The likelihood of being helped or harmed (LHH) ratio is an indirect measure of effect size. It tells the reader how much as likely a patient is to benefit from a treatment as to suffer from an adverse outcome with that treatment; larger values for LHH indicate more favorable treatment outcomes. The numerator for LHH is usually a measure of response or remission with a treatment, and the denominator is usually a measure of all-cause discontinuation or discontinuation due to adverse events; so, there can be more than 1 LHH statistic for a study. As an example, an LHH of 5 could indicate that after removal of placebo effects a patient is 5 times as likely to respond to a treatment as to drop out of treatment because of the experience of an adverse event. This article explains the LHH with the help of a worked example, shows how the LHH can be derived from the numbers needed to treat and harm (NNT, NNH) statistics, discusses practical issues related to the concept, and considers its limitations. The LHH is little used in clinical psychopharmacology, and authors who report or review clinical trial data should consider presenting all the LHH information that is clinically relevant in addition to NNT, NNH, and other information. Because LHH statistics present the results of risk-benefit trade-off analyses, they can help clinicians and patients more easily evaluate potential treatments during decision-making processes.
- The likelihood of being helped or harmed (LHH) ratio is a measure of the unique association of a treatment with a favorable outcome vs its unique association with an unfavorable outcome.
- Unique association, as referred to above, is the risk difference for an outcome between treatment and placebo.
- The favorable outcome is usually response to or remission with treatment; the unfavorable outcome is usually drop out due to adverse events.
- Instead of using risk differences, the LHH can be calculated as NNH/NNT, where these statistics are available. Larger values for LLH imply more favorable treatment results.

ABSTRACT
The likelihood of being helped or harmed (LHH) ratio is an indirect measure of effect size. It tells the reader how much as likely a patient is to benefit from a treatment as to suffer from an adverse outcome with that treatment; larger values for LHH indicate more favorable treatment outcomes. The numerator for LHH is usually a measure of response or remission with a treatment, and the denominator is usually a measure of all-cause discontinuation or discontinuation due to adverse events; so, there can be more than 1 LHH statistic for a study. As an example, an LHH of 5 could indicate that after removal of placebo effects a patient is 5 times as likely to respond to a treatment as to drop out of treatment because of the experience of an adverse event. This article explains the LHH with the help of a worked example, shows how the LHH can be derived from the numbers needed to treat and harm (NNT, NNH) statistics, discusses practical issues related to the concept, and considers its limitations. The LHH is little used in clinical psychopharmacology, and authors who report or review clinical trial data should consider presenting all the LHH information that is clinically relevant in addition to NNT, NNH, and other information. Because LHH statistics present the results of risk-benefit trade-off analyses, they can help clinicians and patients more easily evaluate potential treatments during decision-making processes.
J Clin Psychiatry 2017;78(1):e73-e75
https://doi.org/10.4088/JCP.16f11380
© Copyright 2017 Physicians Postgraduate Press, Inc.
Introduction
In clinical psychopharmacology, useful measures of effect size include statistics such as standardized mean deviation, relative risk, odds ratio, number needed to treat (NNT), and number needed to harm (NNH). The likelihood of being helped or harmed (LHH) is one among these statistics that is less well known and therefore less used (or perhaps it is less used and therefore less well known). Previous articles in this column addressed certain of these measures of effect size and related subjects.1-4 The present article considers the LHH.
Likelihood of Being Helped or Harmed: Concept
Conceptually, the LHH is the ratio of the probability of benefit to the probability of harm. In the context of a randomized controlled trial (RCT) that compares active drug with placebo, the probability of benefit is operationalized as the risk difference (between drug and placebo) for a favorable outcome, and the probability of harm is operationalized as the risk difference (between drug and placebo) for an unfavorable outcome. The favorable outcome is usually treatment response, and the unfavorable outcome is usually treatment discontinuation.
In the context referred to above, if the LHH is greater than 1, the patient is expected to be more likely to respond than to drop out of treatment. If the LHH is less than 1, the patient is expected to be more likely to drop out of treatment than to respond. In the unlikely event that the LHH is exactly 1.0, the patient has an equal chance of responding or dropping out of treatment. The concept of LHH and its interpretation will become clearer in the next section.
