidality data, included 8,953 adult subjects receiving bupropion and 6,520 adult subjects receiving placebo from randomized, placebo-controlled trials with bupropion conducted between 1976 and 2006 across multiple indications, including major depressive disorder (MDD). A text string search of the adverse event database and case report form comments was performed to identify potential suicidal events. FDA search criteria included the following text strings: accident-, injur-, suic-, or overdos-, including all events coded as accidental overdose, attempt, cut, gas, hang, hung, jump, mutilat-, self damag-, self harm, self inflict-, shoot, slash, poison, asphyxiation, suffocation, firearm, burn, drown, gun, immolat-, and monoxide, and the following terms were added by GlaxoSmithKline to the search criteria: accident, lacerat-, MVA, and hospital. The database search included data beginning from the first dose of study medication through 1 day following the last dose.
Data Extraction: Suicidal event narratives were classified using the Columbia Classification Algorithm for Suicide Assessment. Additionally, changes on rating scale items for depressed mood and suicidality were analyzed.
Data Synthesis: In the MDD population, the incidence of suicidal behavior or ideation was 17/3,179 (0.53%) versus 11/2,310 (0.48%) for the bupropion and placebo groups, respectively (OR = 1.28; 95% CI, 0.59-2.86). For suicidal behavior, the incidence was 8/3,179 (0.25%) versus 2/2,310 (0.09%), respectively (OR = 3.52; 95% CI, 0.81-24.48). No suicidal behavior event was noted in the other indications, and no completed suicides were reported during treatment. No significant worsening was observed for bupropion relative to placebo on the rating scale items. No differential treatment effects were observed by gender or age; regardless of treatment, however, the 18- to 24-year-old group had the greatest odds of having a suicide event.
Conclusions: Although no statistically significant differences were observed between bupropion and placebo in expressed suicidal ideation or behavior, we believe that all patients treated with antidepressants should receive careful monitoring for clinical worsening, suicidality, or unusual changes in behavior.
Prim Care Companion J Clin Psychiatry 2010;12(5):e1-e8
© Copyright 2010 Physicians Postgraduate Press, Inc.
Submitted: September 23, 2009; accepted December 29, 2009.
Published online: September 23, 2010 (doi:10.4088/PCC.09m00894blu).
Corresponding author: Jack G. Modell, MD, GlaxoSmithKline, 5.5812, Research Triangle Park, NC 27709-3398 ([email protected]).
Suicide was the 11th leading cause of death in the United States in 2006,1 with virtually all mental disorders posing an increased risk of suicide.2 That treatment with antidepressant medication may be associated with an increased risk of suicidal behavior in depressed patients, particularly during the initial phase of treatment, has long been noted.3,4 To further investigate the relationship between antidepressant treatment and expressed suicidal ideation or behavior (“suicidality”), a meta-analysis was conducted by the US Food and Drug Administration (FDA) in 2004 on 23 randomized placebo-controlled trials of antidepressants in pediatric patients; this analysis found evidence of a modestly increased risk of suicidality odds ratio (OR) (1.95; 95% CI, 1.28-2.98) associated with the use of antidepressant drugs.5 As a result of these findings, in October 2004, the FDA informed manufacturers of all antidepressant medications that revised labeling to include a warning of the potential for increased risk of suicidality in children and adolescents would be required6; specific labeling was provided to manufacturers by the FDA in January 2005.7
In 2005-2006, the FDA conducted a meta-analysis of 372 randomized placebo-controlled trials of 11 antidepressants to examine the relationship between antidepressant medications and expressed suicidality in adult patients.8 The results of their analysis of the complete study population supported the null hypothesis of no treatment effect on suicidality in the complete study population (OR = 0.86; 95% CI, 0.71-1.04; P = .12). The results were similar when only the psychiatric disorders population was examined (OR = 0.83; 95% CI, 0.69-1.00; P = .05); however, when the results were stratified by age, suicidality was positively associated with assignment to treatment with antidepressants in subjects under 25 years of age (OR = 1.62; 95% CI, 0.97-2.71; P = .07), but negatively associated (OR = 0.74; 95% CI, 0.60-0.90; P = .003) with suicidality in subjects 25 years and older. There also appeared to be a further distinction between a modest protective effect in subjects aged 25-64 years (OR = 0.79; 95% CI, 0.64-0.98; P = .03) and a stronger protective effect in subjects 65 years and older (OR = 0.37; 95% CI, 0.18-0.76; P = .007).
Clinical Points
- In this meta-analysis, no statistically significant differences between bupropion and placebo were observed in the incidence of expressed suicidal ideation or behavior within and across individual and multiple indications.
- Regardless of treatment, the 18- to 24-year-old group had greater odds of having a suicide event compared to the ≥ 25-year-old group.
- It is important that all patients who are treated with antidepressants be carefully monitored for clinical worsening, suicidality, or unusual changes in behavior.
