J Clin Psychiatry 2023;84(3):22br14567
To cite: Shi Z, Li X, Kampman KM, et al. Depressive symptomatology is associated with smaller reductions in drug cue reactivity during extended-release naltrexone treatment of opioid use disorder. J Clin Psychiatry. 2023;84(3):22br14567.
To share: https://doi.org/10.4088/JCP.22br14567
© 2023 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of Pennsylvania Perelman School of Medicine, Philadelphia, Pennsylvania
*Corresponding author: Daniel D. Langleben, MD, 3535 Market St Ste 500, Philadelphia, PA 19104 ([email protected]).
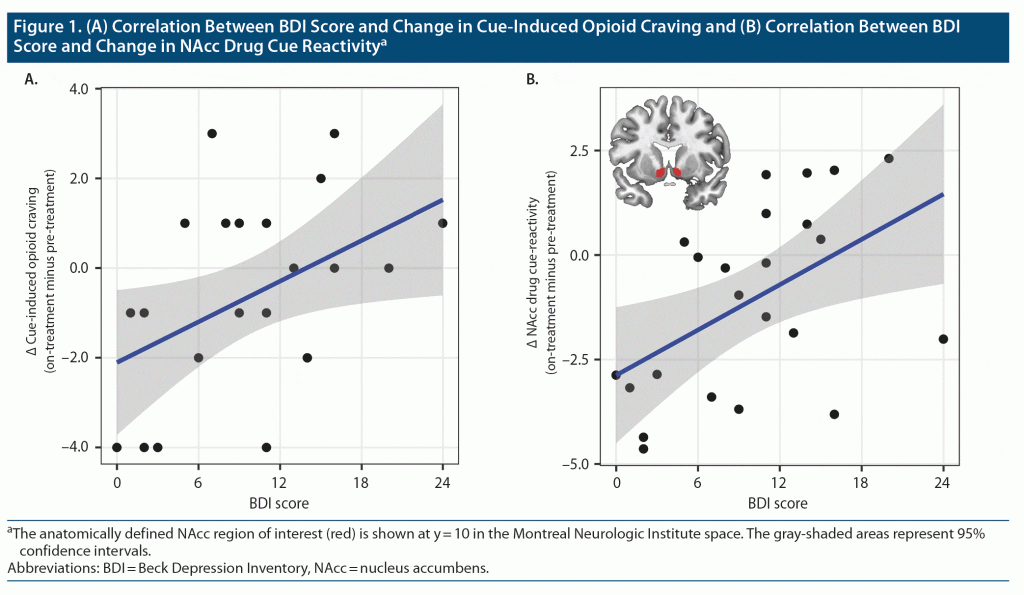
The high incentive value attributed to a drug in substance use disorders leads to the heightened dopaminergic responses to drug-related conditioned stimuli (ie, cue reactivity),1 stimulating drug-seeking behavior and promoting relapse.2 The nucleus accumbens (NAcc) is the key brain region implicated in cue reactivity in general. Specifically, in opioid use disorder (OUD), greater NAcc response to opioid drug cues has been associated with heavier drug use.3 The once-monthly injectable extended-release opioid antagonist naltrexone (XR-NTX) is an effective relapse prevention medication for OUD4,5 that significantly reduces NAcc cue reactivity.6,7 Depression and OUD are highly comorbid,8,9 and both involve endogenous opioid dysregulation.10,11 Patients with more severe depressive symptoms show poorer response to OUD treatment.12–14 Here, we used functional magnetic resonance imaging (fMRI) to test the hypothesis that depressive symptomatology in OUD is associated with reduced sensitivity of subjective or neural indices of drug cue reactivity to the XR-NTX treatment of OUD.
Methods
We performed a secondary analysis on a previously described dataset.6 Briefly, 23 detoxified OUD patients (9 female; 21–47 years old) were offered up to 3 monthly XR-NTX injections. Participants completed pre-treatment fMRI before the first injection and on-treatment fMRI approximately 2 weeks after the first injection. Each fMRI session included a cue reactivity task that presented drug-related, sexual, aversive, and neutral images. Before and after the task, participants reported craving for opioids on a 10-point scale (0 = none, 9 = extremely). Cue-induced craving was indexed by the change from before to after the task. The Beck Depression Inventory (BDI), which demonstrates good reliability and validity in the OUD population,15,16 was administered approximately 1 week after the first injection.
