ABSTRACT
Objective: Therapeutic options are limited for treatment-resistant bipolar depression (TRBD). Insulin resistance (IR) confers increased risk for TRBD. We investigated metformin, an insulin sensitizer, to reverse IR and improve clinical outcomes in TRBD.
Methods: Using a random-assignment (1:1), intent-to-treat, 2-site, quadruple-masked, parallel-group (metformin to 2,000 mg/d or placebo) clinical trial design, patients with DSM-5 bipolar disorder (BD) type I or II and IR received study medication for 26 weeks (February 2016 to October 2019). The primary outcome was the change in depression rating scores (Montgomery-Asberg Depression Rating Scale [MADRS]) at 14 weeks between those who no longer met IR criteria (converters) and those who still did (non-converters). Additional outcomes included scores on the Global Assessment of Functioning (GAF); the Clinical Global Impressions Scale, Bipolar Disorders version (CGI-BP); and the Hamilton Anxiety Rating Scale (HAM-A) and maintenance of improved outcomes up to 26 weeks.
Results: Forty-five BD patients were randomized to metformin (n = 20) or placebo (n = 25), and at 14 weeks or later, 11 subjects no longer met IR criteria (n = 10 with metformin, n = 1 with placebo; P = .0009). These converters experienced significant improvements in MADRS (P values ranged from .031 to .008) and GAF (P values ranged from .045 to .008) scores compared to non-converters beginning at week 6, sustained to week 26. HAM-A (P = .022 at week 14 and .019 at week 26) and CGI-BP change scores (P = .046 at 26 weeks) significantly favored converters over non-converters. Effect sizes were large for the MADRS and GAF (Cohen d > 1 at 14 and 26 weeks) and large for the HAM-A and CGI-BP at 26 weeks. Transient gastrointestinal side effects occurred under both treatment conditions.
Conclusions: Pending replication, this early study suggests that reversal of IR by metformin offers a path out of TRBD. Further characterization of metformin converters with TRBD will prove informative.
Trial Registration: ClinicalTrials.gov identifier: NCT02519543
J Clin Psychiatry 2022;83(2):21m14022
To cite: Calkin CV, Chengappa KNR, Cairns K, et al. Treating Insulin Resistance With Metformin as a Strategy to Improve Clinical Outcomes in Treatment-Resistant Bipolar Depression (the TRIO-BD Study): a randomized, quadruple-masked, placebo-controlled clinical trial. J Clin Psychiatry. 2022;83(2):21m14022.
To share: https://doi.org/10.4088/JCP.21m14022
© Copyright 2022 Physicians Postgraduate Press, Inc.
aFaculty of Medicine, Department of Psychiatry, Dalhousie University, Halifax, Nova Scotia, Canada
bMood and Metabolism Program, QE II Health Sciences Centre, Nova Scotia Health, Halifax, Nova Scotia, Canada
cFaculty of Medicine, Department of Medical Neuroscience, Dalhousie University, Halifax, Nova Scotia, Canada
dDepartment of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania
eWestern Psychiatric Hospital, University of Pittsburgh Medical Centre, Pittsburgh, Pennsylvania
fMood Disorders Program, QE II Health Sciences Center, Nova Scotia Health, Halifax, Nova Scotia, Canada
gThe Centre for Mental Health and Addiction, Toronto, Ontario, Canada
*Corresponding author: Cynthia V. Calkin, MD, CCFP, FRCPC, Departments of Psychiatry and Medical Neuroscience, Director, Mood and Metabolism Program, QE II Health Sciences Centre, Room 4108, Abbie J. Lane Bldg, Halifax, NS B3H 2E2 CANADA ([email protected]).
The clinical trajectory of bipolar disorder (BD) may be complicated by treatment resistance to medications such as lithium, lamotrigine, valproate, or atypical antipsychotics, or to combinations thereof.1 Expert panels have recently defined the more treatment-recalcitrant phase of bipolar illness, ie, treatment-resistant bipolar depression (TRBD).1,2 TRBD criteria consist of failure to reach sustained remission after two 8-week trials of recommended medications, including combination therapy, at therapeutic doses. The new criteria highlight a lack of treatment options available for TRBD as well as a dearth of evidence-based treatments to recommend at this stage of the illness.2,3 Unsurprisingly, TRBD is associated with increased psychiatric hospitalizations, disability, and poor quality of life.2 Therefore, there is an urgent need to develop new treatments to reverse TRBD and reduce morbidity and mortality.
Our group and others have been investigating factors that contribute to treatment resistance in patients with BD—and, specifically, TRBD.4–11 We have focused on the development of insulin resistance (IR) and type 2 diabetes mellitus (T2DM) in BD as significant risk factors. Not only do 22% of BD patients have T2DM, but an additional 32% have IR, conditions that are often missed in clinical practice.4 Our work and that of others suggest that IR and T2DM switch patients from an episodic, relapsing-remitting illness to a more severe, chronic (neuroprogressive) course characterized mainly by major depression, particularly, TRBD.4–11 If IR could be identified and reversed in patients with BD, the intriguing possibility arises that the development of a neuroprogressive illness course, including TRBD, could be treated or, better still, prevented or delayed. We hypothesized that patients with BD I or II who met IR criteria and were experiencing TRBD despite optimal treatment3,12 would respond favorably to treatment with metformin, an insulin-sensitizing medication. Metformin is recommended worldwide as the first-line treatment for T2DM13 and is on the World Health Organization’s list of essential medicines.14 The primary hypothesis was that, compared to placebo, metformin would reverse IR significantly among TRBD patients, and the reversal of IR would result in significant improvement of TRBD and associated clinical outcomes.
METHODS
Study Design
This parallel-group, intent-to-treat, random-assignment (in a 1:1 ratio to metformin or placebo), quadruple-masked (patient, investigator, outcomes assessor, statistician) study was undertaken at two sites (Halifax, Nova Scotia, and Pittsburgh, Pennsylvania) from February 2016 (first patient enrolled) to October 2019 (last patient). The study protocol and consent documents were approved by the Nova Scotia Health Research Ethics Board and the University of Pittsburgh’s Institutional Review Board, and all participants provided written, informed consent prior to initiating study procedures. This study received Health Canada approval and US Food and Drug Administration (FDA) exemption. The Treating Insulin-Resistance With Metformin as a Strategy to Improve Clinical Outcomes in Treatment-Resistant Bipolar Depression (TRIO-BD) study was registered at ClinicalTrials.gov (Identifier: NCT02519543).