Others have also investigated the association between antidepressant use and suicidality in adult patients. The results of a recent meta-analysis of 477 randomized placebo-controlled trials of selective serotonin reuptake inhibitors (SSRIs) looking at suicide, nonfatal self-harm, and suicidal thoughts found no evidence that SSRIs increased suicidal risk.9 That analysis showed that there may be a small risk of nonfatal self-harm in the antidepressant group compared to placebo (OR = 1.57; 95% CI, 0.99-2.55), but no evidence of an increased risk of suicidal thoughts. Another systematic review of the literature that identified 702 randomized, controlled trials of SSRIs used for any clinical condition reported an association between suicide attempts and SSRI use compared to placebo, with an observed odds ratio of 2.28 (95% CI, 1.14-4.55; P = .02).10
The current study was designed to assess a possible relationship between treatment with bupropion (vs placebo) and expressed suicidality during clinical trials. Bupropion is an aminoketone antidepressant that inhibits the reuptake of dopamine and norepinephrine, but has no clinically significant serotonergic effects or effects on postsynaptic receptors. The analysis reported herein covers all indications for use for bupropion, as well as some studies for unlicensed indications. Given bupropion’s unique mechanism of action among antidepressants, it is of interest to assess whether the results of this analysis might differ from those obtained in the FDA’s analyses of all antidepressants considered together or in studies of serotonergic antidepressants alone. It is, however, recognized that, as with all retrospective analyses, the information obtained can only be considered supportive or hypothesis generating, and neither conclusive nor indicative of a cause-and-effect relationship.
METHOD
Types and Sources of Data
The inclusion of data for this analysis was modeled after the FDA’s methodology for their adult suicidality analysis. The database consisted of double-blind, randomized, placebo-controlled trials of bupropion that had a minimum of 30 subjects. Fifty-one trials conducted between 1976 and 2006 comprising 8,953 adult subjects receiving bupropion and 6,520 adult subjects receiving placebo were included in the full analysis. One additional trial (Burroughs-Wellcome trial #83) was included in the text-string search only, due to missing treatment codes. No events were identified. The following primary clinical indications were analyzed: major depressive disorder (MDD), smoking cessation, prevention of seasonal major depressive episodes associated with seasonal affective disorder, and relapse prevention (MDD and smoking cessation).
In addition, data from studies in the following unlicensed indications or populations were analyzed individually as well as combined with all other data for the all-indications analysis: obesity, attention-deficit/hyperactivity disorder (ADHD), sexual dysfunction, bipolar disorder, alcohol dependence, hypertension, and bulimia. The analysis included data from studies across all formulations of bupropion: bupropion immediate-release tablets, bupropion sustained-release tablets, and bupropion extended-release tablets.
Statistical Methods
The statistical methods used to examine suicidality data for bupropion were based on methods previously used by the FDA to examine the effects of antidepressants on emergent suicidality in the pediatric and adolescent populations. The 2 primary analyses that were conducted were the analysis of possible suicide-related events as defined by the Columbia Classification Algorithm for Suicide Assessment11 ratings described below, and the analysis of events defined by rating scale items for suicidality and depressed mood.
Columbia Classification Algorithm for Suicide Assessment analysis. A text-string search of all adverse-event fields (preferred and verbatim terms) and free-text fields for potential suicidal events based on FDA search criteria was conducted in all 52 trials. The FDA search criteria included the following text strings: accident-, injur-, suic-, or overdos-, including all events coded as accidental overdose, attempt, cut, gas, hang, hung, jump, mutilat-, self damag-, self harm, self inflict-, shoot, slash, poison, asphyxiation, suffocation, firearm, burn, drown, gun, immolat-, and monoxide. The following terms were added by GlaxoSmithKline to the search criteria: accident, lacerat-, MVA, and hospital. The database search included data beginning from the first dose of study medication through 1 day following the last dose. In addition, all deaths and other serious adverse events were included for the Columbia classification. For positive matches, subject narratives were prepared by Drug Safety Alliance (a contract research organization with offices in North Carolina) and GlaxoSmithKline clinical scientists. The narratives were blinded and sent to Columbia University Medical Center, where they were reviewed by 3 independent experts who assigned a code according to the following Columbia Classification Algorithm for Suicide Assessment ratings:
- Completed suicide
- Suicide attempt
- Preparatory acts toward imminent suicidal behavior
- Suicidal ideation
- Self-injurious behavior, intent unknown
- Not enough information (fatal)
- Self-injurious behavior, no suicidal intent
- Other, no deliberate self-harm: accident, psychiatric symptoms only, or medical symptoms only
- Not enough information (nonfatal)
For purposes of statistical analysis, 2 events—suicidal behavior or ideation, and suicidal behavior only—were defined. Subjects were classified as having a suicidal behavior or ideation event if their event was assigned a Columbia rating of 1-4 and a suicidal behavior event if the event was assigned a rating of 1-3. If a subject had more than 1 potential event, the event with the greatest severity was used in the analysis. Events assigned to Columbia categories 5-9 were not included in the analysis because reports in these categories lack adequate information to determine their true nature and the events may be more likely to be unrelated to suicidality than events in categories 1-4; as such, inclusion of the additional rating categories in this analysis would render interpretation of the obtained result very difficult.
Point and confidence interval estimates of the common odds ratio across trials were computed. Also, P values for the test of the null hypothesis that the common odds ratio equals 1 were calculated. Exact statistical procedures implemented using StatXact (Cytel Inc; Cambridge, Massachusetts) were used as the primary method to compare treatment groups (bupropion and placebo) controlling for trial. The exact method is reported here because it is designed particularly for sparse datasets such as this one. The incidence of suicidal events (proportion of subjects with an event) was compared between treatment groups. P values for the treatment group comparisons were computed and compared against a 2-sided .05 level of significance. Treatment groups were also compared for each subgroup defined by (1) age group (18-24, 25-64, and ≥ 65 years) and (2) gender. Since the goal of the analysis was detection of a possible safety signal, no correction to the P values was made for multiplicity of tests and comparisons between age groups, gender, or the 2 outcome measures.
