ABSTRACT
Objective: To assess the effects of lurasidone on anxiety symptoms and sleep disruption, and their moderating and mediating roles on treatment response in bipolar depression.
Methods: This post hoc analysis included pooled data from 2 previously published 6-week placebo-controlled trials of lurasidone for bipolar I depression conducted between April 2009 and February 2012. Hamilton Anxiety Rating Scale (HAM-A) “psychic anxiety” (items 1–6, 14) and “somatic anxiety” (items 7–13) subscores were calculated. Functional outcome was assessed by the Sheehan Disability Scale.
Results: All subjects (n = 824) had at least 1 psychic anxiety and 729 (88.5%) had at least 1 somatic anxiety symptom at baseline. 594 subjects (72.1%) experienced baseline sleep disturbance. Lurasidone, as monotherapy (20–60 mg/d and 80–120 mg/d pooled dose groups vs placebo) and adjunctive therapy (20 to 120 mg/d flexibly dosed vs placebo) with lithium or valproate, significantly reduced HAM-A psychic anxiety (−4.82 vs −2.97, P < .001, monotherapy; −5.56 vs −4.26, P = .009, adjunctive therapy) and somatic anxiety (−1.89 vs −1.37, P = .048, monotherapy; −2.22 vs −1.47, P = .006, adjunctive therapy) subcomponents. Improvement in anxiety symptoms mediated reduction in depressive symptoms and functional impairment. Decrease in sleep at baseline predicted change in anxiety symptoms with lurasidone treatment at week 6.
Conclusions: Lurasidone was superior to placebo in reducing psychic and somatic anxiety in the short-term treatment of bipolar depression. Improvement in depressive symptoms and reduction in functional impairment were associated with reduction in anxiety symptoms moderated by baseline sleep disturbance during lurasidone treatment.
Trial Registration: ClinicalTrials.gov identifiers: NCT00868699 and NCT00868452
J Clin Psychiatry 2023;84(4):22m14732
Author affiliations are listed at the end of this article.
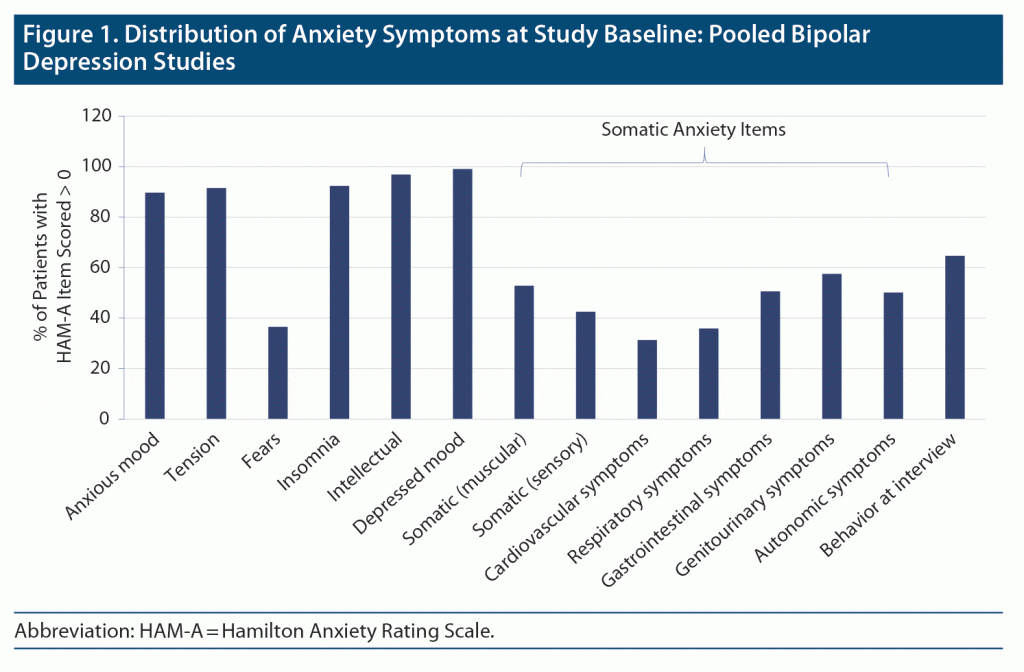
Anxiety and manic symptoms often occur during episodes of bipolar depression and pose important challenges for both diagnosis and treatment. Nearly half of adults with bipolar disorder present with comorbid anxiety symptoms,1,2 leading to higher illness burden,3 more affective episodes,4 decreased responsiveness to treatment,5,6 heightened suicidal ideation or behavior,4,7,8 impaired psychosocial functioning,4,8,9 and poor quality of life.10 Anxiety symptoms can be characterized by core components involving psychic anxiety (mental agitation and psychological distress, eg, fears, worries, irritability, tension, loss of interest and pleasure) and somatic anxiety (physical complaints related to anxiety, eg, palpitations, respiratory symptoms, hot and cold flushes, dry mouth).11
Despite the fact that nearly half of individuals with bipolar disorder meet standard diagnostic criteria for at least 1 comorbid anxiety disorder,2 few clinical trials have addressed the impact of comorbid anxiety phenomena in bipolar disorder. While selective serotonin reuptake inhibitors (SSRIs) are commonly used to treat anxiety symptoms and syndromes in patients without bipolar disorder, the sole empirical data examining SSRI anxiolytic therapy in the context of bipolar depression come from a post hoc analysis involving paroxetine, which failed as an active comparator to quetiapine but did show a provisional signal for anxiolytic properties.12 Other reports have questioned excessive use of monoaminergic antidepressants in patients with bipolar disorder who have concurrent anxiety, given the lack of broadly demonstrated anxiolytic efficacy in this population.9
Most FDA-approved pharmacotherapies for bipolar depression preliminarily addressed improvement in overall anxiety symptoms as a secondary endpoint, as shown previously in studies with lurasidone,13,14 quetiapine,15 olanzapine or olanzapine-fluoxetine combination,16 and cariprazine.17 The present post hoc analysis is intended to better characterize the breadth of lurasidone’s anxiolytic properties in the context of treating bipolar depression.
Comorbid anxiety has previously been identified as a correlate of sleep disturbance in bipolar disorder.8 Sleep disruption is highly prevalent18 and may be identified using instruments designed to assess various dimensions of bipolar depression.19–21 Prior studies of lurasidone for major depressive disorder with mixed features found that sleep disruption constituted a “bridge” symptom fundamentally linking depressive and manic symptoms.22 These findings also suggested that sleep disruption moderated and mediated treatment response to lurasidone when manic and depressive symptoms coexist. Moreover, previous studies found that a majority of bipolar depressive episodes incur subthreshold manic symptoms.23 Because sleep disruption and elements of psychomotor activation are pertinent to anxiety as well as subthreshold hypomania, we sought to examine both the anxiolytic and antidepressant efficacy of lurasidone during treatment for bipolar depression while accounting for possible moderating and mediating effects of sleep disruption.