Preprocessed fMRI data were analyzed by modeling each stimulus category. The NAcc was defined as the a priori region of interest. Neural activity was evaluated by contrasting the drug, sexual, and aversive conditions with the neutral condition. Pearson correlation was performed between BDI scores and changes in cue-induced craving and NAcc drug cue reactivity (on-treatment minus pre-treatment). We also tested the correlation for sexual and aversive stimuli. Whole-brain analysis explored correlation in other brain regions.17
See the Supplementary Material and Shi et al6 for additional details on the methods.
Results
Participants’ BDI scores ranged from 0 (no depression) to 24 (moderate depression) (mean ± SD = 9.91 ± 6.22). A higher BDI score was associated with smaller reductions in cue-induced craving and NAcc cue reactivity at the ROI level (r = 0.44 and 0.50, P = .035 and .014; Figure 1) from pre-treatment to on-treatment. BDI score was not correlated with changes in NAcc response to sexual or aversive stimuli, (r = 0.21 and 0.07, P = .34 and .75). Whole-brain analysis did not show correlation with BDI score in other regions. See the Supplementary Material for additional results.
Discussion
Greater depressive symptoms were associated with smaller reductions in cue-induced craving and NAcc drug cue reactivity during XR-NTX treatment, suggesting that depression may hamper XR-NTX’s ability to restore normal incentive salience processing. The lack of correlation between depressive symptoms and changes in NAcc response to the non-drug stimuli is consistent with our prior observation that XR-NTX effect was specific to drug cues.6 Although there is no evidence of XR-NTX causing depression,18–20 patients with greater depressive symptoms show more drug-related thoughts during XR-NTX treatment.21 Our data corroborate this finding and point to NAcc as a key region mediating the impact of depressive symptoms on XR-NTX effectiveness. Given that depression and OUD are highly comorbid,9 interventions targeting depressive symptoms may improve XR-NTX treatment success.22 Study limitations include small sample size, potential confounds in the visual stimulus parameters, and limited number of follow-up timepoints (see Supplementary Material). Future studies are needed to confirm our findings and explore other factors that contribute to individual differences in relapse vulnerability.
Published online: April 17, 2023.
Relevant financial relationships: None.
Funding/support: This work was supported by the Commonwealth of Pennsylvania CURE grant SAP#4100055577 (Dr Childress) and the following National Institutes of Health grants: DA051709 (Dr Shi), DA028874 (Dr Childress), AA026892 (Dr Wiers), and DA036028 (Dr Langleben).
Role of the sponsor: The funders had no role in the design, analysis, interpretation, or publication of this study.
Previous presentation: Presented as an oral communication at the Annual Meeting of the American Society of Clinical Psychopharmacology; May 31, 2022; Scottsdale, Arizona.
ORCID ID: Zhenhao Shi: https://orcid.org/0000-0001-5697-2034
Supplementary material: Available at Psychiatrist.com.