Worked Example and Interpretation
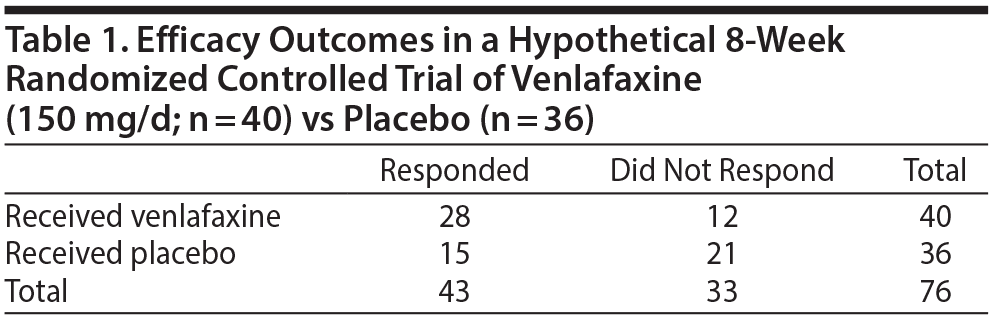
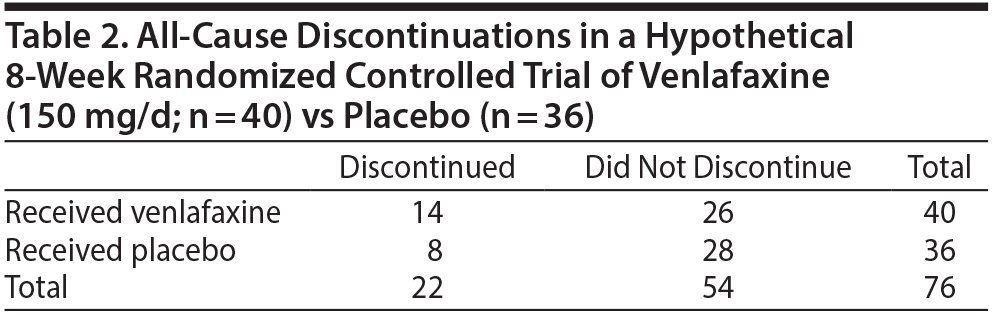
Consider a hypothetical RCT in which 76 depressed patients were randomized to receive either venlafaxine (150 mg/d; n = 40) or placebo (n = 36) for 8 weeks. Treatment response and all-cause discontinuation data are presented in Tables 1 and 2, respectively.
With regard to treatment response (Table 1), the probability of response to venlafaxine was 28/40, or 70%. The probability of response to placebo was 15/36, or 41.7%. The advantage for venlafaxine over placebo, or the unique contribution of venlafaxine toward response (that is, the risk difference) is therefore (70.0 – 41.7)%, or 28.3%. As explained in an earlier article,2 this translates to an NNT of 100/28.3; that is, approximately 3.5.
With regard to all-cause treatment discontinuation (Table 2), the probability of discontinuation with venlafaxine was 14/40, or 35.0%. The probability of discontinuation with placebo was 8/36, or 22.2%. The unique contribution of venlafaxine toward all-cause discontinuation, or the risk difference for discontinuation with venlafaxine (over discontinuation with placebo) is therefore (35.0 – 22.2)%, or 12.8%. As explained in an earlier article,2 this translates to an NNH of 100/12.8; that is, approximately 7.8.
The LHH, as defined earlier, is the probability of benefit divided by the probability of harm, or, more specifically, the ratio of the risk differences for benefit and harm. This works out to 28.3%/12.8%, or 2.2. What this means is that after removal of the placebo effect patients are 2.2 times as likely to respond to venlafaxine as they are to drop out of treatment for any cause. Expressed in another way, for every 2 (extra) patients who respond to venlafaxine, 1 (extra) patient will drop out of treatment for some reason, known or unknown.
Alternate Method of Calculation
As shown in the previous section, the NNT and NNH for the data in Tables 1 and 2 are 3.5 and 7.8, respectively. Note that the LHH can also be calculated as NNH/NNT; that is, 7.8/3.5, or 2.2. It is useful to know this alternate method of calculation because data may not always be available as presented in Tables 1 and 2; instead, NNT and NNH data may be available, as in a journal abstract or a presentation slide. A little bit of mental arithmetic will then quickly yield the LHH.
Usefulness of the LHH
The NNT and NNH express the benefits and risks in the context of the number of patients who are treated. In contrast, the LHH expresses the benefits in the context of the risks, thereby helping both clinician and patient evaluate potential risk-benefit trade-offs with the treatment. There is no advantage or disadvantage of any one of these statistics over the other; each conveys different information to the reader, and so each has its own place.