Prior studies of lurasidone in bipolar depression13,14 demonstrated significant improvement on the Hamilton Anxiety Scale (HAM-A)19 total score compared to placebo. The objective of this post hoc analysis was to investigate the effects of lurasidone on the reduction of psychic and somatic anxiety components of the HAM-A, the relation between anxiety and sleep disruption, and their impact on overall treatment outcomes in patients with bipolar depression presenting with comorbid anxiety symptoms at baseline.
METHODS
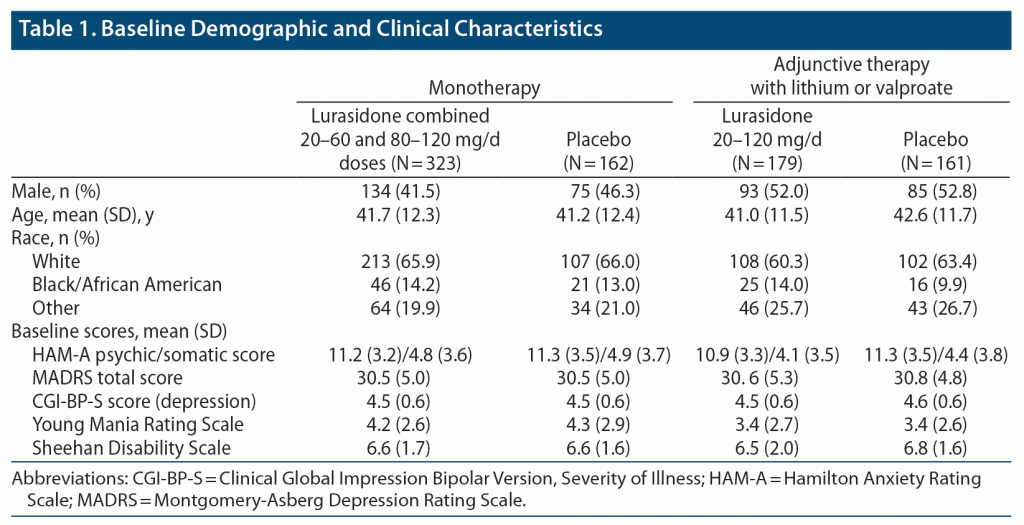
This post hoc analysis included pooled data from 2 placebo-controlled studies of lurasidone as monotherapy (20–60 mg/d and 80–120 mg/d, conducted from April 2009 to February 2012) and as adjunctive therapy (20–120 mg/d, conducted from May 2009 to January 2012) flexibly dosed with lithium or valproate in adult patients with bipolar I depression (ClinicalTrials.gov identifiers: NCT00868699 and NCT00868452).13,14 The key entry criteria for both studies included outpatients, aged 18–75 inclusive, with a DSM-IV-TR diagnosis of bipolar I disorder with current major depressive episode, a Montgomery-Asberg Depression Rating Scale (MADRS)20 score ≥ 20, and a Young Mania Rating Scale (YMRS)21 score ≤ 12; with or without rapid cycling; without psychotic features; and with a history of at least 1 lifetime bipolar manic or mixed manic episode.
Study Design
In the monotherapy study, patients were randomized to 6 weeks of double-blind treatment with flexibly dosed lurasidone (20–60 mg/d or 80–120 mg/d) or placebo.13 In the adjunctive therapy study, patients on stable therapy with either lithium or valproate were randomized to 6 weeks of double-blind treatment with flexibly dosed lurasidone (20–120 mg/d) or placebo.14
Improvement in anxiety symptoms was assessed by the change from baseline to week 6 on the HAM-A.19 A “psychic anxiety” symptom component was assessed by a sum of HAM-A items 1–6 and item 14 for mental agitation and psychological distress. A “somatic anxiety” symptom component was assessed by a sum of HAM-A item 7–13 for physical complaints related to anxiety.19 Based on median split, lower HAM-A was defined as HAM-A total < 14,19,24 lower psychic anxiety was defined as HAM-A psychic subcomponent of HAM-A < 12, and lower somatic anxiety was defined as HAM-A somatic subcomponent of HAM-A < 4.
Antidepressant efficacy was assessed by change in MADRS ratings20 and in CGI-BP-S score (Depression).25 Functional impairment was assessed using the Sheehan Disability Scale (SDS), assessed at baseline and week 6 endpoint.26 The YMRS was included as a secondary endpoint to assess the occurrence of any manic symptoms during the study.21
The presence of sleep disturbance was defined as a rating of “reduced sleep” (assessed by MADRS item 4); “insomnia” (assessed by HAM-A item 4); and “decrease in sleep” (assessed by YMRS item 4), with all items rated > 0. We examined both the potential moderating and mediating effects of “decrease in sleep” (YMRS item 4) on changes in anxiety (HAM-A) and depressive (MADRS) symptoms based on prior studies pointing to the sleep item 4 on YMRS as a significant correlate of reduced sleep (insomnia) and exerting an important influence over treatment outcomes in bipolar disorder.22,27–29 YMRS item 4 was rated on a 5-point scale from “0” for “Reports no decrease in sleep,” 1 for “Sleeping less than normal amount by up to one hour,” “2” for “Sleeping less than normal by more than one hour,” “3” for “Reports decreased need for sleep,” and “4” for “Denies need for sleep.” YMRS item 4 > 0 indicated the presence of “decrease in sleep” or reduced sleep time.
Institutional review board approval was obtained at each study site for the parent study, as described in the previous publications addressing the primary outcome measure of antidepressant efficacy.13,14
Statistical Analysis
In this post hoc analysis, we evaluated the relationship between symptoms of anxiety and depression in patients with bipolar depression, and their implications for treatment response to lurasidone. Bivariate correlations (eg, “psychic anxiety” or “somatic anxiety” component scores and “decrease in sleep”) were presented in univariate analyses as Spearman coefficients.
An analysis of covariance (ANCOVA) model was applied to evaluate effectiveness of lurasidone (vs placebo) treatment for improvement in psychic anxiety and somatic anxiety subcomponents of HAM-A assessed at baseline and week 6 endpoint, and sleep disturbance.
The potential moderating effect of decrease in sleep at study baseline on the relationship between treatment group and change from baseline in anxiety outcome measures and SDS score was evaluated by a statistical interaction test for treatment group and “decrease in sleep” (baseline YMRS item 4 > 0 vs = 0 reports no decrease in sleep) in the ANCOVA model.
The relation between antidepressant response to lurasidone (assessed by change in MADRS and CGI-BP-S score [Depression] at week 6 endpoint) and baseline anxiety was evaluated using a mixed model for repeated measures (MMRM) with interaction effects for treatment group and stratified baseline anxiety based on median split.
We conducted exploratory analyses to investigate changes in depressive symptoms and anxiety symptoms as potential mediator variables for improvement in functioning outcome during lurasidone treatment. The ANCOVA model included terms for treatment, baseline SDS score, week 6 change in MADRS from baseline, week 6 change in HAM-A from baseline, and pooled sites. The associations between changes in depressive symptoms, anxiety symptoms, and functioning were also explored by analyzing changes in depressive symptoms and SDS as mediator variables for changes in HAM-A in an ANCOVA model.