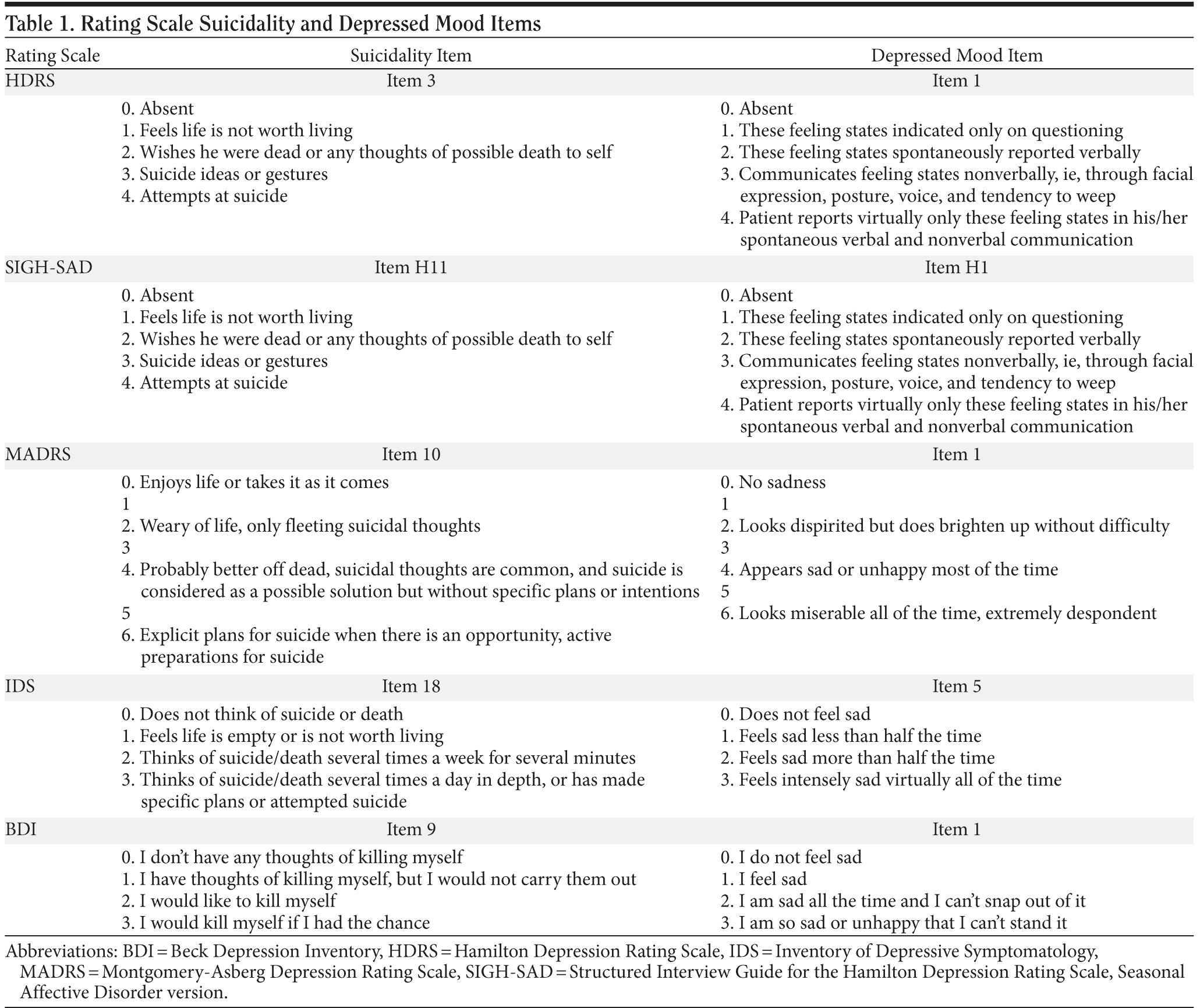
Rating scale item analysis. Additionally, analysis of the rating scale depressed mood and suicide item scores was conducted for trials in which an individual item score was available at baseline and at least 1 individual item score was available postbaseline. The trials analyzed included the MDD trials (n = 18), seasonal affective disorder prevention trials (n = 3), and smoking cessation trials (n = 4). Data were obtained from the following rating scales (Hamilton Depression Rating Scale [HDRS], Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorder version [SIGH-SAD], Montgomery-Asberg Depression Rating Scale [MADRS], Inventory of Depressive Symptomatology [IDS, used in 1 MDD trial], and Beck Depression Inventory [BDI]). Table 1 lists the specific items from these scales that correspond with suicidality and depressed mood.
Six events were defined for analysis based on changes in the rating scale items. Four were defined as any of the following categorical rating changes occurring during the double-blind treatment phase of the trial:
- Increase in suicidal ideation: increase of ≥ 1 from baseline in suicide item score.
- Increase in suicidal ideation: increase of ≥ 2 from baseline in suicide item score.
- Increase in depressed mood: increase of ≥ 1 from baseline in depressed mood item score.
- Increase in depressed mood: increase of ≥ 2 from baseline in depressed mood item score.
Two events were based on the last evaluable categorical rating assessment that occurred during the double-blind treatment phase of the trial:
- Decrease in suicidal ideation: decrease of ≥ 1 from baseline in suicide item score.
- Decrease in depressed mood: decrease of ≥ 1 from baseline in depressed mood item score.
The treatment groups were compared using the exact statistical methods described above.
Age effect. A post hoc analysis was conducted to compare suicidal events based on the Columbia ratings in young adults (18-24 years of age) with those in subjects 25 years and older from the MDD trials.