Participants
Adults aged 18 years or older with DSM-515 BD I or II, based on the Schedule for Affective Disorders and Schizophrenia–Lifetime version (SADS-L) interview,16 review of chart records, and discussions with referring clinicians, were recruited from two academic mood/psychotic disorder clinics. All had unremitting depressive symptoms (Montgomery-Asberg Depression Rating Scale [MADRS]17 score ≥ 15) for at least 4 weeks despite optimal treatment. Optimal treatment was defined as prescription of mood-stabilizing monotherapy or medication combinations at stable doses as per the 2013 Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines12 (the most recent available guidelines at the time of study start-up) for at least 4 weeks. Subjects were screened for IR using the Homeostatic Model Assessment-–Insulin-Resistance (HOMA-IR) equation, described by us previously.5 Concurrent fasting plasma glucose (FPG) and serum insulin (FSI) concentrations were measured to obtain HOMA-IR values, defining IR using the HOMA-IR cutoff of ≥ 1.8 since metabolic syndrome becomes clinically significant at this value.18 Subjects without IR or with T2DM were screened out of the study. Additional exclusionary criteria included rapid cycling (DSM-5 criteria), presence of manic symptoms (Young Mania Rating Scale [YMRS]19 score ≥ 15), current suicidal ideation rating of 5 on the Columbia-Suicide Severity Rating Scale (C-SSRS),20 receiving metformin within 2 weeks of study entry, allergy to metformin, liver function tests ≥ 3 times the reference value, estimated glomerular filtration rate ≤ 30 mL/min/1.73m2, pregnancy, and breastfeeding.
Interventions
Once all eligibility criteria were satisfied, subjects received 500 mg metformin (oral immediate-release) or identical-looking placebo capsules, provided in blister packs, with breakfast and supper daily for 1 week (1,000 mg/d) and titrated to 1,000 mg metformin or placebo twice daily (2,000 mg/d), if tolerated, for 25 additional weeks. Slower titration was permitted for tolerability, and all subjects were maintained on a minimum of 1,500 mg/d.
Assessments for Primary and Secondary Outcomes
To support the primary hypothesis, the MADRS was administered and IR was measured at baseline (pre-randomization), 2 weeks after randomization, and every 4 weeks thereafter (at weeks 6, 10, 14 [primary outcome time point], 18, 22, and 26 [end of study]). It is pertinent to note that after baseline assessments, outcomes raters were blinded to FPG, FSI, and HOMA-IR, since the effects of metformin on these laboratory values could potentially unblind the study. The YMRS was used to monitor emergent manic symptoms, and the C-SSRS (since last visit version) was used for suicidal ideation at the aforementioned time points. Secondary outcomes included the Clinical Global Impressions Scale, Bipolar Disorders version (CGI-BP),21 and the Global Assessment of Functioning (GAF).22 Anxiety symptoms were assessed using the Hamilton Anxiety Rating Scale (HAM-A)23 at baseline and at weeks 14 and 26.
Safety and Laboratory Monitoring
In addition to a physical examination and review of medical and psychiatric history, we obtained measures of psychopathology and psychosocial functioning from rating scales, FPG and FSI, and baseline liver and renal functions. We assessed serum human chorionic gonadotrophin levels for pregnancy in reproductive-aged women, urine for illicit drug use, electrocardiograms, complete blood cell counts, thyroid functions, lipids, and blood mood stabilizer levels. Body weight, body mass index (BMI), and blood pressure were measured at baseline and each scheduled visit.
Adherence to Study Medication
Returned pill counts were used to determine adherence to study medication, with significant nonadherence defined as 7 consecutive days of not taking the medications.
Data and Statistical Analyses
Baseline demographic and illness characteristics were compared between randomized groups to assess randomization. The primary hypothesis that metformin would have a significant effect in reversing IR (conversion) compared to placebo was tested using the Fisher exact test. Our follow-on hypothesis, that converters would experience significant improvements in MADRS scores (and additional outcomes—GAF, HAM-A, and CGI-BP scores), was tested using a random-intercept random-slope mixed-effect model, using the interaction between time (categorical, weeks 0, 2, 6, 10, 14, 18, 22, and 26) and IR reversal (converters, non-converters) as a fixed effect of interest. Treatment group (metformin, placebo) and site (Halifax, Pittsburgh) were added to the model as independent variables because they were design features. Age, age at onset, and baseline BMI were added for being theoretically associated with IR, and baseline MADRS scores were added to control for depression severity at study entry in the longitudinal analyses. Marital status was controlled for as there was a trend level association. Estimated marginal means and their linear contrasts were used to describe outcomes of interest when they were significant (P < .05) in the model. All subjects in the trial were included in the analysis, regardless of whether they dropped out. The mixed-effect model handles missing values and dropouts by using full maximum likelihood information; that is, it includes all available information in the data without removing subjects because of missing values at specific time points. All analyses were conducted in R version 4.0.224 with packages lme4,25 lmerTest,26 and emmeans.27
RESULTS
Patient Population
Eighty-eight subjects were screened for eligibility and consented, and 50 patients were randomized (Supplementary Figure 1). The efficacy and safety data for 45 randomized patients are reported; 20 were randomly assigned to metformin and 25 to placebo. No subjects withdrew because of side effects or noncompliance. Thirty-nine subjects (87%) completed the study to the 14-week primary outcome endpoint. Seventeen subjects (metformin = 7, placebo = 10) left the study for lack of improvement between weeks 14 and 26, and 28 subjects completed the full 26 weeks (placebo n = 15, metformin n = 13). Adherence as measured by returned pill counts was 97% for both treatment groups.