References (22)

- Courtney KE, Schacht JP, Hutchison K, et al. Neural substrates of cue reactivity: association with treatment outcomes and relapse. Addict Biol. 2016;21(1):3–22. PubMed CrossRef
- Li Q, Li W, Wang H, et al. Predicting subsequent relapse by drug-related cue-induced brain activation in heroin addiction: an event-related functional magnetic resonance imaging study. Addict Biol. 2015;20(5):968–978. PubMed CrossRef
- Shi Z, Jagannathan K, Padley JH, et al. The role of withdrawal in mesocorticolimbic drug cue reactivity in opioid use disorder. Addict Biol. 2021;26(4):e12977. PubMed CrossRef
- Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone for opioid dependence: a double-blind, placebo-controlled, multicentre randomised trial. Lancet. 2011;377(9776):1506–1513. PubMed CrossRef
- Krupitsky E, Nunes EV, Ling W, et al. Injectable extended-release naltrexone (XR-NTX) for opioid dependence: long-term safety and effectiveness. Addiction. 2013;108(9):1628–1637. PubMed CrossRef
- Shi Z, Wang AL, Jagannathan K, et al. Effects of extended-release naltrexone on the brain response to drug-related stimuli in patients with opioid use disorder. J Psychiatry Neurosci. 2018;43(4):254–261. PubMed CrossRef
- Langleben DD, Ruparel K, Elman I, et al. Extended-release naltrexone modulates brain response to drug cues in abstinent heroin-dependent patients. Addict Biol. 2014;19(2):262–271. PubMed CrossRef
- Volkow ND, Jones EB, Einstein EB, et al. Prevention and treatment of opioid misuse and addiction: a review. JAMA Psychiatry. 2019;76(2):208–216. PubMed CrossRef
- Substance Abuse and Mental Health Services Administration. 2020 National Survey on Drug Use and Health. 2022. https://pdas.samhsa.gov/#/survey/NSDUH-2020-DS0001/crosstab/?column=UDYR5OPI&results_received=false&row=AMDEYR&run_chisq=false&weight=ANALWTQ1Q4_C.
- Jelen LA, Stone JM, Young AH, et al. The opioid system in depression. Neurosci Biobehav Rev. 2022;140:104800. PubMed CrossRef
- Emery MA, Akil H. Endogenous opioids at the intersection of opioid addiction, pain, and depression: the search for a precision medicine approach. Annu Rev Neurosci. 2020;43(1):355–374. PubMed CrossRef
- Havard A, Teesson M, Darke S, et al. Depression among heroin users: 12-Month outcomes from the Australian Treatment Outcome Study (ATOS). J Subst Abuse Treat. 2006;30(4):355–362. PubMed CrossRef
- Huhn AS, Sweeney MM, Brooner RK, et al. Prefrontal cortex response to drug cues, craving, and current depressive symptoms are associated with treatment outcomes in methadone-maintained patients. Neuropsychopharmacology. 2019;44(4):826–833. PubMed CrossRef
- Rounsaville BJ, Weissman MM, Crits-Christoph K, et al. Diagnosis and symptoms of depression in opiate addicts. Course and relationship to treatment outcome. Arch Gen Psychiatry. 1982;39(2):151–156. PubMed CrossRef
- Hesse M. The Beck Depression Inventory in patients undergoing opiate agonist maintenance treatment. Br J Clin Psychol. 2006;45(Pt 3):417–425. PubMed CrossRef
- Moffett LA, Radenhausen RA. Assessing depression in substance abusers: Beck Depression Inventory and SCL-90R. Addict Behav. 1990;15(2):179–181. PubMed CrossRef
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. PubMed CrossRef
- Krupitsky E, Zvartau E, Blokhina E, et al. Anhedonia, depression, anxiety, and craving in opiate dependent patients stabilized on oral naltrexone or an extended release naltrexone implant. Am J Drug Alcohol Abuse. 2016;42(5):614–620. PubMed CrossRef
- Latif ZE, Šaltyte Benth J, Solli KK, et al. Anxiety, depression, and insomnia among adults with opioid dependence treated with extended-release naltrexone vs buprenorphine-naloxone: a randomized clinical trial and follow-up study. JAMA Psychiatry. 2019;76(2):127–134. PubMed CrossRef
- Zaaijer ER, van Dijk L, de Bruin K, et al. Effect of extended-release naltrexone on striatal dopamine transporter availability, depression and anhedonia in heroin-dependent patients. Psychopharmacology (Berl). 2015;232(14):2597–2607. PubMed CrossRef
- Dean AJ, Saunders JB, Jones RT, et al. Does naltrexone treatment lead to depression? Findings from a randomized controlled trial in subjects with opioid dependence. J Psychiatry Neurosci. 2006;31(1):38–45. PubMed
- Na PJ, Scodes J, Fishman M, et al. Co-occurring depression and suicidal ideation in opioid use disorder: prevalence and response during treatment with buprenorphine-naloxone and injection naltrexone. J Clin Psychiatry. 2022;83(3):21m14140. PubMed CrossRef
This PDF is free for all visitors!