Due to the exploratory nature of these analyses, adjustment for multiple comparisons was not performed. Results were summarized by basic summary statistics of mean and standard error (SE), 95% confidence interval (CI) of the effect estimates, Cohen d placebo-corrected effect sizes, and nominal P values.
RESULTS
Baseline characteristics of the study group are presented in Table 1.
Symptoms of anxiety were highly prevalent in patients with bipolar depression at study baseline, with 100% (n = 824) having at least 1 psychic anxiety symptom (HAM-A items 1–6 and item 14, mean 11.2), and 88.5% (n = 729) having at least 1 somatic anxiety symptom (HAM-A items 7–13, mean 4.6). Prevalence rates for each of the individual HAM-A items are depicted in Figure 1.
Concomitant Anxiety and Sleep Symptoms During Depression
The prevalence of “decrease in sleep” (YMRS item 4 > 0) at study baseline was 72.5% (597/824) for the pooled sample of monotherapy and adjunctive therapy studies, with 76.3% (370/485) in the monotherapy and 67.0% (227/339) in the adjunctive therapy studies. A total of 494 (60.0%) patients reported “Sleeping less than normal by more than one hour” (YMRS item 4 = 2), with 62.3% (302/485) in the monotherapy and 56.6% (192/339) in the adjunctive therapy studies.
A total of 594 (72.1%) patients in the pooled sample (n = 369, 76.1% in the monotherapy study and n = 225, 66.4% in the adjunctive therapy study) had “sleep disturbance” (sleep item 4 > 0 on HAM-A, MADRS, and YMRS) at study baseline with high correlations between these symptoms (r = 0.41 to 0.68, all P < .001). Only 47 (5.7%) patients did not have any of these sleep-related symptoms. Both “decrease in sleep” (YMRS item 4) and “insomnia” (by HAM-A item 4) correlated with MADRS total score at baseline.
In univariate analyses, the HAM-A psychic anxiety component correlated with baseline symptoms of “reduced sleep” (MADRS item 4, r = 0.30, P = .010, Spearman correlation) and MADRS total score (r = 0.45, P < .001). There were significant correlations between related psychic anxiety and depressive symptoms, including depressed mood (HAM-A item 6 and MADRS items 1 and 2, r = 0.33–0.38, P < .001), tension (HAM-A item 2 and MADRS item 3, r = 0.62, P < .001), intellectual difficulty (HAM-A item 5 and MADRS item 6, r = 0.57, P < .001), and insomnia (HAM-A item 4 and MADRS item 4, r = 0.70, P < .001).
The somatic anxiety component of the HAM-A (items 7–13) correlated with baseline symptoms of “reduced sleep” (MADRS item 4, r = 0.12, P < .001), MADRS total (r = 0.239, P < .001), and “decrease in sleep” (YMRS item 4, r = −0.134, P = .003). Further, the somatic anxiety score also correlated with inner tension related to depression (MADRS item 3, r = 0.26, P < .001) and tension in psychic anxiety (HAM-A item 2, r = 0.40, P < .001).
Treatment of Anxiety Symptoms
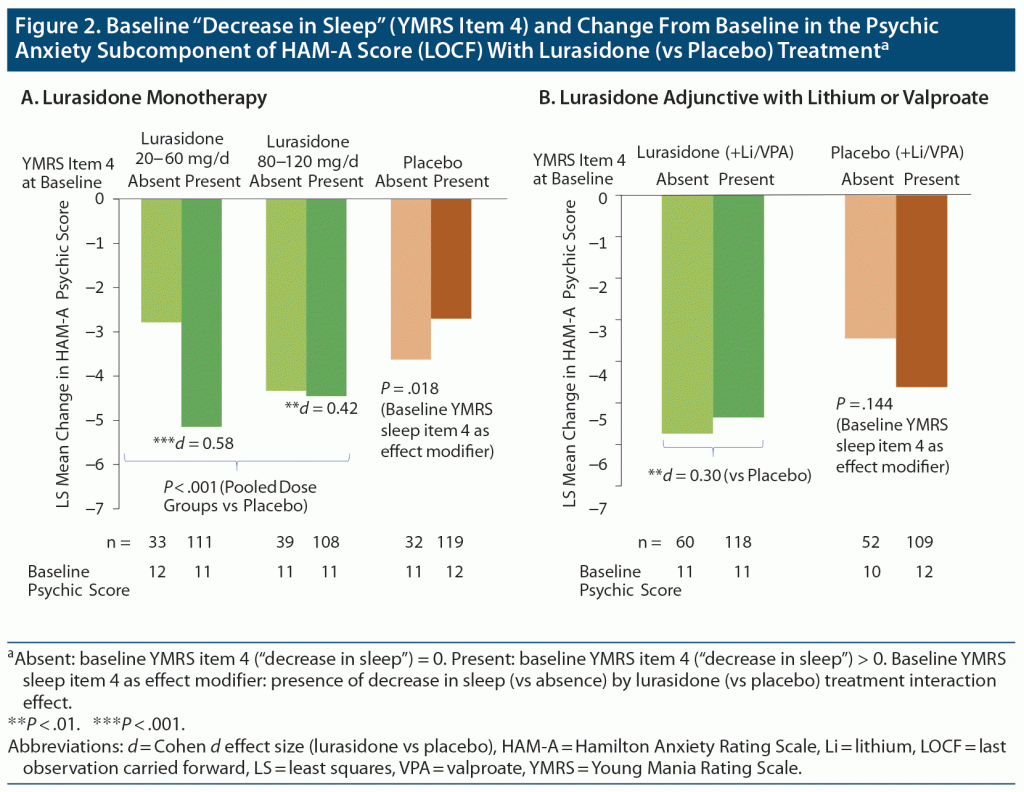
Lurasidone was associated with significant change in the HAM-A psychic anxiety component score from baseline to week 6 (Figure 2) as monotherapy (P < .001; 95% CI for between treatment group difference: −2.69, −1.00; LSM = −4.82 ± 0.37 for lurasidone vs −2.97 ± 0.28 for placebo) and as adjunctive therapy with lithium or valproate (P = .009; 95% CI for between treatment group difference: −2.27, −0.32; LSM = −5.56 ± 0.41 for lurasidone vs −4.26 ± 0.42 for placebo) (Figure 2).