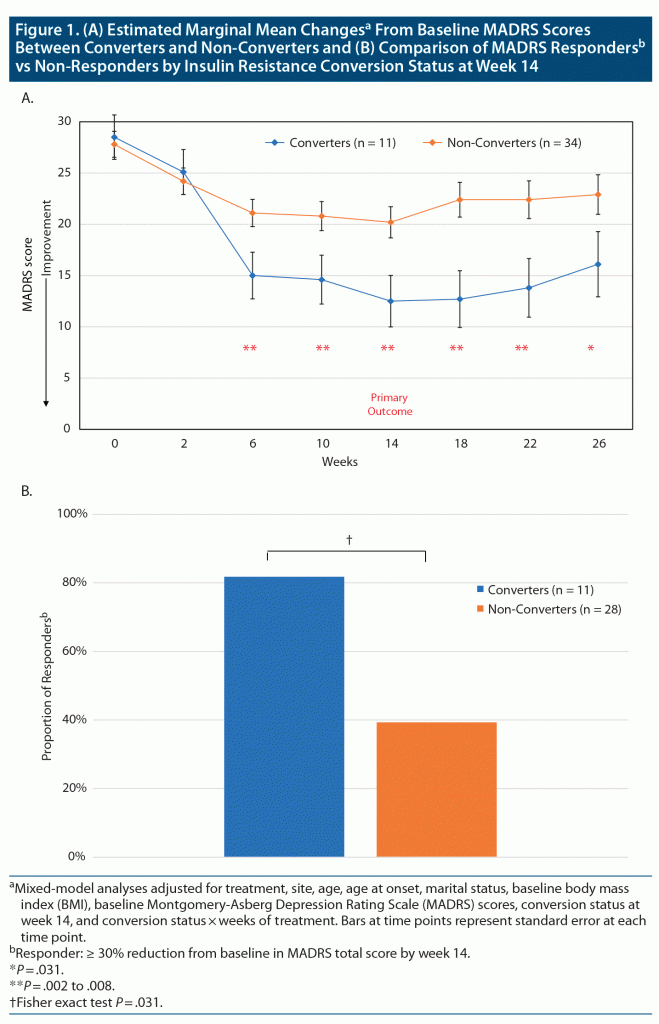
Baseline demographic and clinical characteristics were similar between randomized treatment groups (Table 1). The mean age of the cohort was 47.5 years, 76% were women, and participants had 15 years of education. Patients had an illness duration of 26 years, 64% had BD I, and 64% had received disability benefits. Mean lifetime failed medication trials for BD was 8.6. Patients were taking a mean of 2.5 (failed) medications at study entry. A total of 91.2% of patients failed drug trials from at least 3 psychotropic drug classes (lithium, antiepileptics, antipsychotics, antidepressants), with 55.6% failing drugs from all 4 drug classes. Baseline MADRS, GAF, HAM-A, CGI-BP, and YMRS scores were similar between the randomized groups (with mean MADRS scores > 28 [representing moderate, bordering on severe depression28], mean HAM-A scores > 16 [representing moderate anxiety29], and mean GAF scores < 50 [denoting serious symptoms and/or impairment in social/occupational functioning22]). Eighty-nine percent had a chronic or interepisode symptomatic course, with only 11% having periods of remission. Similarly, there were no significant baseline differences in body weight, BMI, FPG, FSI, or HOMA-IR between randomized treatment groups. Additionally, there were no significant baseline differences in levels of lithium or lamotrigine, the 2 main mood stabilizers that study patients received (data not shown). There were no significant between-site (Halifax and Pittsburgh) differences in any of the demographic or clinical variables (data not shown). Based on the lack of significant differences between the treatment groups (all P values > .05), randomization was effective across potential confounders, such as BMI and BD subtype.
Efficacy
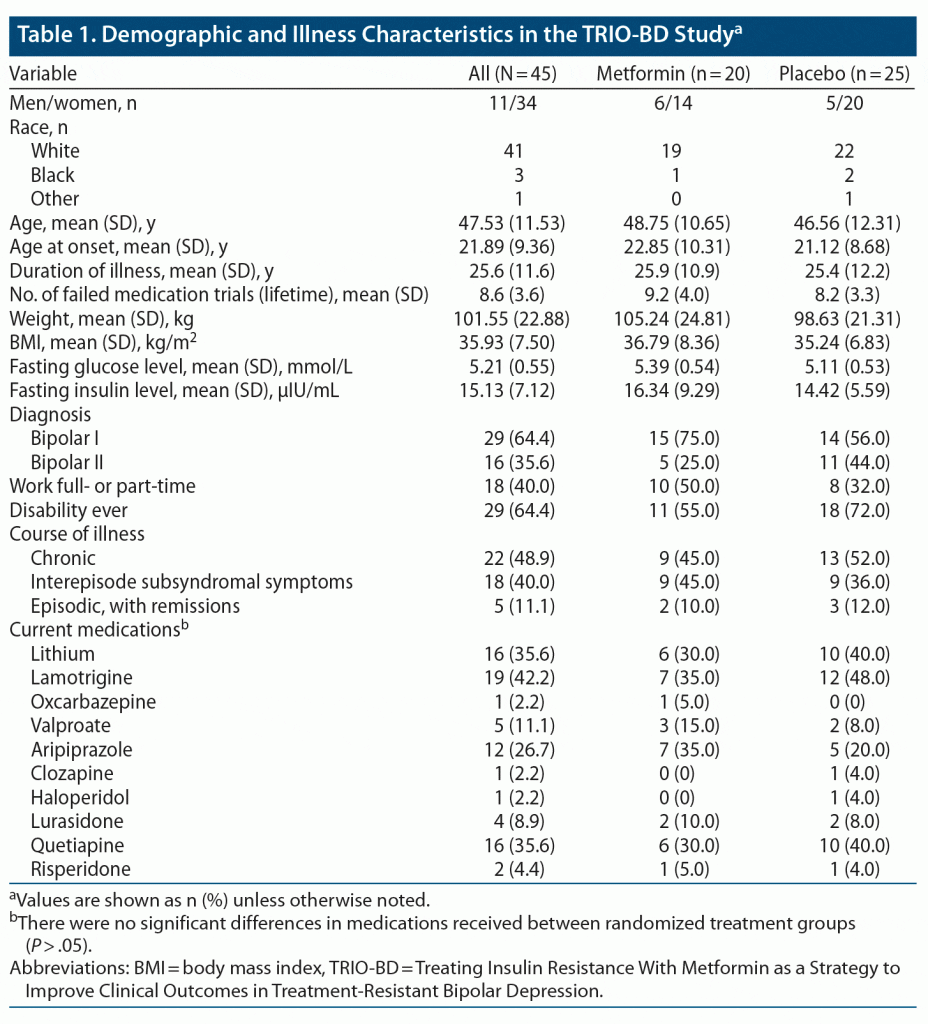
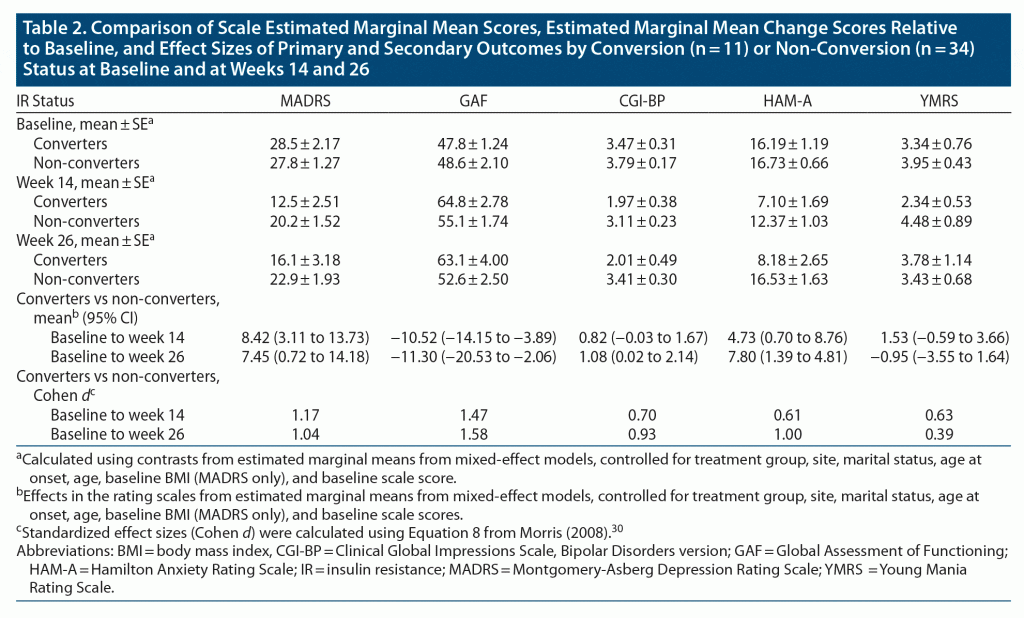
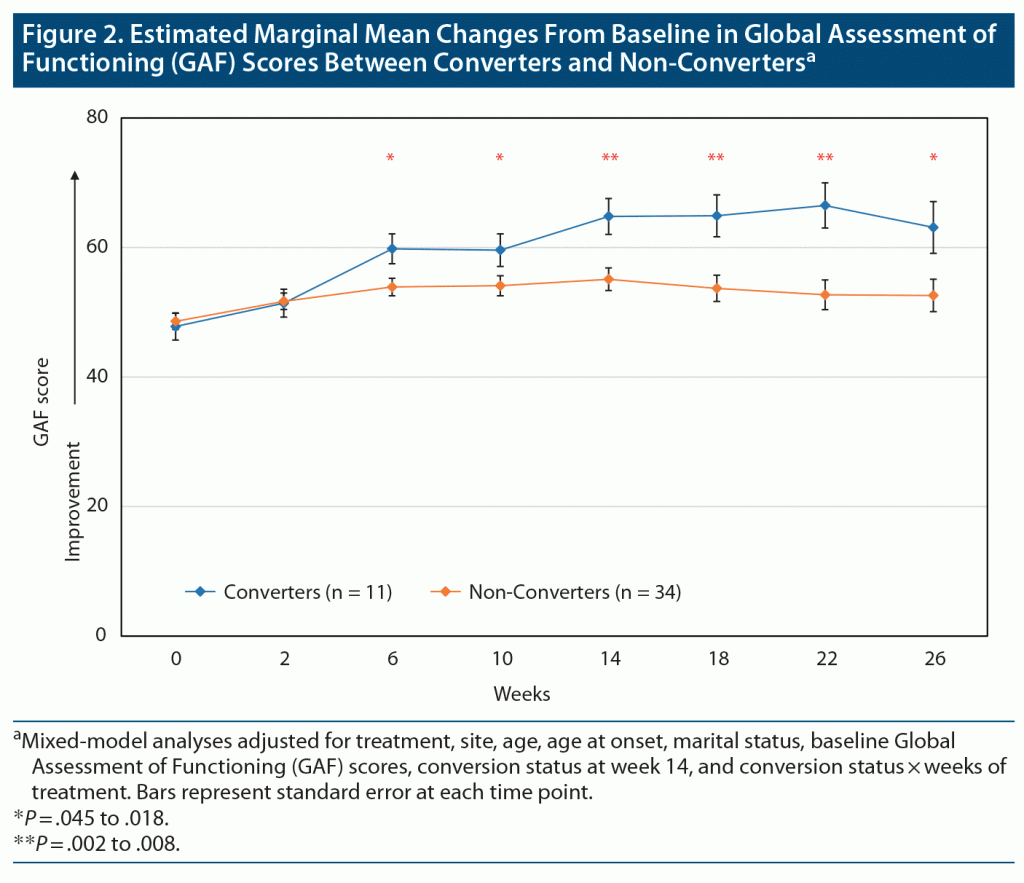
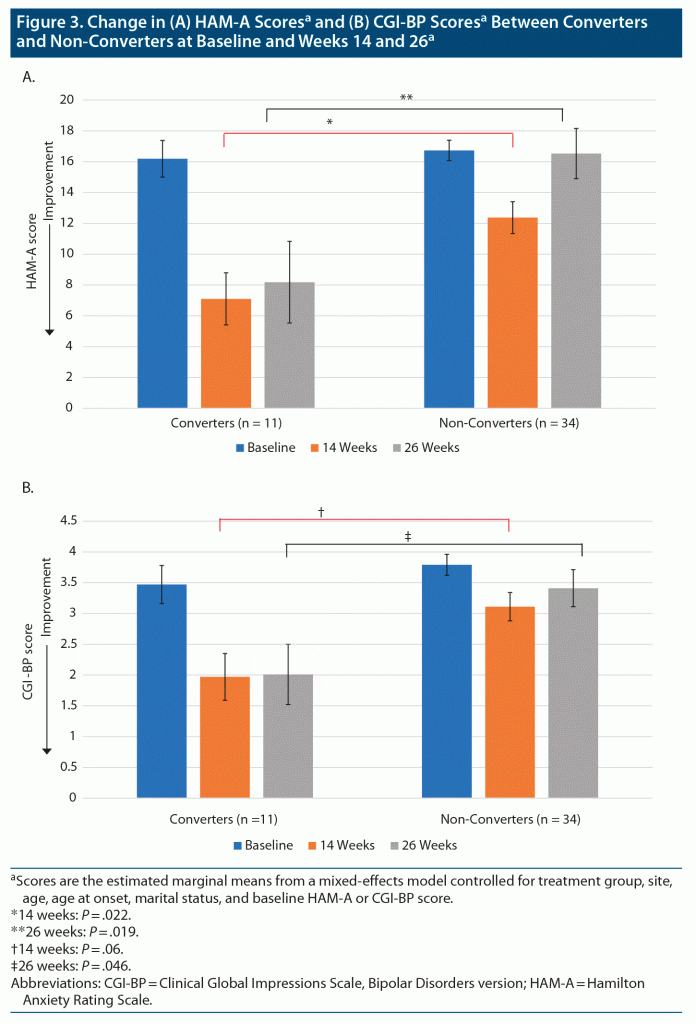
Ten metformin-treated patients (50%) no longer met IR criteria (became converters) at week 14, the primary outcome endpoint, compared to 1 placebo-assigned patient (4%) (Fisher exact P = .0009). The daily dose of metformin received by the converters was 1,500 mg in 3 patients and 2,000 mg in 7 patients. The converters (n = 11) achieved a significantly greater reduction in the primary outcome depression rating scale measure (MADRS total scores), as compared to non-converters, beginning at week 6. This improvement was maintained until the end of the study at week 26 (Figure 1A and Table 2 with estimated marginal means). Significantly more converters (n = 9 [81.8%]) were treatment responders (defined as reduction in baseline MADRS scores of ≥ 30%, in view of TRBD, at week 14) than non-converters (n = 11 [39.3%], Fisher exact P = .031) (Figure 1B). The 