Observational Study of the Impact of Short-Term Duloxetine Treatment on Body Weight in Patients With Major Depressive Disorder: A Taiwanese Perspective
To the Editor: The impact of antidepressants on body weight interferes with patients’ adherence to these medications. In the past, tricyclic antidepressants were associated with weight gain.1 In recent years, even with advances, antidepressants have still struggled solving this complex issue. Selective serotonin reuptake inhibitors (SSRIs) can cause short-term body weight loss, but long-term body weight gain can be a significant concern in this treatment strategy.2 Serotonin-norepinephrine reuptake inhibitor (SNRI) agents, such as venlafaxine and sibutramine, have different effects on body weight. Sibutramine is approved by the US Food and Drug Administration (FDA) for obesity treatment,3 but the effect of venlafaxine on body weight is still controversial.
Duloxetine hydrochloride, another SNRI, lacks significant affinity for muscarinic, histamine-1, α1-adrenergic, 5-HT2C, and opioid receptors, which are associated with body weight homeostasis.4 An analysis of 10 clinical studies (using repeated-measurement analyses) revealed that short-term duloxetine treatment would cause an average 0.5 kg body weight loss in Western, including Caucasian, Hispanic, and African American, individuals.5 To investigate the short-term effect of duloxetine treatment on body weight in depressed Taiwanese patients and establish preliminary data for Taiwanese subjects, my colleagues and I conducted this open-label observational study.
Method. This study was approved by the ethics committee and institutional review board of the Buddhist Tzu Chi General Hospital Taipei Branch, and all patients completed consent forms. In 2008, we enrolled 24 outpatients (7 male subjects and 17 female subjects, mean ± SD age 40.8 ± 13.8 years old) who met the following criteria: age ranging from 18 to 65 years, major depressive disorder as defined by DSM-IV, no psychotropic medicine use 2 weeks (fluoxetine: 4 weeks) prior to enrollment, first-episode major depression, no concurrent significant systemic physical illness influencing body weight, no concurrent diet therapy for body weight control, no concurrent diet pill therapy, no other psychiatric diagnosis comorbidity, and Clinical Global Impressions-Severity of Illness scale (CGI-S)6 rating ≥ 4 (moderate). The mean ± SD body weight change before treatment was −2.6 ± 1.2 kg, according to the subjective description, and mean ± SD pretreatment body mass index (BMI) was 28.4 ± 3.2. All the patients received duloxetine treatment with a dose ranging from 30 to 60 mg/d, based on their clinical symptoms and clinician judgment. Concomitant medications included benzodiazepines or hypnotics. We estimated the subjects’ body weight at baseline and at the first, second, third, fourth, sixth, and eighth weeks. Body weight assessment procedures were as follows: (1) coat removal, (2) shoes off, (3) underwear remaining, (4) fasting condition, (5) concurrent body height assessment by the same rule (for the BMI), (6) no exercise 4 hours beforehand, (7) all measurements made using the same electronic scale, (8) resetting to the null point before weighing. All the body weight change data were analyzed by SPSS statistical software version 12 (SPSS, Inc., Chicago, IL) and plotted by SigmaPlot version 10 (Cranes Software International Ltd, Bangalore, India). The intraclass correlation coefficient between cardinal data of body weight (Cronbach α: .995) revealed that the reliable intraclass body weight data were consistent.