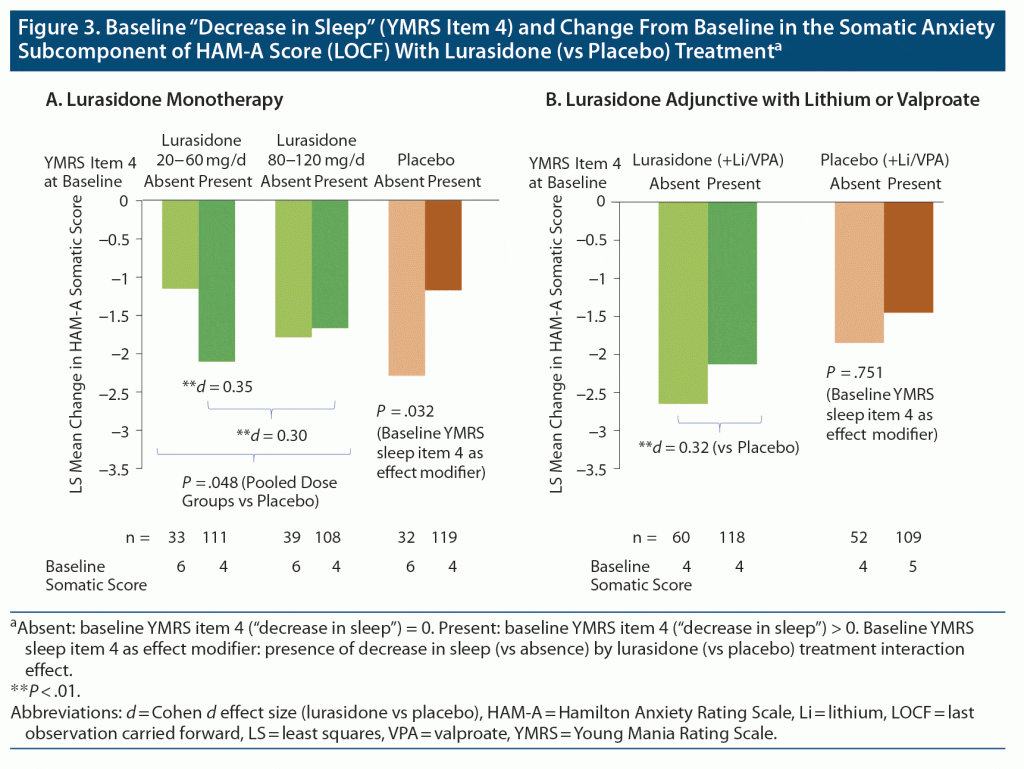
Lurasidone was associated with significant change in the HAM-A somatic anxiety component score from baseline to week 6 (Figure 3), as monotherapy (P = .048; 95% CI for between treatment group difference: −1.04, −0.01; LSM = −1.89 ± 0.17 for lurasidone vs −1.37 ± 0.23 for placebo, adjusted for decrease in sleep, baseline anxiety severity, and region) and as adjunctive therapy with lithium or valproate (P = .006; 95% CI for between treatment group difference: −1.29, −0.23; LSM = −2.22 ± 0.22 for lurasidone vs −1.47 ± 0.23 placebo, adjusted for decrease in sleep, baseline anxiety severity, and region).
Changes from baseline in HAM-A (P < .001, F1, 430 = 511.21) as well as psychic (P < .001, F1, 430 = 790.06) and somatic (P <.001, F1, 430 = 94.76) subcomponents of HAM-A were significantly associated with change in MADRS score with lurasidone monotherapy and adjunctive treatment (all P < .001).
Changes from baseline in HAM-A (P < .001, F1, 396 = 167.97) as well as psychic (P < .001, F1, 396 = 208.44) and somatic (P < .001, F1, 396 = 47.06) subcomponents of HAM-A were significantly associated with change in SDS score with lurasidone monotherapy as well as adjunctive treatment (all P < .001). Furthermore, changes from baseline in SDS score mediated changes in HAM-A with lurasidone monotherapy (P < .001, F1, 396 = 240.39) and adjunctive treatment (P < .001, F1, 275 = 132.42).
The mediation association between changes in HAM-A and SDS scores was significant for lurasidone monotherapy (P = .044, F1,395 = 4.08) after accounting for improvement in MADRS total score (P < .001, F1,395 = 117.29) but not for adjunctive therapy with lithium or valproate (P = .519).
Baseline Anxiety and Antidepressant Efficacy
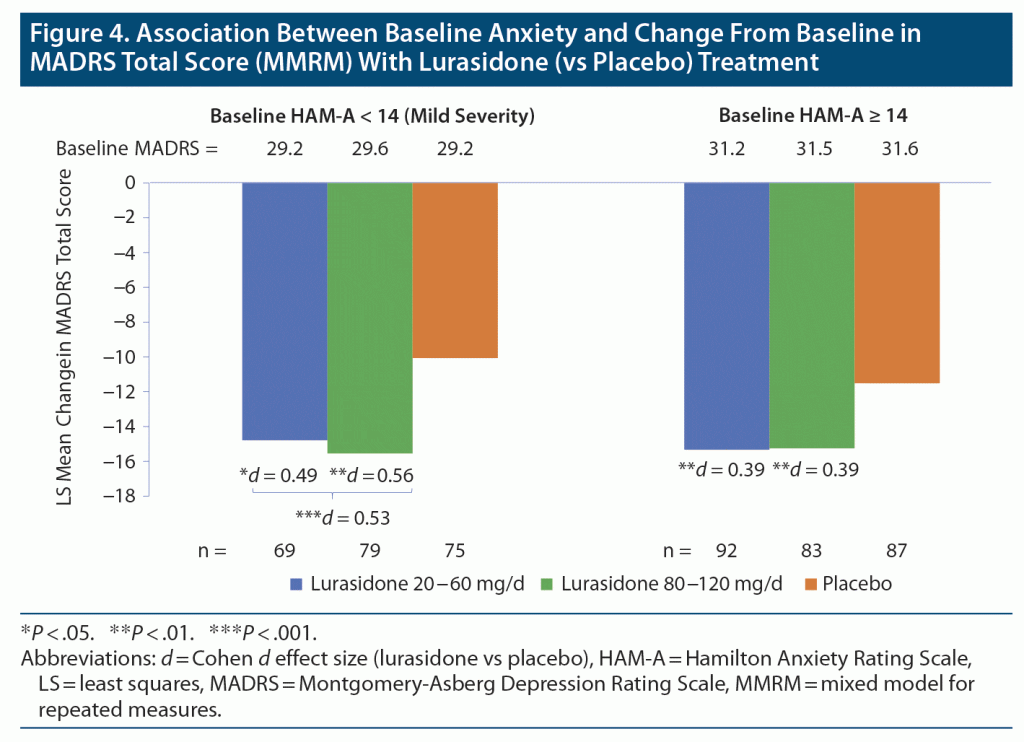
Treatment with lurasidone (vs placebo) significantly improved MADRS total score in both the lower anxiety (HAM-A total < 14 mild severity, median split, n = 223) and higher anxiety (n = 262) groups. Among participants in the monotherapy study, treatment effect size for improvement in depressive symptoms was 0.53 (P = .001, t444 = −3.3; 95% CI for between treatment group difference: −8.15, −2.07; LSM = −15.19 for lurasidone and −10.08 for placebo) for participants with lower baseline anxiety symptoms, and 0.39 (P = .005, t444 = −2.8; 95% CI for between treatment group difference: −6.39, −1.16; LSM = −15.28 for lurasidone and −11.51 for placebo) for participants with higher anxiety symptoms at baseline (Figure 4).