2 metformin-treated converters who did not meet the ≥ 30% response threshold improved by 21% and 28%, respectively. Among the non-converters, there were no significant differences in those who met the 30% response criteria between metformin (n = 4/10 [40%]) or placebo (n = 7/24 [29.2%]). The size of the treatment effect (Cohen d) for MADRS (depression) scores for converters was large30: d = 1.17 at week 14 and d = 1.04 at week 26 (Table 2). Consistent with improvements in depression scores, the GAF scores improved significantly for converters compared to non-converters, beginning at week 6, and this was sustained until week 26 (Figure 2 and Table 2), with large treatment effects at weeks 14 (d = 1.47) and 26 (d = 1.58) (Table 2). Similarly, HAM-A scores also improved significantly in favor of converters compared to non-converters at weeks 14 (d = 0.61) and 26 (d = 1.0) (Figure 3A and Table 2). CGI-BP change scores improved in favor of converters compared to non-converters, but less consistently over time; nonetheless, this improvement was significantly better for converters at week 26, with an effect size d = 0.93 (Figure 3B and Table 2). YMRS mean scores were low at baseline and remained low for converters and non-converters alike throughout the study (Table 2).
Safety and Tolerability
Vital signs remained stable and did not differ significantly between randomized treatment groups (data not shown). Lipid profiles and thyroid-stimulating hormone did not differ by randomized group. Adverse events occurring at a frequency of ≥ 5% in either group (see Supplementary Table 1) included loose stool/diarrhea (40% in the metformin group), nausea (35% with metformin), and vomiting (10% with metformin); gastrointestinal side effects were expected in the metformin-treated group, and, interestingly, high rates also occurred in the placebo group, with no significant between-treatment differences using the Fisher exact test (Supplementary Table 1). Headaches and constipation were more frequent in the placebo-assigned group, with no significant between-treatment differences. Suicidal ideation scores did not worsen in the majority of patients (n = 40); in 5 subjects, scores fluctuated but did not result in study withdrawal. At week 14, relative to baseline, metformin-treated patients lost 1.67 kg whereas placebo-assigned patients gained 1.35 kg, a statistically significant but not visibly noticeable difference (mean ± SD difference: 3.02 ± 1.31 kg; t122.99 = 2.29, P = .023). Weight differences in favor of metformin compared to placebo continued to week 26 but were no longer statistically significant (mean ± SD difference: 2.54 ± 1.93 kg; t53.28 = 1.32, P = .194). Similarly, at week 14, BMI differences trended in favor of the metformin-treated group compared to the placebo-treated group (mean ± SD difference in BMI: 0.78 ± 0.43 kg/m2; t119.88 = 1.804, P = .074), but the difference in BMI at 26 weeks favoring the metformin-treated group compared to the placebo-treated group was no longer statistically significant (mean ± SD difference in BMI: 0.74 ± 0.64 kg/m2; t51.99 = 1.150, P = .256).