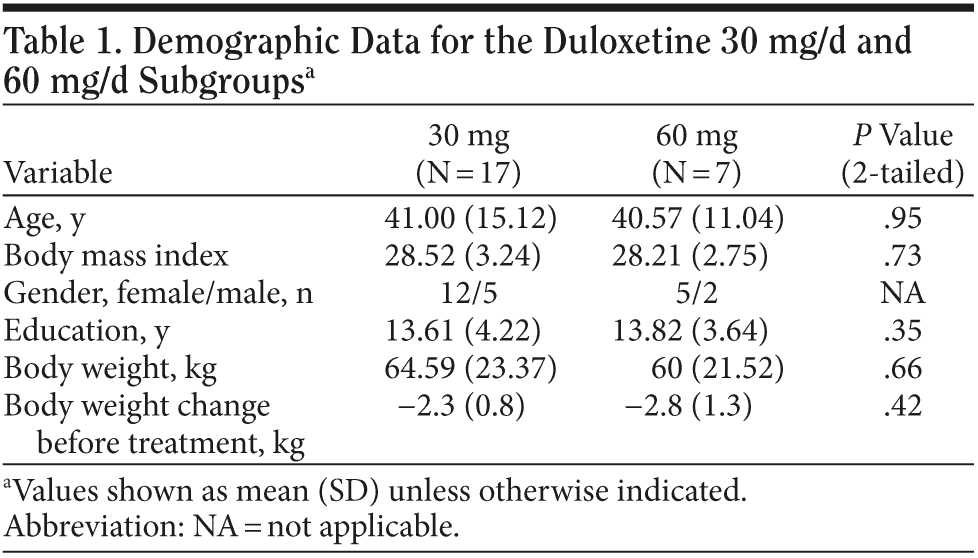
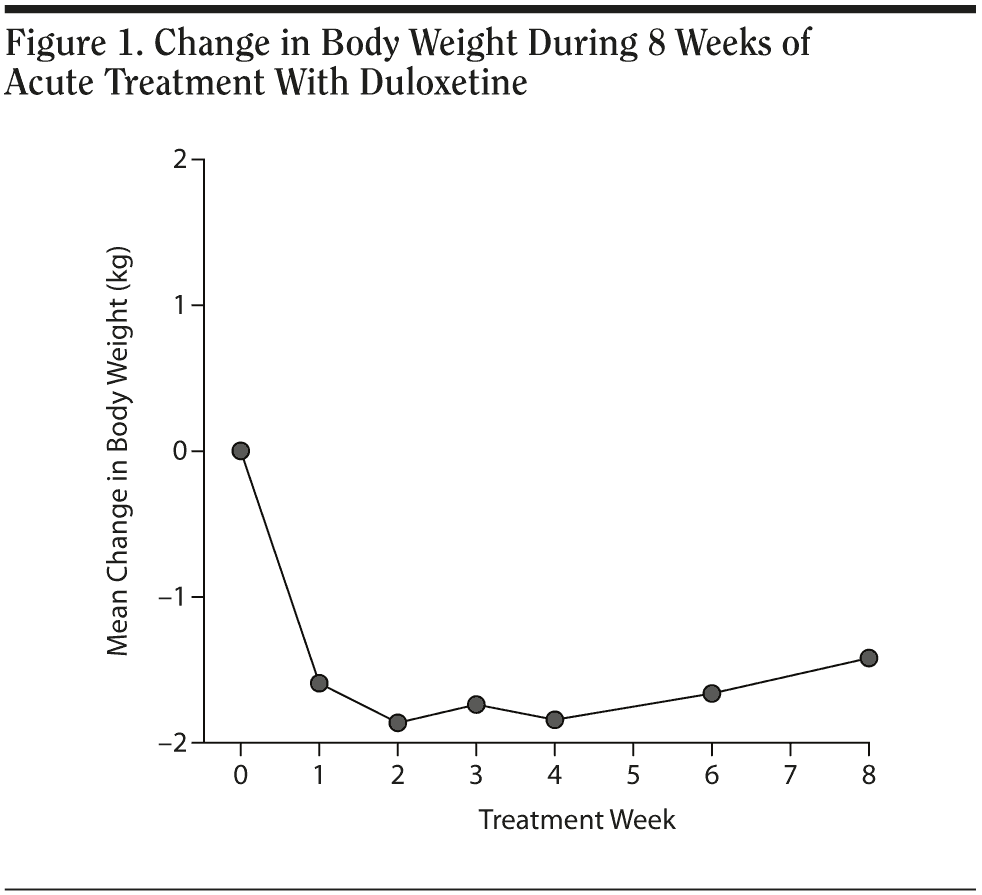
Results. The duloxetine treatment dose was classified into 2 subgroups: 30 mg and 60 mg (30 mg: 17 subjects; 60 mg: 7 subjects). Demographic data from each subgroup were compared were each other; comparisons revealed no significant difference between groups in baseline body weight, body weight change before treatment, years of education, and age (Table 1). Every patient achieved was much improved (Clinical Global Impressions-Improvement scale [CGI-I] score ≤ 2) in the eighth week. The patients’ descriptive statistics revealed a steep decline of body weight after beginning oral use of duloxetine, and the effect seemed to persist until the fourth week. The mean body weight loss from baseline was about 1.6 kg in the first week, 1.8 kg in the second week, 1.7 kg in the third week, 1.8 kg in the fourth week, 1.6 kg in the sixth week, and 1.4 kg in the eighth week. The plotting curve showed a fast body weight loss effect in the first week (Figure 1), with the effect becoming more obvious in the second week and the profile remaining the same until the fourth week. The sixth week and eighth week profile revealed gradual body weight gain after an initial duration of relatively clinically significant body weight loss. Body weight loss never exceeded 2 kg during this 8-week observational study.