In a sensitivity analysis using a high HAM-A cutoff value of 17 (moderate to severe), treatment effect size for change in MADRS total score was 0.56 (P < .001; LSM = −15.33 for lurasidone and −9.79 for placebo) for monotherapy study participants with lower baseline anxiety symptoms (HAM-A total < 17, n = 304) and 0.32 (P = .045; n = 181; LSM = −15.21 for lurasidone and −11.81 for placebo) for monotherapy study participants with higher anxiety symptoms at baseline. Mean change in MADRS score in the placebo group increased from −11.51 for baseline HAM-A > 14 to −12.9 for a more extreme cutoff value of 20 (baseline HAM-A > 20), suggesting regression to mean might contribute to placebo response.
Treatment with lurasidone (vs placebo) significantly improved MADRS total score in both the lower psychic anxiety (psychic anxiety subcomponent of HAM-A < 12, median split, n = 273) and higher psychic anxiety (n = 212) groups. Among participants in the monotherapy study, treatment effect size for improvement in depressive symptoms was 0.45 (P < .001) for participants with lower baseline psychic anxiety symptoms and 0.43 (P = .004) for participants with higher psychic anxiety symptoms at baseline.
Treatment with lurasidone (vs placebo) significantly improved MADRS total score in both the lower somatic anxiety (somatic anxiety subcomponent of HAM-A < 4, median split, n = 205) and higher somatic anxiety (n = 280) groups. Among participants in the monotherapy study, treatment effect size for improvement in depressive symptoms was 0.53 (P < .001) for participants with lower baseline somatic anxiety symptoms and 0.38 (P = .003) for participants with higher somatic anxiety symptoms at baseline.
Treatment with lurasidone (vs placebo) significantly improved CGI-BP-S score (Depression) in both the lower anxiety (HAM-A total < 14, median split, n = 223) and higher anxiety (n = 262) groups. Among participants in the monotherapy study, treatment effect size for improvement in CGI-BP-S score (Depression) was 0.61 (P < .001) for participants with lower baseline anxiety symptoms and 0.43 (P = .001) for participants with higher anxiety symptoms at baseline.
Moderating Effects of Decrease in Sleep
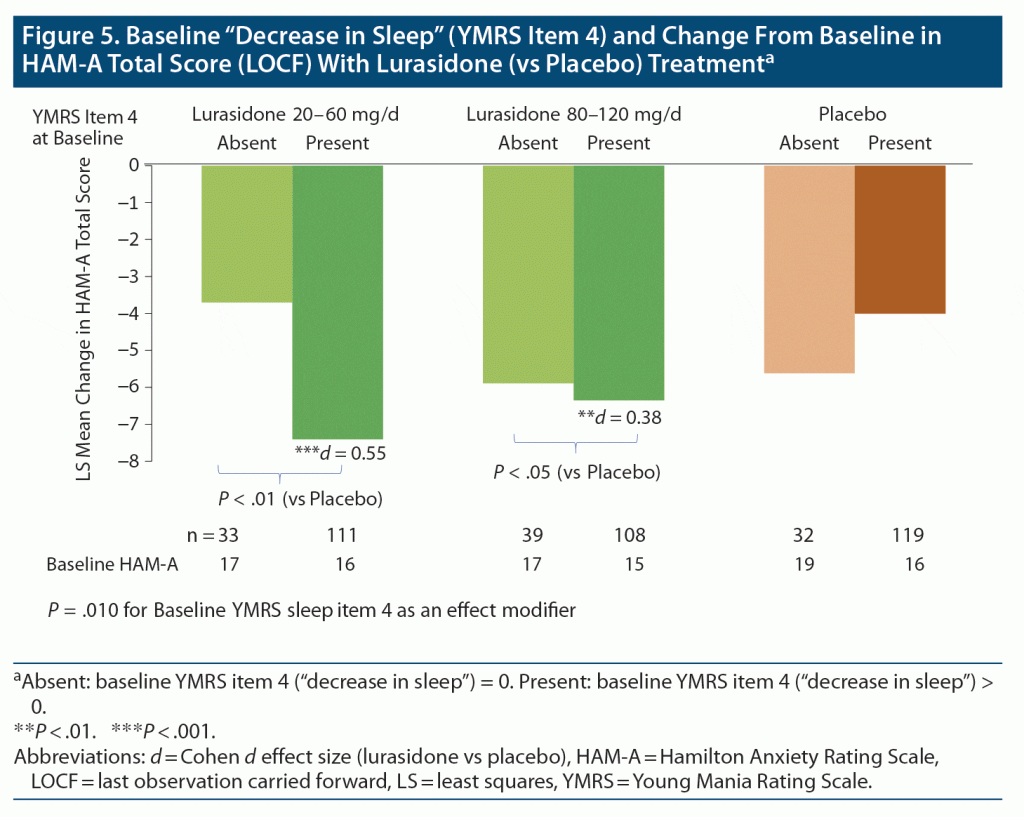
A significant interaction was found between baseline decrease in sleep (presence vs absence) and lurasidone monotherapy treatment groups for change in overall anxiety score between baseline and study endpoint (P = .010 for baseline YMRS item 4 “decrease in sleep” interaction with treatment, F2, 428 = 4.69) (Figure 5).
When baseline decrease in sleep was present, lurasidone monotherapy improved overall anxiety score; with medium effect size for the pooled dose group (Cohen d = 0.47, P < .001) and for the low dose range (20–60 mg/d) (d = 0.55, P < .001) or high dose range (80–120 mg/d) (Cohen d = 0.38, P = .008) (Figure 5). However, during adjunctive therapy, no significant interaction effect was observed involving baseline decrease in sleep with lurasidone (versus placebo added to lithium or valproate) for the change in HAM-A total score (Figure 5).
Psychic Anxiety
A significant interaction was found between baseline decrease in sleep (presence vs absence) and lurasidone monotherapy treatment groups for change in psychic anxiety score between baseline and study endpoint (P = .018 for baseline YMRS item 4 “decrease in sleep” interaction with treatment, F2, 428 = 4.03) (Figure 2).
When baseline decrease in sleep was present, lurasidone monotherapy improved psychic anxiety; medium effect sizes were observed for the pooled dose group (Cohen d = 0.50, P < .001) and for the low dose range (20–60 mg/d) (Cohen d = 0.58, P < .001) or high dose range (80–120 mg/d) (Cohen d = 0.42, P = .002) (Figure 2). However, during adjunctive therapy, no significant interaction effect was observed involving baseline decrease in sleep with lurasidone (versus placebo added to lithium or valproate) treatment for the change in psychic anxiety component score (P = .144, F1, 296 = 2.15) (Figure 2).