DISCUSSION
As hypothesized, successful reversal of IR by metformin resulted in a statistically significant and clinically meaningful reduction in depression rating scale scores in patients suffering from TRBD. Fifty percent of insulin-resistant TRBD patients treated with metformin converted to insulin-sensitive (reversed their IR), and improvements in depression ratings that were first noted at 6 weeks were sustained up to 26 weeks. Eighty percent of TRBD patients treated with metformin (and 1 patient assigned to placebo) who converted also met the 30% response threshold for treatment-resistant depression symptom improvements; the 2 metformin-treated subjects who did not meet that threshold improved by 28% and 21%, respectively. Coincident with improvements in depression outcomes, GAF ratings improved significantly for the converters at identical time points, with scores moving from serious impairments in social/occupational functioning to mild impairments by 26 weeks. Anxiety, which often complicates BD and bipolar depression, also diminished significantly among converters, as reflected by HAM-A scores at 14 and 26 weeks. Reassuringly, improvements in depression ratings were not accompanied by increases in mania ratings, and low YMRS scores were maintained throughout the 6-month trial in both randomized treatment groups. The treatment effect sizes for IR conversion in the primary and secondary outcomes were predominantly large. These outcomes are particularly promising, as nearly 89% of patients at study entry had a non-remitting bipolar course (49% were chronic; 40% had significant interepisode symptomatology). Despite the small sample size, these effect sizes are likely reliable, as our study was hypothesis-driven.
No serious adverse events occurred over the course of this trial. Adverse events that did occur were primarily gastrointestinal symptoms commonly associated with metformin (diarrhea, nausea, and vomiting) and were equally prevalent in the placebo group.
Our previous work4 found that BD patients with IR have equally poor outcomes as those with more advanced metabolic dysregulation (T2DM). It is our hypothesis that reversal of IR needs to occur before frank T2DM sets in; there may be a narrow window of opportunity to intervene, as it appears the benefits of IR reversal do not positively impact treatment-resistant BD outcomes once T2DM is diagnosed.31 This early study demonstrates that such intervention may have lasting clinical benefits.