RESULTS
Columbia Classification Algorithm for Suicide Assessment Analysis
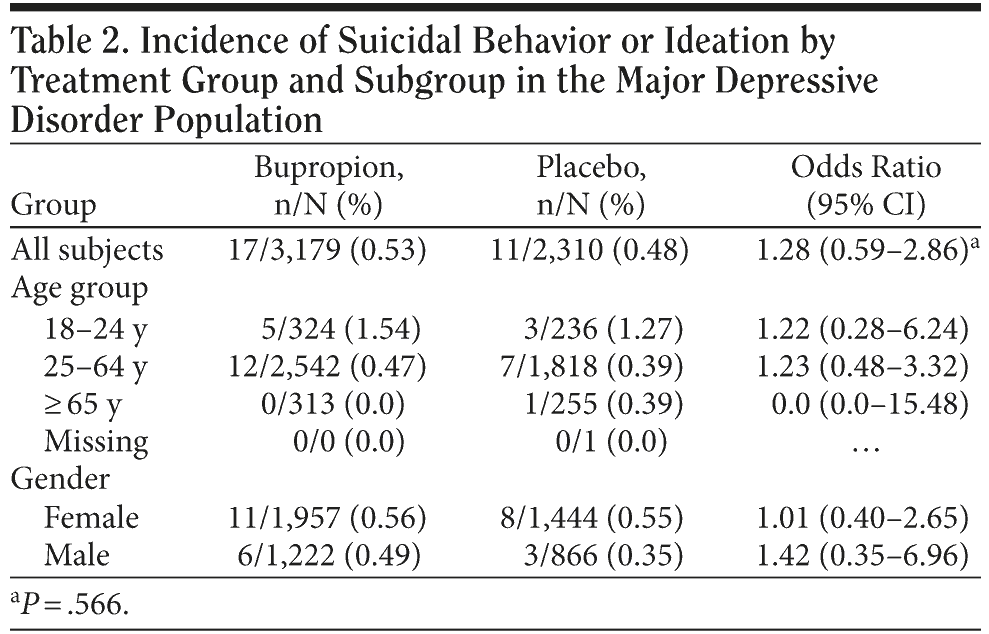
Major depressive disorder population. As shown in Table 2, in the MDD population for the suicidal behavior or ideation endpoint (Columbia rating 1-4), there was no statistically significant difference between bupropion and placebo (17/3,179 [0.53%] vs 11/2,310 [0.48%]; OR = 1.28; 95% CI, 0.59-2.86). For the age group and gender subgroup analyses, the 95% confidence intervals for the odds ratios also did not indicate any statistically significant differences between the treatment groups.
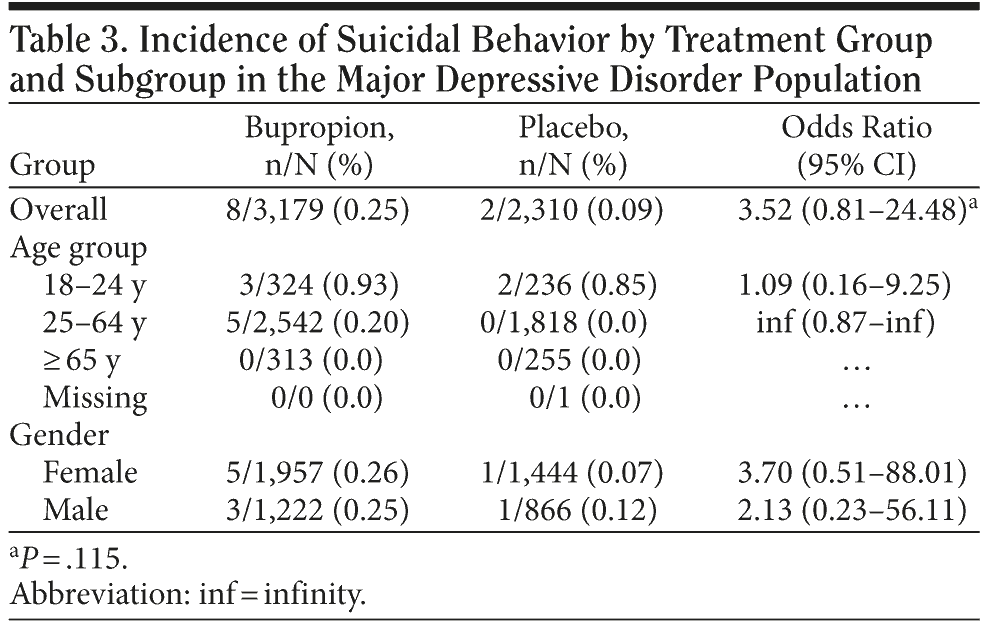
As shown in Table 3, for the suicidal behavior endpoint in the MDD population (Columbia rating 1-3), there was a higher frequency of events in bupropion versus placebo (8/3,179, 0.25% vs 2/2,310, 0.09%); however, the confidence interval was large, and the difference between the groups was not statistically significant (OR = 3.52; 95% CI, 0.81-24.48). In addition, the absolute incidence of events was very low. For the age group and gender subgroups, the 95% confidence intervals for the odds ratios also did not indicate any statistically significant differences between the treatment groups. There was no completed suicide reported during treatment in any of the trials.