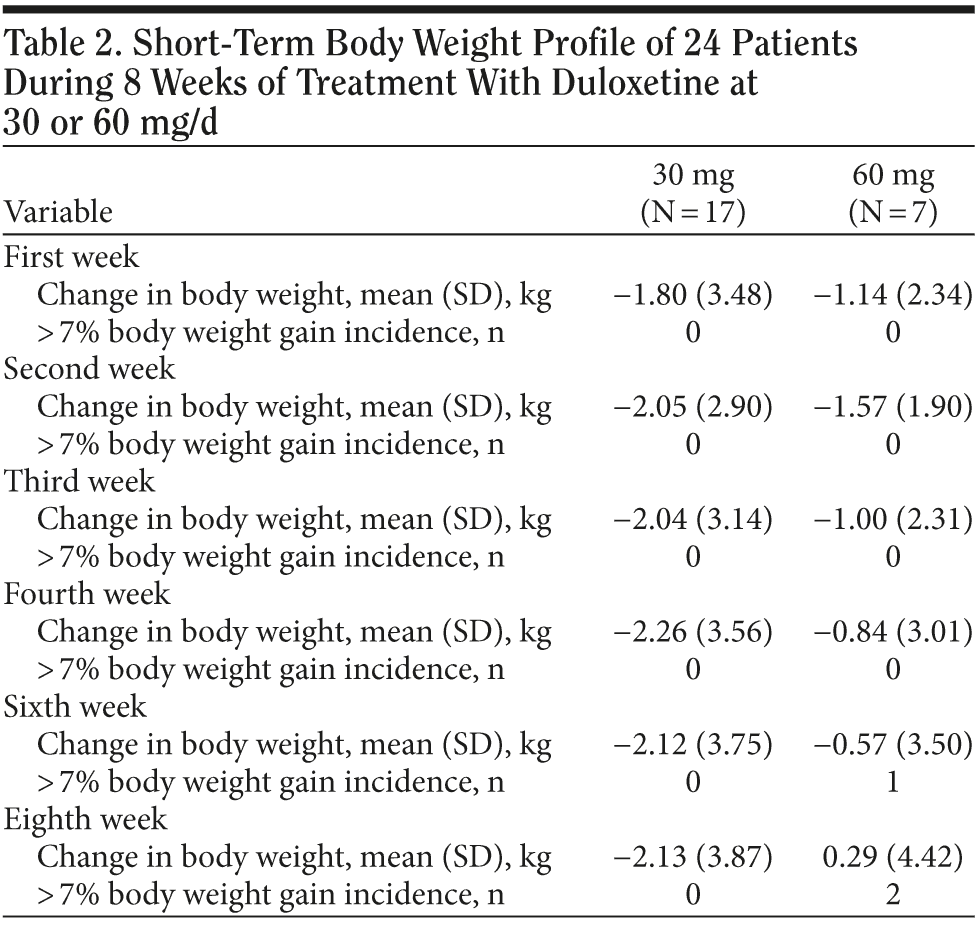
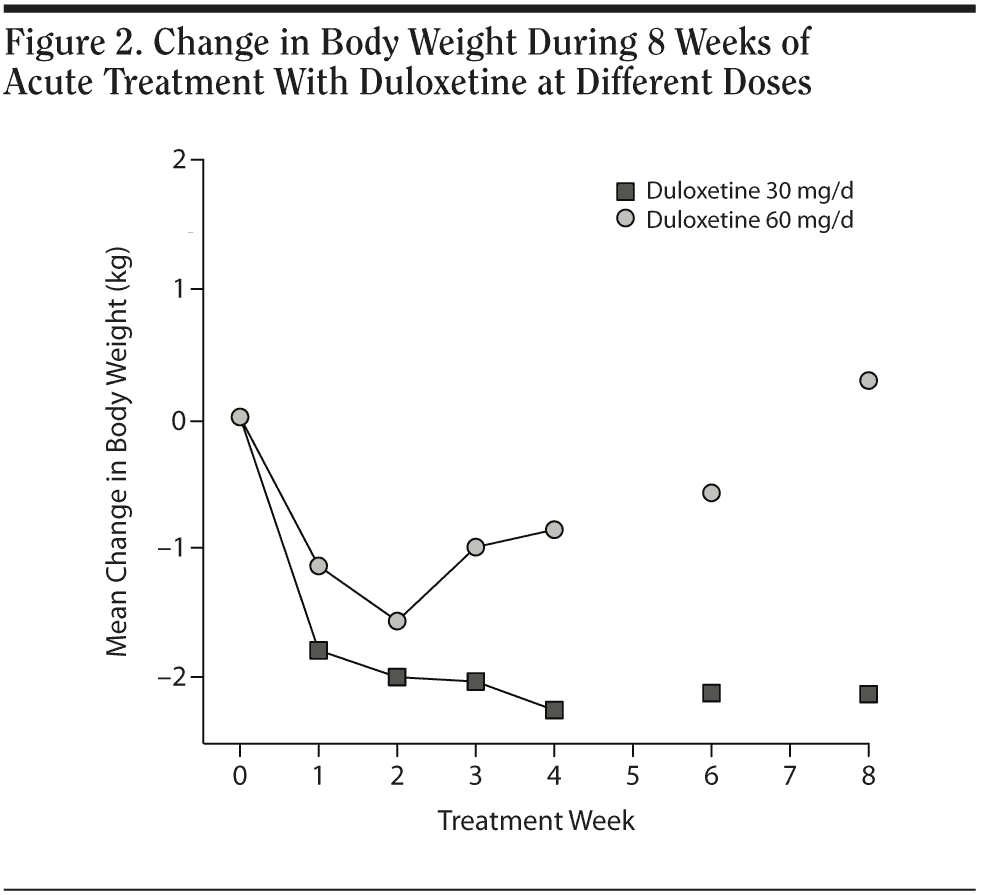
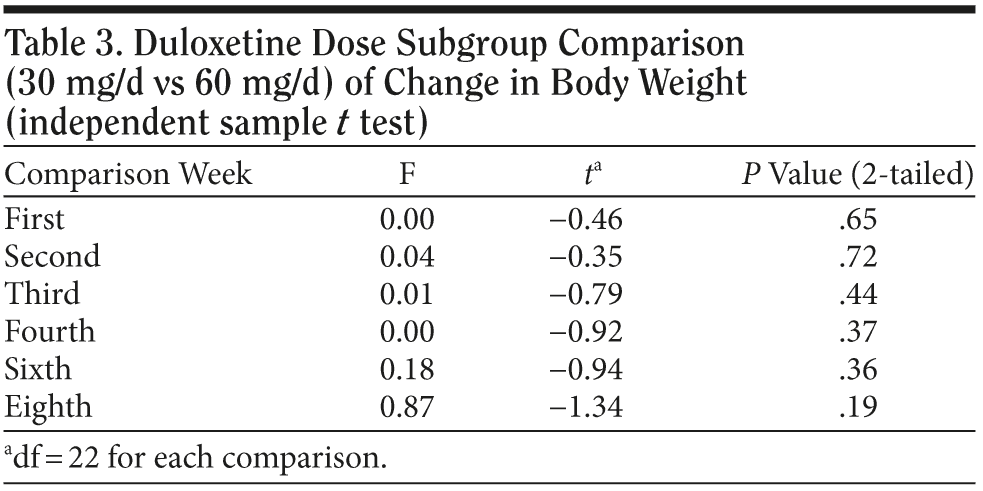
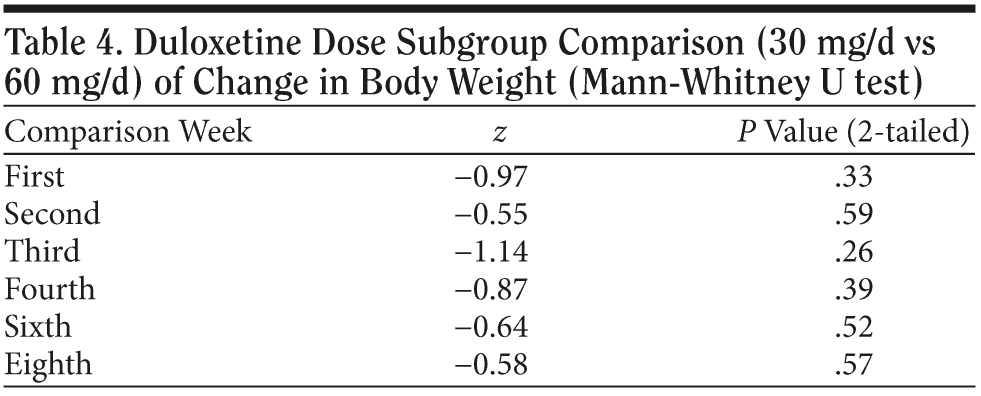
In the 30 mg daily dose subgroup, body weight was maintained at a similar level with a maximum loss of 2.2 kg during the 8 weeks. A body weight return phenomenon was not observed in this subgroup. Contrary to the 30 mg daily dose subgroup result, the 60 mg daily dose subgroup showed prominent body weight loss of approximately 1.6 kg in second week followed by a body weight return phenomenon beginning in the second week, with a mild gain of around 0.3 kg in the eighth week. A difference in the proportion of patients with body weight gain more than 7% was noted between the 2 dose subgroups. (Table 2). In the plot curve, a duloxetine dose group difference in body weight was noted (Figure 2). No statistically significant difference was found between the 2 dosage groups, either by independent sample t test (Table 3) or by Mann-Whitney U test (Table 4).