Many medications used to treat BD increase risk of IR/T2DM, contributing to treatment resistance. Of the insulin-sensitizing medications, metformin is an appealing choice to help break this cycle, as it not only increases insulin-sensitivity and helps with weight loss, but also has an established safety profile (including during pregnancy).13 Additionally, metformin is already in use in psychiatric practice as a weight maintenance strategy for patients receiving antipsychotics32 and has been shown to improve insulin sensitivity and dysglycemia in schizophrenia spectrum patients33 and in animal models of antipsychotic-induced metabolic dysregulation.34,35 Positive effects on cognition and improvement of depressive symptoms have also been reported following metformin treatment in people with T2DM.36 Regarding bipolar depression, previous clinical trials37–39 have assessed the efficacy of another insulin-sensitizing medication, the peroxisome proliferator–activated receptor (PPAR)-γ agonist pioglitazone. In the two studies reporting positive findings,37,38 it remained unclear whether insulin sensitization was the mechanism underlying pioglitazone’s apparent antidepressant effects. The TRIO-BD study is the first to indicate that insulin sensitization, to the point of IR reversal, is key to obtaining significant improvement in TRBD.
Insulin resistance contributes to endothelial dysfunction,40 leading to pathologically permeable blood-brain barrier (BBB), allowing excessive entry of proinflammatory cytokines into the central nervous system (CNS), causing neuroinflammation, neuronal dysfunction, and degeneration.41 Further, BBB insulin receptors are down-regulated, limiting insulin transport into the brain, disrupting CNS insulin signaling.42 In our recent study43 using dynamic contrast-enhanced magnetic resonance imaging to measure BBB permeability, all BD patients with extensive BBB leakage had comorbid IR, a chronic course, more severe depression and anxiety, and poorer functioning compared to those with normal BBB permeability. BBB leakage may be a mechanism by which BD patients with IR develop a more treatment-resistant neuroprogressive course. Although we did not find that metformin itself affected depression scores in the absence of IR reversal, metformin may target the BBB directly. Increased levels of matrix metalloproteinase-9 (MMP-9)—an enzyme that degrades the BBB—were demonstrated in bipolar depression,44 and metformin is thought to suppress the action of MMP-9.45 Further, PPAR is thought to mediate the insulin-sensitizing action of metformin by modulating the insulin-like growth-factor axis.46 In animal studies, PPAR agonists have been shown to protect the BBB; reduce neuro-inflammation, oxidative stress, and neuronal injury47; and improve neurologic outcomes.48 Subjects in the TRIO-BD study may have also had BBB leakage, and reversal of IR in the converters group may have facilitated BBB repair, leading to improvement in TRBD. For a detailed review of hypothesized mechanisms linking IR, insulin-sensitizing agents, and BBB function, please see Calkin et al (2021).49
Current Treatment Options for TRBD
Historically, best-practice guidelines3,12,50 have not specifically addressed treatment options for TRBD. In recent guidelines from an International College of Neuropsychopharmacology expert panel,2 recommendations were made for first-line use of lithium and lamotrigine combination therapy or adjunctive lamotrigine, modafinil, or pramipexole in treating TRBD. The recommended second-line treatment, notably, was pioglitazone, suggesting an important role for insulin signaling in TRBD (it must be noted that the 2018 CANMAT treatment guidelines3 do not provide specific recommendations regarding insulin-sensitizing agents, such as pioglitazone). Although much additional evidence is needed to refine treatment selection for patients with TRBD,2 IR may provide an effective treatment target for certain patients. Metabolic dysregulation in bipolar disorder is seen in over 50% of patients and is closely associated with poor clinical outcomes4; hence, metabolic phenotyping may yield targeted interventions that benefit a fairly large specific subgroup in TRBD and may be particularly useful in addressing heterogeneity of treatment response in resistant psychiatric disorders.
Limitations
Limitations of this trial include the small sample size, which prevented further exploration of demographic, clinical, or IR characteristics that might have defined which metformin-treated TRBD patients were more likely to benefit. Second, only a 50% reversal rate of IR was achieved using immediate-release metformin in doses ranging from 1,500 to 2,000 mg/d. It is not known whether higher daily dosages of metformin or extended-release preparations might provide more benefit to insulin-resistant TRBD patients and what tolerability and safety concerns might arise or improve. While metformin reversed IR and improved outcomes for 50% of patients in the short and intermediate term (26 weeks), it may not be an adequate long-term treatment for all patients.
CONCLUSIONS AND FUTURE DIRECTIONS
Pending replication, this clinical trial suggests that reversal of IR by metformin significantly improves depressive and anxiety symptoms and general functioning in a significant percentage of TRBD patients. Our findings introduce a number of questions, suggesting the importance of further study. We know that diet, exercise, and weight loss can effectively reverse IR,31,51 and in psychiatric patients, diet and exercise are linked to positive outcomes52,53; however, whether this improvement in outcome is via the mechanism of reversing IR, by improving a sense of general well-being through the production of endorphins, or due to other factors is unclear. For those patients not responding to metformin, glucagon-like peptide-1 agonists (for example, semaglutide) may offer a better therapeutic option. The efficacy of semaglutide for reducing weight and FSI in obesity or T2DM has recently been demonstrated in a large-scale clinical trial.54 We are currently studying the effects of semaglutide on outcome in those who fail to reverse IR with metformin, and in those failing semaglutide, we are studying the effect of pioglitazone. It is likely that improved outcomes result from reversing IR by any means (in other words, correcting the aberrant underlying mechanism) and are not treatment specific. One particularly pressing question is whether treating IR as soon as it is identified, even in euthymic BD patients, may prevent progression of BD to a more treatment-resistant neuroprogressive state. Further, for patients who respond to metformin in the short-term, how long will insulin sensitivity be adequately maintained? Finally, could these results be applicable to other treatment-resistant psychiatric disorders, eg, major depressive disorder,55 schizophrenia,56 posttraumatic stress disorder,57 obsessive-compulsive disorder?58 Hopefully, continued research will help answer these questions and further elucidate the role of IR in TRBD and other psychiatric illness.
Submitted: April 2, 2021; accepted August 30, 2021.
Published online: February 1, 2022.
Potential conflicts of interest: Dr Chengappa reports that the University of Pittsburgh is pursuing intellectual property for the use of an herbal extract in schizophrenia in which he is listed as a co-inventor and that is not connected with this study; he has no conflicts of interest with this report or study. None of the remaining authors has any conflicts of interest to disclose in connection with this study.
Funding/support: We are thankful to the Stanley Medical Research Institute (grant number 14T-008 to Dr Calkin, PI and Sponsor of the TRIO-BD study) for funding this study, allowing for the advancement of psychiatric research.
Role of the sponsor: The Stanley Medical Research Institute had no role in conducting the clinical trial, data collection, analyses, or reporting of study results.
Previous presentations: Some of the data were presented at the 2021 ASCP Virtual Annual Meeting on June 2, 2021, and the 2021 ISBD Virtual Conference on May 15, 2021.