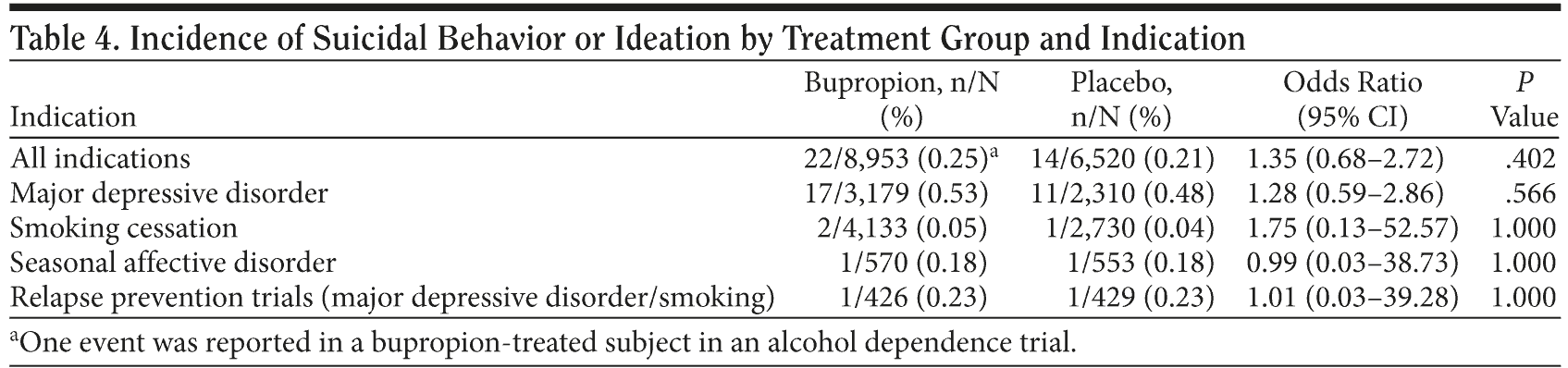
Other populations. In the smoking cessation, seasonal affective disorder prevention, and relapse prevention (MDD and smoking cessation) trials, and for all indications combined, there were no statistically significant differences between bupropion and placebo for the suicidal behavior or ideation endpoint (Table 4). Among the remaining populations for unlicensed indications (obesity, ADHD, sexual dysfunction, bipolar disorder, alcohol dependence, hypertension, and bulimia), only 1 suicidality behavior or ideation event was identified, and it occurred in a bupropion-treated subject in an alcohol dependence trial. For the suicidal behavior endpoint, no events were reported in the smoking cessation, seasonal affective disorder, or relapse prevention trials or in any other populations. No completed suicide was reported during treatment in any of the trials.
Depression Rating Scale Analysis
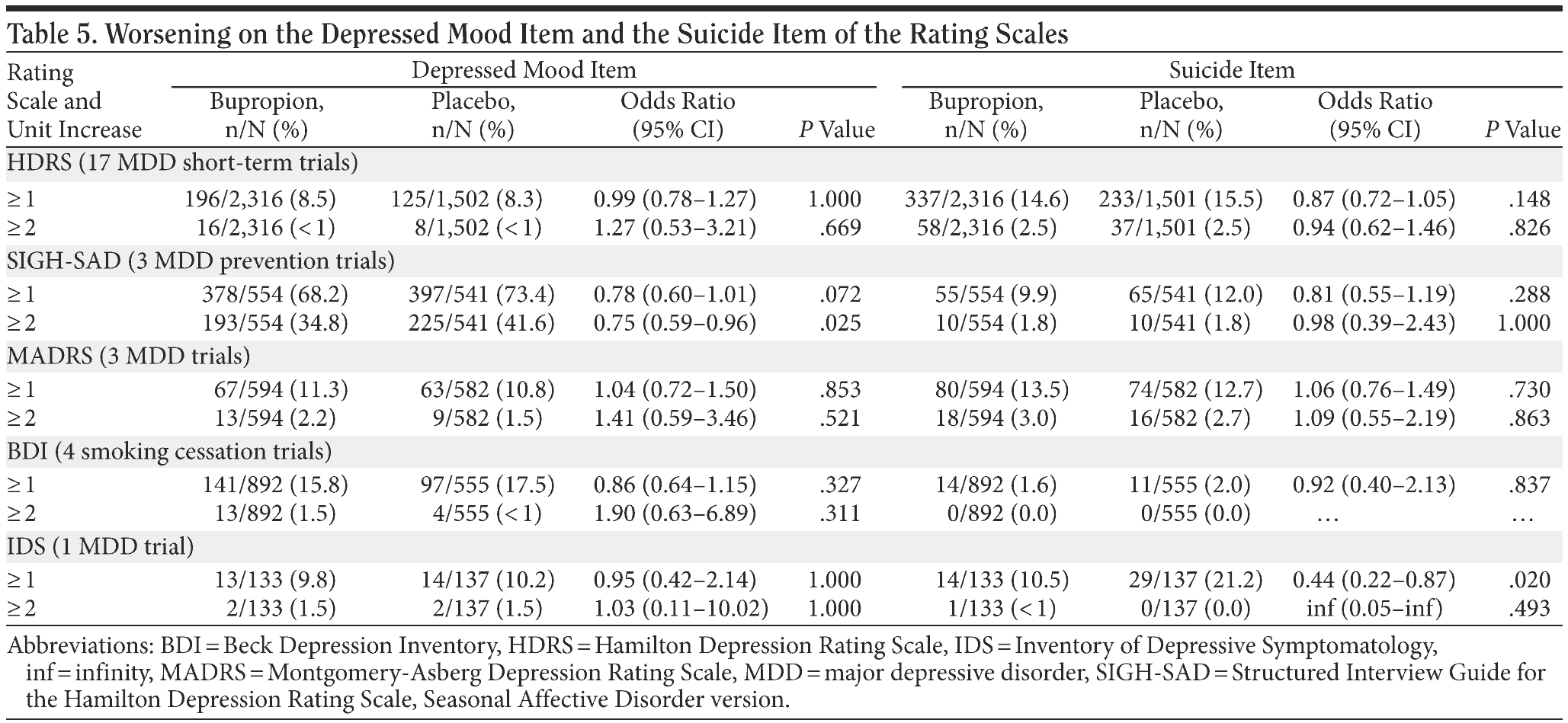
Suicidality item. Analysis of the suicidality item of the rating scales showed no statistically significant differences between bupropion and placebo for worsening (an increase of ≥ 1 or ≥ 2 units) on the rating scale, with 1 exception. In the MDD trial in which the IDS was used, a statistically significant difference was observed for an increase of ≥ 1, with the placebo group having a greater proportion of subjects showing worsening than the bupropion group. There were no other statistically significant differences between treatment groups (Table 5) regardless of indication or rating scale analyzed.