Duloxetine was reported to be associated with the side effects of nausea and anorexia, which might be the confounding factors influencing body weight during duloxetine treatment. According to an analysis of 10 clinical studies, acute treatment with duloxetine in major depressive disorder patients would cause a mean body weight loss of 0.5 kg (0.2 kg with placebo).5 These were all Western, including Caucasian, African American, and Hispanic, subjects; their duloxetine dose range was 40-120 mg daily. Our Taiwanese data showed a mean body weight loss of 1.4 kg until the eighth week, and our duloxetine dose range was 30-60 mg daily. Our dose range was clearly less than that in the Western published data, but the average body weight loss of Taiwanese individuals was around 3-fold more than that of Western subjects in the acute treatment phase. Duloxetine is mostly metabolized by cytochrome P450 2D6,7 which is believed to be more active in Western populations.8 Thus, the dramatic body weight loss of the Taiwanese subjects might be related to this variance by race in enzyme activity, but further study would be needed to confirm this supposition.
In terms of the impact of duloxetine dose on body weight, the higher dose (60 mg) subgroup seemed to display a different body weight change profile compared with the lower dose (30 mg) subgroup, though without statistical significance. Published data on long-term treatment revealed that a 60-120 mg dose would show an almost 4 kg increase at around 100 weeks.9 Our data suggested that a higher dose might give our major depressive disorder subjects a faster “recovery” of body weight earlier, beginning in the second week. Further study is needed to clarify this dose impact issue.