Acknowledgments: The investigative team would like to thank Ms Julie Garnham, RN, BN, who played a critical role at study commencement as research program coordinator (Halifax site) in preparing the submission of the study protocol to Health Canada and to the Nova Scotia Health Authority (NSHA) Research Ethics Board (REB), in aiding in procuring study drug and placebo, in facilitating investigative pharmacy and laboratory services, and in coordinating and participating in the start-up visit at the Pittsburgh site. We thank Drs Patricia Pearce, MD; Külli Põder, MD; and Sreenivasa Bhaskara, MBBS, and other psychiatrists and clinical staff at Nova Scotia Health’s Mental Health Outpatient clinics for referring patients under their care to the TRIO-BD study and we thank the research assistants and students for contributing to scheduling participant follow-up and data collection (Halifax). We thank the late Ms Patricia Schlicht, RN, MA, who played a pivotal role as the research program coordinator (Pittsburgh site), and Drs Mohammad Ismael, MD; Jatinder Babbar, MD; Marcia DeLeo, CRNP; and Holly Swartz, MD, and other clinical staff and doctors at the Bellefield Clinic and CRS Oxford Clinic for referring patients under their care to the TRIO-BD study (Pittsburgh).We gratefully thank Ms Joan Spinogatti, AAS, for coordinating all Institutional Review Board processes and US Food and Drug Administration (FDA) communications and facilitating Pittsburgh/Halifax and Data and Safety Monitoring Board (DSMB) meetings and coordination among the authors prior to manuscript submission. We thank Dr Jaspreet Brar, MBBS, MPH, PhD, for completing study procedures of the last couple of subjects upon Ms Schlicht’s passing. We would also like to thank the pharmacy staff of Ford’s Pharmacy for undertaking all investigative pharmacy services associated with the study medications. We are grateful for oversight provided by the DSMB and would like to thank Drs Samuel Gershon, MD (chair); Paul Grof, MD; and Thomas Ransom, MD, as well as the study monitors (Darlene Baxendale, BScN [Halifax], and Joan Rea, RN, BSN [Pittsburgh]). We also thank Dr Carl Jarvis, MD, and Dr Paige Forrest, MD, for their medical oversight in Halifax and Pittsburgh, respectively. None of the acknowledged persons has any conflicts of interest to disclose.
Supplementary material: Available at Psychiatrist.com.
Clinical Points
- Insulin resistance (IR) may be a previously unrecognized mechanism underlying treatment-resistant bipolar depression (TRBD).
- Reversal of IR may improve depression and other clinical outcomes in TRBD.
- Treatment of modifiable clinical risk factors for IR in TRBD—for example, obesity or long-term antipsychotic use—should be considered.
References (58)

- Hidalgo-Mazzei D, Berk M, Cipriani A, et al. Treatment-resistant and multi-therapy-resistant criteria for bipolar depression: consensus definition. Br J Psychiatry. 2019;214(1):27–35. PubMed CrossRef
- Fountoulakis KN, Yatham LN, Grunze H, et al. The CINP guidelines on the definition and evidence-based interventions for treatment-resistant bipolar disorder. Int J Neuropsychopharmacol. 2020;23(4):230–256. PubMed CrossRef
- Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170. PubMed CrossRef
- Calkin CV, Ruzickova M, Uher R, et al. Insulin resistance and outcome in bipolar disorder. Br J Psychiatry. 2015;206(1):52–57. PubMed CrossRef
- Cairns K, McCarvill T, Ruzickova M, et al. Course of bipolar illness worsens after onset of insulin resistance. J Psychiatr Res. 2018;102:34–37. PubMed
- Calkin CV. Insulin resistance takes center stage: a new paradigm in the progression of bipolar disorder. Ann Med. 2019;51(5-6):281–293. PubMed CrossRef
- Steardo L Jr, Fabrazzo M, Sampogna G, et al. Impaired glucose metabolism in bipolar patients and response to mood stabilizer treatments. J Affect Disord. 2019;245:174–179. PubMed CrossRef
- Cuperfain AB, Kennedy JL, Gonçalves VF. Overlapping mechanisms linking insulin resistance with cognition and neuroprogression in bipolar disorder. Neurosci Biobehav Rev. 2020;111:125–134. PubMed CrossRef
- Ruzickova M, Slaney C, Garnham J, et al. Clinical features of bipolar disorder with and without comorbid diabetes mellitus. Can J Psychiatry. 2003;48(7):458–461. PubMed CrossRef
- Calkin C, van de Velde C, Růzicková M, et al. Can body mass index help predict outcome in patients with bipolar disorder? Bipolar Disord. 2009;11(6):650–656. PubMed CrossRef
- Calkin CV, Gardner DM, Ransom T, et al. The relationship between bipolar disorder and type 2 diabetes: more than just co-morbid disorders. Ann Med. 2013;45(2):171–181. PubMed CrossRef
- Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15(1):1–44. PubMed CrossRef
- Rojas LB, Gomes MB. Metformin: an old but still the best treatment for type 2 diabetes. Diabetol Metab Syndr. 2013;5(1):6. PubMed CrossRef
- World health organization model list of essential medicines. 2019;21:43.
- American Psychiatric Association. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
- Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35(7):837–844. PubMed CrossRef
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. PubMed CrossRef
- Esteghamati A, Ashraf H, Khalilzadeh O, et al. Optimal cut-off of homeostasis model assessment of insulin resistance (HOMA-IR) for the diagnosis of metabolic syndrome: third national surveillance of risk factors of non-communicable diseases in Iran (SuRFNCD-2007). Nutr Metab (Lond). 2010;7(1):26. PubMed CrossRef
- Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(5):429–435. PubMed CrossRef
- Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. PubMed CrossRef
- Spearing MK, Post RM, Leverich GS, et al. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73(3):159–171. PubMed CrossRef
- American Psychiatric Association. DSM-III-R Diagnostic and Statistical Manual of Mental Disorders. Third Edition, Revised. American Psychiatric Association; 1987.
- Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50–55. PubMed CrossRef
- R Core Team. R: A language and environment for statistical computing. R Found Stat Comput Vienna, Austria. 2020. R-Project website. https://www.r-project.org/. Accessed February 12, 2021.
- Bates D, Mächler M, Bolker BM, et al. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. CrossRef
- Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest Package: Tests in Linear Mixed Effects Models. J Stat Softw. 2017;82(13):1–26. CrossRef
- Lenth R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package. 2020. R-Project website. https://cran.r-project.org/package=emmeans. Accessed February 12, 2021.