Somatic Anxiety
A significant interaction was found between baseline decrease in sleep (presence vs absence) and lurasidone monotherapy treatment groups for change in somatic anxiety score between baseline and study endpoint (P = .032 for baseline YMRS item 4 “decrease in sleep” interaction with treatment, F2, 428=3.47) (Figure 3).
When baseline decrease in sleep was present, lurasidone monotherapy improved somatic anxiety with effect size of 0.3; a larger effect size was seen at the low dose range (20–60 mg/d: Cohen d = 0.35, P = .008) than at the higher dose range (80–120 mg/d; Cohen d = 0.24, P = .069). Placebo response was smaller in patients who had baseline “decrease in sleep” with lower somatic anxiety (median 4.0 vs 6.0 for YMRS = 0) at baseline (Figure 3).
During adjunctive therapy, no significant interaction effect was observed involving baseline decrease in sleep with lurasidone (vs placebo added to lithium or valproate) treatment for the change in somatic anxiety component score (P = .751, F1, 296 = 0.10) (Figure 3).
Mediating Effects of Decrease in Sleep
Reduction in YMRS item 4 (“decrease in sleep”) from baseline was significantly associated with improvement in the HAM-A (P < .001, F1, 401 = 92.16) and MADRS total scores (P < .001, F1, 444 = 98.81) with lurasidone monotherapy treatment. Similarly, significant associations between improvement in decrease in sleep and these outcome measures were observed for lurasidone adjunctive therapy with lithium or valproate treatment (all P < .001).
Lurasidone monotherapy or adjunctive therapy with lithium/valproate treatment (vs placebo) was associated with significantly greater improvement in “reduced sleep” (MADRS item 4, all P = .005 for the pooled monotherapy dose groups vs placebo, P = .015 for adjunctive therapy vs placebo), “insomnia” (HAM-A item 4, P = .006 for the pooled monotherapy dose groups vs placebo, P = .016 for adjunctive therapy vs placebo), and “decrease in sleep” (YMRS item 4, P = .002 for lurasidone 20–60 mg/d vs placebo; P = .214 for lurasidone 80–120 mg/d vs placebo; P = .043 for adjunctive therapy vs placebo).
DISCUSSION
The results of this post hoc analysis of 2 placebo-controlled clinical trials indicated that lurasidone treatment, as monotherapy (20–60 mg/d and 80–120 mg/d vs placebo, pooled dose groups) and as adjunctive therapy (20 to 120 mg/d flexibly dosed vs placebo) with lithium or valproate, was efficacious in treating both psychic and somatic anxiety (subcomponents of HAM-A) over a 6-week treatment period. These data extend previously reported findings on the efficacy of lurasidone in improving overall anxiety symptom severity (assessed by HAM-A total)13,14 and suggest that lurasidone may be useful for the treatment of both psychological and physical symptoms of anxiety in patients with bipolar depression.
Significant improvement in depressive symptom severity associated with lurasidone monotherapy (vs placebo) treatment was observed in patients with higher and lower anxiety symptom scores at study baseline. Reduction in depressive symptoms with lurasidone treatment in patients with low anxiety symptom severity at study baseline suggests a specific antidepressant effect associated with lurasidone treatment independent of antianxiety effect.
While the mechanism for the antianxiety effects of lurasidone are not well understood, preclinical studies have suggested possible anxiolytic properties associated with agents (such as lurasidone) that exhibit potent 5-HT7 antagonism,30,31 5-HT1A partial agonism,32,33 and/or D2 antagonism.34
Most patients (72.1%) in the pooled samples from monotherapy and adjunctive studies were found to have sleep disturbances at study baseline as assessed by HAM-A item 4 (“insomnia”), MADRS item 4 (“reduced sleep”), and YMRS item 4 (“decrease in sleep”) (all rated > 0). These data are consistent with prior findings that sleep disturbance is frequently present in patients with bipolar depression.6
YMRS, HAM-A, and MADRS sleep items were correlated in this study, suggesting that they measure an overlapping construct in patients with bipolar depression, ie, sleep disturbance of a nonspecific nature including reduced sleep time and other forms of insomnia.
“Decrease in sleep” (YMRS item 4) at study baseline was a significant effect modifier for improvement in anxiety symptoms at week 6 endpoint during lurasidone monotherapy (vs placebo) treatment, with greater anxiolytic efficacy when sleep disturbance was present at baseline. The presence (vs absence) of decrease in sleep at study baseline predicted changes in anxiety symptoms with lurasidone (vs placebo) treatment at week 6 endpoint. These findings were explained, in part, by mediation associations between a significant reduction of sleep disturbance and improvement in anxiety and depressive symptoms at study endpoint.
Our findings are consistent with recent symptom network analyses demonstrating a key role of sleep disturbance (YMRS item 4) as an effect modifier and mediator for improvement in overall depression severity with lurasidone (vs placebo) treatment in adult patients with MDD and mixed features22 or children and adolescents with bipolar depression.27 Taken together, these findings suggest that sleep disturbance plays a likely role in the development of anxiety symptom, and can help predict treatment response in patients with bipolar depression.22,27–29
All patients included in the current post hoc analysis (n = 825, 100%) were found to have 3 or more symptoms of anxiety at baseline, with at least 1 psychic anxiety symptom in all patients and at least 1 somatic anxiety symptom in 88.5% of patients. These findings are consistent with prior reports that a variety of anxiety symptoms occur frequently in association with bipolar depressive episodes.23,35–37 It would be useful for future studies to further examine the potential anxiolytic effects of lurasidone in a cohort of euthymic bipolar disorder patients with residual or comorbid anxiety symptoms, in order to provide more definitive information about the specificity of lurasidone’s putative anxiolytic properties in bipolar disorder patients.
Concurrent anxiety symptoms with bipolar disorders have been associated with a family history of mood disorders and early age at onset of bipolar disorder, leading to decreased treatment response and poorer quality of life.4,38 Moreover, from a pragmatic standpoint, clinicians often struggle to identify useful and efficacious treatments for anxiety symptoms in bipolar depression and may favor unstudied options such as SSRIs or benzodiazepines without necessarily appreciating that at least some existing FDA-approved treatments for bipolar depression may in themselves render anxiolytic efficacy.
It is unknown whether or not second-generation antipsychotics exert a class effect on treating anxiety symptoms in bipolar disorder. A review by Rakofsky and Dunlop39 observed that risperidone was no better than placebo in treating anxiety in bipolar disorder, while olanzapine appeared superior to lamotrigine. In studies of cariprazine for bipolar depression, concomitant anxiolytic efficacy using the HAM-A was evident at low doses (1.5 mg/d) but not high doses (3.0 mg/d).17 Quetiapine dosed at 300 or 600 mg/d was superior to placebo in reducing HAM-A scores in registration trials for depression, although a separate randomized trial of quetiapine for bipolar disorder with comorbid generalized anxiety disorder found no differences in change from baseline HAM-A scores between drug and placebo.40
Limitations
The current investigation has several limitations. Our findings are based on a post hoc analysis of monotherapy and adjunctive therapy placebo-controlled trials in patients with bipolar depression that were not specifically designed to investigate the role of anxiety symptoms or sleep disturbance in moderating and mediating treatment response. While these trials involved a similar patient population treated for 6 weeks with comparable lurasidone dose ranges, it is possible that response to lurasidone may differ for monotherapy versus adjunctive treatment.