Regarding the impact of depression on body weight, all patients in this study were at least “minimally improved.” While depression responded to medication treatment, improved appetite might influence body weight condition. We should refine this issue in further study to decrease possible confounding bias from depressive symptoms themselves.10
The main limitations of this study are the small sample size, lack of response scores from rating scales for our patients, diet not standardized, lack of control of BMI for analysis, and lack of a placebo control group. The weight loss experienced by the patients with lower-dosage duloxetine treatment could be secondary to the weight loss inherent in depression itself. Perhaps higher duloxetine dose would be more effective to reverse depressive symptoms, including weight loss. But all of these patients had clinically significant improvement (CGI ≤ 2), so the weight change might not be explained only by depression itself, but possibly is also related to duloxetine dose. The inclusion of a comparison group will be a goal of future studies to make our results more persuasive. Besides, the 2 groups were not randomized and gender matched. As such, this was a pilot study, and our findings should be interpreted with caution. The major strength of this study is that we can chart the body weight change profile of Taiwanese subjects using duloxetine treatment and gain more local information about this new medication.
In conclusion, this study has shown a short-term (8-week) effect on body weight loss with duloxetine treatment in major depressive disorder patients and a possible dose-related interaction, although the latter without a statistically significant difference. Further placebo-controlled, gender-matched, randomized, BMI-controlled, diet-standardized, and detailed response-rating studies should be carried out to confirm this short-term effect.
References
1. Berken GH, Weinstein DO, Stern WC. Weight gain: a side-effect of tricyclic antidepressants. J Affect Disord. 1984;7(2):133-138. PubMed doi:10.1016/0165-0327(84)90031-4
2. Fava M, Judge R, Hoog SL, et al. Fluoxetine versus sertraline and paroxetine in major depressive disorder: changes in weight with long-term treatment. J Clin Psychiatry. 2000;61(11):863-867. PubMed
3. Finer N, Bloom SR, Frost GS, et al. Sibutramine is effective for weight loss and diabetic control in obesity with type 2 diabetes: a randomised, double-blind, placebo-controlled study. Diabetes Obes Metab. 2000;2(2):105-112. PubMed doi:10.1046/j.1463-1326.2000.00071.x
4. Turcotte JE, Debonnel G, de Montigny C, et al. Assessment of the serotonin and norepinephrine reuptake blocking properties of duloxetine in healthy subjects. Neuropsychopharmacology. 2001;24(5):511-521. PubMed doi:10.1016/S0893-133X(00)00220-7
5. Guy W. ECDEU Assessment Manual for Psychopharmacology. US Dept Health, Education, and Welfare publication (ADM) 76-338. Rockville, Md: National Institute of Mental Health; 1976:218-222.
6. Wise TN, Perahia DG, Pangallo BA, et al. Effects of the antidepressant duloxetine on body weight: analyses of 10 clinical studies. Prim Care Companion J Clin Psychiatry. 2006;8(5):269-278. PubMed
7. Skinner MH, Kuan HY, Pan A, et al. Duloxetine is both an inhibitor and a substrate of cytochrome P4502D6 in healthy volunteers. Clin Pharmacol Ther. 2003;73(3):170-177. PubMed doi:10.1067/mcp.2003.28
8. Bertilsson L. Geographical/interracial differences in polymorphic drug oxidation: current state of knowledge of cytochromes P450 (CYP) 2D6 and 2C19. Clin Pharmacokinet. 1995;29(3):192-209. PubMed
9. Wohlreich MM, Mallinckrodt CH, Prakash A, et al. Duloxetine for the treatment of major depressive disorder: safety and tolerability associated with dose escalation. Depress Anxiety. 2007;24(1):41-52. PubMed doi:10.1002/da.20209
10. Fernstrom MH, McConaha C, Kupfer DJ. Perception of appetite and weight change during treatment for depression. Appetite. 1989;13(1):71-77. PubMed doi:10.1016/0195-6663(89)90027-5
Author affiliations: Department of Psychiatry, Buddhist Tzu-Chi General Hospital, Taipei Branch, Taipei, Taiwan.
Potential conflicts of interest: None reported.
Financial funding/support: None reported.
Published online:February 11, 2010 (10.4088/PCC.08l00768blu).
Prim Care Companion J Clin Psychiatry 2010;12(1):e1-e3
© Copyright 2010 Physicians Postgraduate Press, Inc.