- Müller MJ, Himmerich H, Kienzle B, et al. Differentiating moderate and severe depression using the Montgomery-Asberg depression rating scale (MADRS). J Affect Disord. 2003;77(3):255–260. PubMed CrossRef
- Matza LS, Morlock R, Sexton C, et al. Identifying HAM-A cutoffs for mild, moderate, and severe generalized anxiety disorder. Int J Methods Psychiatr Res. 2010;19(4):223–232. PubMed CrossRef
- Morris SB. Estimating effect sizes from pretest-posttest-control group designs. Organ Res Methods. 2008;11(2):364–386. CrossRef
- Calkin CV, Alda M. Insulin resistance in bipolar disorder: relevance to routine clinical care. Bipolar Disord. 2015;17(6):683–688. PubMed CrossRef
- de Silva VA, Suraweera C, Ratnatunga SS, et al. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry. 2016;16(1):341. PubMed CrossRef
- Agarwal SM, Panda R, Costa-Dookhan KA, et al. Metformin for early comorbid glucose dysregulation and schizophrenia spectrum disorders: a pilot double-blind randomized clinical trial. Transl Psychiatry. 2021;11(1):219. PubMed CrossRef
- Boyda HN, Procyshyn RM, Tse L, et al. Differential effects of 3 classes of antidiabetic drugs on olanzapine-induced glucose dysregulation and insulin resistance in female rats. J Psychiatry Neurosci. 2012;37(6):407–415. PubMed CrossRef
- Remington GJ, Teo C, Wilson V, et al. Metformin attenuates olanzapine-induced hepatic, but not peripheral insulin resistance. J Endocrinol. 2015;227(2):71–81. PubMed CrossRef
- Guo M, Mi J, Jiang QM, et al. Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin Exp Pharmacol Physiol. 2014;41(9):650–656. PubMed CrossRef
- Kemp DE, Schinagle M, Gao K, et al. PPAR-γ agonism as a modulator of mood: proof-of-concept for pioglitazone in bipolar depression. CNS Drugs. 2014;28(6):571–581. PubMed CrossRef
- Zeinoddini A, Sorayani M, Hassanzadeh E, et al. Pioglitazone adjunctive therapy for depressive episode of bipolar disorder: a randomized, double-blind, placebo-controlled trial. Depress Anxiety. 2015;32(3):167–173. PubMed CrossRef
- Aftab A, Kemp DE, Ganocy SJ, et al. Double-blind, placebo-controlled trial of pioglitazone for bipolar depression. J Affect Disord. 2019;245:957–964. PubMed CrossRef
- Janus A, Szahidewicz-Krupska E, Mazur G, et al. In: Rosales C, ed. Insulin Resistance and Endothelial Dysfunction Constitute a Common Therapeutic Target in Cardiometabolic Disorders. Mediators Inflamm; 2016.
- Kleinridders A, Ferris HA, Cai W, et al. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63(7):2232–2243. PubMed CrossRef
- Begg DP, Mul JD, Liu M, et al. Reversal of diet-induced obesity increases insulin transport into cerebrospinal fluid and restores sensitivity to the anorexic action of central insulin in male rats. Endocrinology. 2013;154(3):1047–1054. PubMed CrossRef
- Kamintsky L, Cairns KA, Veksler R, et al. Blood-brain barrier imaging as a potential biomarker for bipolar disorder progression. Neuroimage Clin. 2019;26:102049. PubMed
- Rybakowski JK, Remlinger-Molenda A, Czech-Kucharska A, et al. Increased serum matrix metalloproteinase-9 (MMP-9) levels in young patients during bipolar depression. J Affect Disord. 2013;146(2):286–289. PubMed CrossRef
- Lakhan SE, Kirchgessner A, Tepper D, et al. Matrix metalloproteinases and blood-brain barrier disruption in acute ischemic stroke. Front Neurol. 2013;4:32. PubMed CrossRef
- Kang HS, Cho HC, Lee JH, et al. Metformin stimulates IGFBP-2 gene expression through PPARalpha in diabetic states. Sci Rep. 2016;6(1):23665. PubMed CrossRef
- Zhao Y, Wei X, Song J, et al. Peroxisome proliferator-activated receptor γ agonist rosiglitazone protects blood-brain barrier integrity following diffuse axonal injury by decreasing the levels of inflammatory mediators through a caveolin-1-dependent pathway. Inflammation. 2019;42(3):841–856. PubMed CrossRef
- Mandrekar-Colucci S, Sauerbeck A, Popovich PG, et al. PPAR agonists as therapeutics for CNS trauma and neurological diseases. ASN Neuro. 2013;5(5):e00129. PubMed CrossRef
- Calkin C, McClelland C, Cairns K, et al. Insulin resistance and blood-brain barrier dysfunction underlie neuroprogression in bipolar disorder. Front Psychiatry. 2021;12:636174. PubMed CrossRef
- American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry. 2002;159(suppl 4):1–50. PubMed
- Mason C, Foster-Schubert KE, Imayama I, et al. Dietary weight loss and exercise effects on insulin resistance in postmenopausal women. Am J Prev Med. 2011;41(4):366–375. PubMed CrossRef
- Melo MCA, Garcia RF, de Araújo CFC, et al. Physical activity as prognostic factor for bipolar disorder: an 18-month prospective study. J Affect Disord. 2019;251:100–106. PubMed CrossRef
- Firth J, Solmi M, Wootton RE, et al. A meta-review of “lifestyle psychiatry”: the role of exercise, smoking, diet and sleep in the prevention and treatment of mental disorders. World Psychiatry. 2020;19(3):360–380. PubMed CrossRef
- Davies M, Færch L, Jeppesen OK, et al; STEP 2 Study Group. Semaglutide 2–4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–984. PubMed CrossRef
- Okamura F, Tashiro A, Utumi A, et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism. 2000;49(10):1255–1260. PubMed
- Dasgupta A, Singh OP, Rout JK, et al. Insulin resistance and metabolic profile in antipsychotic naïve schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1202–1207. PubMed CrossRef
- Blessing EM, Reus V, Mellon SH, et al. Biological predictors of insulin resistance associated with posttraumatic stress disorder in young military veterans. Psychoneuroendocrinology. 2017;82:91–97. PubMed CrossRef
- Albert U, Aguglia A, Chiarle A, et al. Metabolic syndrome and obsessive-compulsive disorder: a naturalistic Italian study. Gen Hosp Psychiatry. 2013;35(2):154–159. PubMed CrossRef
This PDF is free for all visitors!