Sleep disturbance was an effect modifier for lurasidone monotherapy (vs placebo) but not for adjunctive treatment with lithium and valproate. Given the small magnitudes of placebo-corrected treatment effect sizes for improvement in anxiety symptoms in the adjunctive therapy study (in both the presence and absence of sleep disturbance), there was a lack of statistical power for detecting the moderating effects of sleep disturbance in the adjunctive therapy study.
A larger change in depressive symptom severity was seen in placebo-treated patients with higher (vs lower) HAM-A scores (based on median split) at study baseline, leading to a small observed difference in placebo-corrected effect size for improvement in MADRS score between the higher and lower anxiety groups. A sensitivity analysis found that progressively higher HAM-A score thresholds at study baseline were associated with greater reductions in depressive severity in placebo-treated patients. This finding was likely influenced by regression-to-mean effects.
Sleep disturbance was not assessed based on a specific sleep disorders questionnaire or polysomnography, which may limit the reliability of the reported findings. Psychic and somatic anxiety symptoms were assessed using a single scale (HAM-A). It is possible that measures specific for these anxiety-related subcomponents would permit a more comprehensive assessment of these symptoms.
Given many YMRS items were rated 0 (absent) for most participants at study baseline, this limited the analysis of clustering patterns of manic/hypomanic symptoms (as assessed by the 11 YMRS items) and their links to sleep disturbance in this study sample.
In this post hoc analysis, we found mediation associations between changes in anxiety symptoms, depressive symptoms, and functioning with lurasidone monotherapy and adjunctive treatment over a 6-week treatment period in patients with bipolar depression. However, improvement in anxiety symptoms may reflect in part improvement in the underlying depressive syndrome in patients with bipolar depression. Importantly, there were mediation associations between improvement in anxiety symptoms and reduction in functional impairment with lurasidone monotherapy treatment after accounting for improvement in depressive symptoms.
No multiplicity adjustments were utilized for the post hoc analyses included in this report. Further prospective trials are needed to confirm the exploratory findings reported here.
CONCLUSION
The current post hoc analysis findings derived from 2 placebo-controlled trials suggest that lurasidone as monotherapy or adjunctive therapy with lithium or valproate was effective in reducing anxiety symptoms (psychic and somatic anxiety subcomponents of the HAM-A) in patients with bipolar depression presenting with comorbid anxiety symptoms. Improvement in anxiety symptoms was associated with reduction in depressive symptoms and functional impairment during lurasidone treatment. The presence of “decrease in sleep” moderated antianxiety responses to lurasidone monotherapy (vs placebo) treatment. This analysis underscores the importance of targeting concurrent anxiety and sleep disturbance symptoms in the treatment of patients with bipolar depression.
ARTICLE INFORMATION
Published Online: June 7, 2023. https://doi.org/10.4088/JCP.22m14732
© 2023 Physicians Postgraduate Press, Inc.
Submitted: November 17, 2022; accepted February 20, 2023.
To Cite: Goldberg JF, Siu C, Tocco M, et al. The effect of lurasidone on anxiety symptoms in patients with bipolar depression: a post hoc analysis. J Clin Psychiatry. 2023;84(4):22m14732.
Author Affiliations: Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, New York (Goldberg); COS and Associates, Central, Hong Kong and Toronto, Ontario, Canada (Siu); Sunovion Pharmaceuticals Inc., Teaneck, New Jersey, and Marlborough, Massachusetts (Tocco, Pikalov, Loebel).
Corresponding Author: Joseph F. Goldberg, MD, 128 East Ave, Norwalk, CT 06851 ([email protected]).
Relevant Financial Relationships: Dr Goldberg has received honoraria/speaking fees from Abbvie, Alkermes, Axsome, and Intracellular Therapies; consulting fees from BioXcel, Neurelis, Neumora, Sage Pharmaceuticals, Sunovion, Supernus; and royalties from American Psychiatric Publishing and Cambridge University Press. Dr Siu reports having received consulting fees from Sunovion and the Chinese University of Hong Kong in the past 3 years. Drs Tocco, Pikalov, and Loebel are employees of Sunovion Pharmaceuticals Inc.
Funding/Support: The study was funded, conducted, and supported by Sunovion Pharmaceuticals, Inc.
Role of the Funder/Sponsor: The study sponsor was involved in the design, collection, and analysis of the data. Interpretation of the results was by the authors, and the decision to submit this manuscript for publication in The Journal of Clinical Psychiatry was made by the authors.
Clinical Points
- Anxiety symptoms and sleep disturbance (insomnia or decrease in sleep) are prevalent in patients with bipolar depression.
- Anxiety symptoms can be categorized as psychic anxiety (mental agitation and psychological distress, eg, fears, worries, irritability, tension, loss of interest and pleasure) and somatic anxiety (physical complaints related to anxiety, eg, palpitations, respiratory symptoms, hot and cold flushes, dry mouth).
- In a placebo-controlled monotherapy trial of patients with bipolar depression, decrease in sleep at study baseline predicted improvement in anxiety symptoms with lurasidone at week 6 endpoint.
- Reductions in depressive and anxiety symptoms were associated with improvement in decrease in sleep with lurasidone (alone or added to lithium or valproate).
- Lurasidone (alone or added to lithium or valproate) was associated with improvement in both psychological and physical symptoms of anxiety and sleep disturbance in patients with bipolar depression.