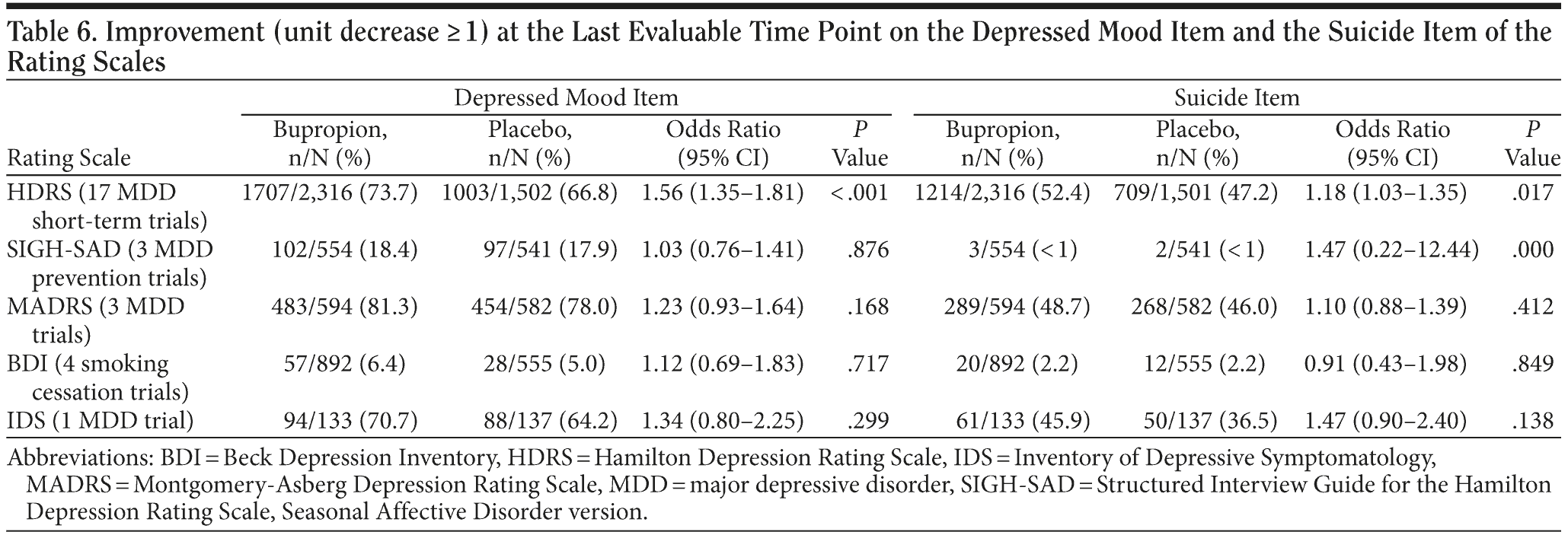
A second analysis of the suicidality item of the rating scales assessed improvement in suicidal thoughts (a decrease of ≥ 1 unit) at the last evaluable time point during the trial (Table 6). A statistically significant difference was noted on the HDRS in the MDD short-term studies in favor of bupropion over placebo (P = .017). No statistically significant differences between treatment groups were noted in the remaining rating scales for this analysis (SIGH-SAD, MADRS, BDI, IDS).
Depression mood item. Analysis of the depressed mood item of the rating scales showed no statistically significant differences in the proportion of subjects showing worsening on the depressed mood item (an increase of ≥ 1 or ≥ 2 units) of the rating scale, with 1 exception. For the SIGH-SAD depressed mood item in the seasonal affective disorder prevention studies, a statistically significant difference was observed for an increase of ≥ 2 units, with the placebo group having a greater proportion of subjects showing worsening than those taking bupropion. There were no other statistically significant differences, regardless of indication or rating scale analyzed.
A second analysis of the depressed mood item of the ratings scales assessed improvement in mood (a decrease of ≥ 1 unit) at the last evaluable time point. A statistically significant difference was noted on the HDRS across all studies using this scale in favor of bupropion over placebo (P < .001). No statistically significant differences between treatment groups were noted in the other ratings scales for this analysis (SIGH-SAD, MADRS, BDI, IDS).
Age Effect
As noted previously, there were no statistically significant differences in rates of suicidal ideation or behavior (Columbia ratings 1-4) between bupropion and placebo by age group. To further explore the relationship between age and suicidal ideation or behavioral events, a post hoc comparison was made between young adults (18-24 years of age) and older adults (≥ 25 years of age) in the MDD trials, controlling for treatment group. This analysis showed no statistically significant treatment-by-age group interaction; therefore, age groups were compared regardless of treatment group. A statistically significant difference was found between the age groups in the frequency of observed events, with an overall higher risk of an event in the 18- to 24-year-old group than in the ≥ 25-year-old group (1.43% vs 0.41%; OR = 3.55; 95% CI, 1.47-7.96; P = .005).