The LHH for specific efficacy vs harm outcomes can be compared across treatments and studies. A limitation here is that the populations from which the samples were drawn (in the studies of the different treatments) should be similar. As an example, it would be wrong to compare the LHH for venlafaxine and duloxetine when the venlafaxine study was conducted in youth or in selective serotonin reuptake inhibitor-resistant patients and the duloxetine study was conducted in elderly subjects or in nonresistant patients.
The Numerator and the Denominator
In the worked example described earlier, benefit was operationalized as treatment response; it could well have been remission with treatment. Likewise, harm was operationalized as all-cause discontinuation; it could well have been discontinuation due to adverse events. Whereas there are not many contenders for the numerator (beyond response and remission), there are many choices for the denominator.
Because response and remission are substantial benefits, a case could be made for choosing a clinically significant measure of harm for the denominator. An example of a meaningful choice could be dropout due to problematic adverse effects, or even merely the experience of problematic adverse effects, whether resulting in dropout or not. Therefore, when calculating the LHH, what the numerator and denominator should be will depend on what the reader wants from the data, to the extent that the desired data are available from the source. Authors and reviewers might wish to present more than 1 LHH value for a study, depending on the nature of the outcomes of clinical interest related to the treatment under consideration.
An Extended Explanation of the LHH
Just as the NNT and the NNH are incomplete without the inclusion of details of the trial (drug, dose, treatment duration, etc), so too is the LHH incomplete without these details. In the example presented in Tables 1 and 2, the LHH is more completely explained as follows: for every 2 extra depressed patients who respond to venlafaxine in the dose of 150 mg/d and administered for 8 weeks, 1 extra patient will drop out of treatment for whatever reason.
Limitations
The LHH is a ratio and so provides no information whatsoever about the actual rates of response and remission or of discontinuation due to any cause. In this regard, the LHH is no better or worse than the NNT and NNH.2,3 As already stated, different statistics serve different purposes, and each has its own value.
Parting Notes
The LHH is a relatively neglected statistic. Few authors present LHH information for treatments that they have trialed or reviewed. As an example of one referral to the LHH, in a pooled analysis of vilazodone RCTs, the NNT for response was 8 and the NNH for discontinuation due to adverse events was 27. The LHH was therefore 27/8, or 3.4. That is, patients with major depression who receive vilazodone at 40 mg/d for 8 weeks are 3.4 times as likely to respond as to drop out because of adverse events. Or, for every 3 extra patients who respond to vilazodone, 1 extra patient will drop out because of adverse events.5
As another example, data from a meta-analysis of 4 regulatory RCTs of duloxetine (80 mg/day) for stress urinary incontinence yielded LHH values of approximately 1; that is, for every extra woman who benefited (operationalized as a patient rating of much or very much better), 1 extra woman discontinued due to adverse events, and for every extra woman who benefited, 1 extra woman had problematic, activation-related adverse effects.6
An earlier and briefer discussion on LHH was provided by Akobeng.7
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Andrade C. Understanding relative risk, odds ratio, and related terms: as simple as it can get. J Clin Psychiatry. 2015;76(7):e857-e861. PubMed doi:10.4088/JCP.15f10150
2. Andrade C. The numbers needed to treat and harm (NNT, NNH) statistics: what they tell us and what they do not. J Clin Psychiatry. 2015;76(3):e330-e333. PubMed doi:10.4088/JCP.15f09870
3. Andrade C. Dr Andrade replies [letter]. J Clin Psychiatry. 2015;76(9):e1137. PubMed doi:10.4088/JCP.15lr10001a
4. Andrade C. A primer on confidence intervals in psychopharmacology. J Clin Psychiatry. 2015;76(2):e228-e231. PubMed doi:10.4088/JCP.14f09751
5. Citrome L. Vilazodone for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antidepressant—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2012;66(4):356-368. PubMed doi:10.1111/j.1742-1241.2011.02885.x
6. Maund E, Guski L, Gotzsche P. Considering benefits and harms of duloxetine for treatment of stress urinary incontinence: a meta-analysis of clinical study reports [published online ahead of print November 14, 2016]. CMAJ. doi:10.1503/cmaj.151104.
7. Akobeng AK. Communicating the benefits and harms of treatments. Arch Dis Child. 2008;93(8):710-713. PubMed doi:10.1136/adc.2008.137083
This PDF is free for all visitors!