References (40)

- Lee JH, Dunner DL. The effect of anxiety disorder comorbidity on treatment resistant bipolar disorders. Depress Anxiety. 2008;25(2):91–97. PubMed CrossRef
- Pavlova B, Perlis RH, Alda M, et al. Lifetime prevalence of anxiety disorders in people with bipolar disorder: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2(8):710–717. PubMed CrossRef
- Boylan KR, Bieling PJ, Marriott M, et al. Impact of comorbid anxiety disorders on outcome in a cohort of patients with bipolar disorder. J Clin Psychiatry. 2004;65(8):1106–1113. PubMed CrossRef
- Sala R, Goldstein BI, Morcillo C, et al. Course of comorbid anxiety disorders among adults with bipolar disorder in the US population. J Psychiatr Res. 2012;46(7):865–872. PubMed CrossRef
- Feske U, Frank E, Mallinger AG, et al. Anxiety as a correlate of response to the acute treatment of bipolar I disorder. Am J Psychiatry. 2000;157(6):956–962. PubMed CrossRef
- Henry C, Van den Bulke D, Bellivier F, et al. Anxiety disorders in 318 bipolar patients: prevalence and impact on illness severity and response to mood stabilizer. J Clin Psychiatry. 2003;64(3):331–335. PubMed CrossRef
- Simon NM, Pollack MH, Ostacher MJ, et al. Understanding the link between anxiety symptoms and suicidal ideation and behaviors in outpatients with bipolar disorder. J Affect Disord. 2007;97(1-3):91–99. PubMed CrossRef
- Hawke LD, Provencher MD, Parikh SV, et al. Comorbid anxiety disorders in Canadians with bipolar disorder: clinical characteristics and service use. Can J Psychiatry. 2013;58(7):393–401. PubMed CrossRef
- Schaffer A, Cairney J, Veldhuizen S, et al. Comparison of antidepressant use between subjects with bipolar disorder and major depressive disorder with or without comorbid anxiety. J Clin Psychiatry. 2007;68(11):1785–1792. PubMed CrossRef
- Yoldi-Negrete M, Morera D, Palacios-Cruz L, et al. Subsyndromal anxiety: does it affect the quality of life? a study on euthymic patients with bipolar disorder. Eur J Psychiatry. 2019;33(4):159–164. CrossRef
- Gelenberg AJ. Psychiatric and somatic markers of anxiety: identification and pharmacologic treatment. Prim Care Companion J Clin Psychiatry. 2000;2(2):49–54. PubMed CrossRef
- McElroy SL, Weisler RH, Chang W, et al; EMBOLDEN II (Trial D1447C00134) Investigators. A double-blind, placebo-controlled study of quetiapine and paroxetine as monotherapy in adults with bipolar depression (EMBOLDEN II). J Clin Psychiatry. 2010;71(2):163–174. PubMed CrossRef
- Loebel A, Cucchiaro J, Silva R, et al. Lurasidone monotherapy in the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):160–168. PubMed CrossRef
- Loebel A, Cucchiaro J, Silva R, et al. Lurasidone as adjunctive therapy with lithium or valproate for the treatment of bipolar I depression: a randomized, double-blind, placebo-controlled study. Am J Psychiatry. 2014;171(2):169–177. PubMed CrossRef
- Hirschfeld RMA, Weisler RH, Raines SR, et al; BOLDER Study Group. Quetiapine in the treatment of anxiety in patients with bipolar I or II depression: a secondary analysis from a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2006;67(3):355–362. PubMed CrossRef
- Tohen M, Calabrese J, Vieta E, et al. Effect of comorbid anxiety on treatment response in bipolar depression. J Affect Disord. 2007;104(1-3):137–146. PubMed CrossRef
- Earley W, Burgess MV, Rekeda L, et al. Cariprazine treatment of bipolar depression: a randomized double-blind placebo-controlled phase 3 study. Am J Psychiatry. 2019;176(6):439–448. PubMed CrossRef
- Harvey AG, Talbot LS, Gershon A. Sleep disturbance in bipolar disorder across the lifespan. Clin Psychol (New York). 2009;16(2):256–277. PubMed CrossRef
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. PubMed CrossRef
- Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. PubMed CrossRef
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429–435. PubMed CrossRef
- Goldberg JF, Siu C, Mao Y, et al. Major depressive disorder with mixed features and treatment response to lurasidone: a symptom network model. J Affect Disord. 2020;277:1045–1054. PubMed CrossRef
- Goldberg JF, Perlis RH, Bowden CL, et al. Manic symptoms during depressive episodes in 1,380 patients with bipolar disorder: findings from the STEP-BD. Am J Psychiatry. 2009;166(2):173–181. PubMed CrossRef
- Chen YC, Chen CK, Wang LJ. Quetiapine fumarate augmentation for patients with a primary anxiety disorder or a mood disorder: a pilot study. BMC Psychiatry. 2012;12(1):162. PubMed CrossRef
- Spearing MK, Post RM, Leverich GS, et al. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73(3):159–171. PubMed CrossRef
- Sheehan DV. The Anxiety Disease. Bantam Books; 1983.
- Singh MK, Siu C, Tocco M, et al. Sleep disturbance, irritability, and response to lurasidone treatment in children and adolescents with bipolar depression [online ahead of print September 27, 2022]. Curr Neuropharmacol. PubMed
- Freeman D, Sheaves B, Waite F, et al. Sleep disturbance and psychiatric disorders. Lancet Psychiatry. 2020;7(7):628–637. PubMed CrossRef
- Gold AK, Sylvia LG. The role of sleep in bipolar disorder. Nat Sci Sleep. 2016;8:207–214. PubMed CrossRef
- Balcer OM, Seager MA, Gleason SD, et al. Evaluation of 5-HT7 receptor antagonism for the treatment of anxiety, depression, and schizophrenia through the use of receptor-deficient mice. Behav Brain Res. 2019;360:270–278. PubMed CrossRef
- Ishibashi T, Horisawa T, Tokuda K, et al. Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther. 2010;334(1):171–181. PubMed CrossRef
- Depoortère R, Bardin L, Varney MA, et al. Serotonin 5HT1A receptor biased agonists display differential anxiolytic activity in a rat social interaction model. ACS Chem Neurosci. 2019;10(7):3101–3107. PubMed CrossRef
- Strauss CV, Vicente MA, Zangrossi H Jr. Activation of 5-HT1A receptors in the rat basolateral amygdala induces both anxiolytic and antipanic-like effects. Behav Brain Res. 2013;246:103–110. PubMed CrossRef
- Dong M-X, Chen G-H, Hu L. Dopaminergic system alteration in anxiety and impulsive disorders: a systematic review of neuroimaging studies. Front Neurosci. 2020;14:608520. PubMed CrossRef
- Goldberg D, Fawcett J. The importance of anxiety in both major depression and bipolar disorder. Depress Anxiety. 2012;29(6):471–478. PubMed CrossRef
- Goghari VM, Harrow M. Anxiety symptoms across twenty-years in schizoaffective disorder, bipolar disorder, and major depressive disorder. Psychiatry Res. 2019;275:310–314. PubMed CrossRef
- Goodwin GM. The overlap between anxiety, depression, and obsessive-compulsive disorder. Dialogues Clin Neurosci. 2015;17(3):249–260. PubMed CrossRef
- Schaffer JB, Rodolfa E, Owen J, et al. The examination for professional practice in psychology: new data–practical implications. Train Educ Prof Psychol. 2012;6(1):1–7. CrossRef
- Rakofsky JJ, Dunlop BW. Treating nonspecific anxiety and anxiety disorders in patients with bipolar disorder: a review. J Clin Psychiatry. 2011;72(1):81–90. PubMed CrossRef
- Gao K, Wu R, Kemp DE, et al. Efficacy and safety of quetiapine-XR as monotherapy or adjunctive therapy to a mood stabilizer in acute bipolar depression with generalized anxiety disorder and other comorbidities: a randomized, placebo-controlled trial. J Clin Psychiatry. 2014;75(10):1062–1068. PubMed CrossRef
This PDF is free for all visitors!