DISCUSSION
The overall rates of reported suicidal behavior and ideation and of suicidal behavior alone in subjects taking bupropion were low in all indications, and there were no completed suicides reported during treatment in any of the trials. In the analysis of the incidence of suicidal behavior or ideation (Columbia ratings 1-4), there were no statistically significant differences between bupropion and placebo. For the analysis of suicidal behavior (Columbia ratings 1-3), the bupropion treatment group had slightly greater odds of having an event compared to the placebo group; however, the difference was not statistically significant.
These results should be put in context of other analyses of pooled data that examined suicidality, ideation, and behaviors. In the 2005-2006 analysis conducted by the FDA, data were pooled from placebo-controlled clinical trials of 11 antidepressants: 6 SSRIs (citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, and sertraline), 2 serotonin-norepinephrine reuptake inhibitors (duloxetine and venlafaxine), and 3 other antidepressants with differing mechanisms of action (bupropion, mirtazapine, and nefazodone). In the patient population diagnosed with any psychiatric diagnosis in the FDA analysis, the odds ratio of a suicide event (ideation or behavior) for active drug relative to placebo was 0.83 (95% CI, 0.69-1.00; P = .05).8 Individual analyses, by antidepressant, of the data set of patients with psychiatric disorders were conducted using conditional logistic regression, and odds ratios relative to placebo ranged from 2.44 (95% CI, 0.90-6.63; P = .08) for escitalopram to 0.51 (95% CI, 0.29-0.91; P = .02) for sertraline across the 11 products, with the odds ratio of a suicide event in the bupropion group compared to placebo of 1.35 (95% CI, 0.45-4.06; P = .59). These data, along with other previously published analyses,9,10 raise the possibility that expressed suicidality may vary across antidepressants and that analysis of individual products or products grouped by a similar mechanism of action would be of scientific interest and could potentially provide better information for patients than analysis of combined classes of medications.
In this bupropion analysis, changes during treatment in the rating scale items for depressed mood and suicidality did not show any statistically significant differences between bupropion and placebo in worsening of the items, with the exception of the SIGH-SAD depressed mood item and the IDS suicidality item in the seasonal affective disorder studies, which both showed rates of worsening for placebo greater than for bupropion. The finding in the seasonal affective disorder studies is not unexpected given that these studies were prevention studies in which patients with seasonal affective disorder were enrolled in the autumn and treated prior to the onset of a seasonal depressive episode. Across these 3 studies, the rate of recurrence of a wintertime major depressive episode was significantly (44%) lower in patients treated with bupropion than in those treated with placebo.12
In addition, in the MDD short-term studies, statistically significant differences between bupropion and placebo in improvement of the suicidality and depressed mood items of the HDRS were noted. Both items showed rates of improvement for bupropion greater than for placebo, which may reflect a therapeutic effect of the drug on depression.
In the FDA’s analysis including all antidepressants, an interaction was found between treatment and age, with an odds ratio > 1 for expressed suicidality (both ideation or behavior and, behavior alone) observed with antidepressant treatment relative to placebo in subjects under 25 years of age (OR = 1.62; 95% CI, 0.97-2.71; P = .07), an odds ratio < 1 in subjects 25-64 years of age (OR = 0.79; 95% CI, 0.64-0.98; P = .03), and the lowest odds ratio observed in subjects ≥ 65 years of age (OR = 0.37; 95% CI, 0.18-0.76; P = .007).12 Our data, in contrast, did not show an interaction between treatment and age for expressed suicidality in subjects under 25 years of age (OR = 1.22; 95% CI, 0.28-6.24), 25-64 years of age (OR = 1.23; 95% CI, 0.48-3.32), or ≥ 65 years of age (OR = 0.0; 95% CI, 0.0-15.48). The number of events observed in the ≥ 65-year-old group—0/313 receiving bupropion and 1/255 receiving placebo—is too small to make conclusions about a possible protective effect of bupropion in this population subgroup. A post hoc evaluation of the effect of age on suicidal ideation or behavior did show that, regardless of treatment, the 18- to 24-year-old group had greater odds of having a suicide event compared to the ≥ 25-year-old group.
It is possible that bupropion’s lack of effect on serotonin neurotransmission in the brain may be a factor in the difference between our results and those reported by the FDA with respect to age by treatment effect and suicidality. Neither the dopaminergic nor noradrenergic systems have been strongly implicated in suicidality; however, 3 different indices of serotonergic function appear to correlate with suicidal behavior in patients independent of diagnosis: cerebrospinal fluid 5-HIAA, the prolactin response to fenfluramine, and platelet 5-HT2A receptor binding.13 Additionally, a polymorphism in the gene for tryptophan hydroxylase, the rate-limiting enzyme in the biosynthesis of serotonin, has been shown to be associated with suicidal behavior in impulsive alcoholic criminals14 as well as in individuals with a major depression.15 Additional studies on the role of individual neurotransmitter systems in suicidality would be helpful.
There are some limitations to the analyses reported within this article and their interpretations. The risk of suicidal behavior is inherent in patients with psychiatric disorders, and parsing out a drug effect versus a symptom of the illness itself is not possible in this retrospective analysis. Clinical trials also do not typically enroll patients who are actively suicidal, which may restrict the generalizability of these results to clinical populations. Another limitation was the collection period: the data analyzed were collected over a 29-year period using collection methodology that was not standard across all trials. Finally, the overall low incidence and small absolute number of events limit the power of statistical analyses to detect small differences between treatments.
CONCLUSION
No statistically significant differences between bupropion and placebo were observed in the incidence of expressed suicidal ideation or behavior (combined) within and across individual and multiple indications combined, including MDD, smoking cessation, prevention of seasonal depressive episodes, and relapse prevention, and no differential treatment effects were observed by gender or age. The only suicidal behavior events occurred in the MDD population, in which there was a greater incidence of events in the bupropion group compared to the placebo group; however, the incidence of events was low, and the difference was not statistically significant.
It is not possible to determine whether differences between the results of our analyses and those observed in previous analyses of antidepressants might be due to the unique mechanism of action of bupropion among the antidepressants, although this possibility could be considered for future research. Finally, we would like to emphasize the importance of carefully monitoring all patients who are treated with antidepressants for clinical worsening, suicidality, or unusual changes in behavior; these results should not be construed as suggesting otherwise.
Drug names: bupropion (Wellbutrin, Aplenzin, and others), citalopram (Celexa and others), duloxetine (Cymbalta), escitalopram (Lexapro and others), fluoxetine (Prozac and others), fluvoxamine (Luvox and others), mirtazapine (Remeron and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others), venlafaxine (Effexor and others).
Author affiliations: Medicine Development Center, Neuroscience, GlaxoSmithKline, Research Triangle Park, North Carolina.
Potential conflicts of interest: All authors are employed by GlaxoSmithKline. They report no additional financial or other relationship relevant to the article.
Funding/support: This work was supported by GlaxoSmithKline, Research Triangle Park, North Carolina.
Previous presentation: Preliminary results were presented at the 18th Annual Meeting of the American Neuropsychiatric Association; February 20, 2007; Tucson, Arizona.
REFERENCES
1. Centers for Disease Control and Prevention. National Vital Statistics Report. 2006;57(14). 80 pp. (PHS) 2009-1120. http://www.cdc.gov/nchs/products/nvsr.htm. Accessibility verified July 29, 2010.
2. Harris EC, Barraclough B. Suicide as an outcome for mental disorders: a meta-analysis. Br J Psychiatry. 1997;170(3):205-228. PubMed doi:10.1192/bjp.170.3.205
3. Damluji NF, Ferguson JM. Paradoxical worsening of depressive symptomatology caused by antidepressants. J Clin Psychopharmacol. 1988;8(5):347-349. PubMed doi:10.1097/00004714-198810000-00007
4. Teicher MH, Glod C, Cole JO. Emergence of intense suicidal preoccupation during fluoxetine treatment. Am J Psychiatry. 1990;147(2):207-210. PubMed
5. Hammad TA, Laughren T, Racoosin J. Suicidality in pediatric patients treated with antidepressant drugs. Arch Gen Psychiatry. 2006;63(3):332-339. PubMed doi:10.1001/archpsyc.63.3.332
6. US Food and Drug Administration. Suicidality in Children and Adolescents Being Treated With Antidepressant Medications. October 15, 2004. http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm161679.htm. Accessibility verified July 29, 2010.
7. US Food and Drug Administration. Class Suicidality Labeling Language for Antidepressants. January 26, 2005. http://www.fda.gov/downloads/Drugs/DrugSafety/InformationbyDrugClass/UCM161641.pdf. Accessibility verified July 29, 2010.
8. Laughren TP. Department of Health and Human Services, Public Health Service, Food and Drug Administration Center for Drug Evaluation and Research. Overview for December 13 meeting of Psychopharmacologic Drugs Advisory Committee. http://www.fda.gov/ohrms/dockets/ac/06/briefing/2006-4272b1-01-FDA.pdf. November 16, 2006. Accessibility verified July 29, 2010.
9. Gunnell D, Saperia J, Ashby D. Selective serotonin reuptake inhibitors (SSRIs) and suicide in adults: meta-analysis of drug company data from placebo controlled, randomised controlled trials submitted to the MHRA’s safety review. BMJ. 2005;330(7488):385-388. PubMed doi:10.1136/bmj.330.7488.385
10. Ferguson D, Doucette S, Glass KC, et al. Association between suicide attempts and selective serotonin reuptake inhibitors: systematic review of randomised controlled trials. BMJ. 2005;330(7):396-399. PubMed
11. Posner K, Oquendo MA, Gould M, et al. Columbia Classification Algorithm of Suicide Assessment (C-CASA): classification of suicidal events in the FDA’s pediatric suicidal risk analysis of antidepressants. Am J Psychiatry. 2007;164(7):1035-1043. PubMed doi:10.1176/appi.ajp.164.7.1035
12. Modell JG, Rosenthal NE, Harriett AE, et al. Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biol Psychiatry. 2005;58(8):658-667. PubMed doi:10.1016/j.biopsych.2005.07.021
13. Mann JJ. The neurobiology of suicide. Nat Med. 1998;4(1):25-30. PubMed doi:10.1038/nm0198-025
14. Nielsen DA, Goldman D, Virkkunen M, et al. Suicidality and 5-hydroxyindoleacetic acid concentration associated with a tryptophan hydroxylase polymorphism. Arch Gen Psychiatry. 1994;51(1):34-38. PubMed
15. Mann JJ, Malone KM, Nielsen DA, et al. Possible association of a polymorphism of the tryptophan hydroxylase gene with suicidal behavior in depressed patients. Am J Psychiatry. 1997;154(10):1451-1453. PubMed