Objective: Perinatal depression is a common and costly health concern with serious implications for the mother and child. We sought to quantify the “Perinatal Depression Treatment Cascade”—the cumulative shortfalls in clinical recognition, initiation of treatment, adequacy of treatment, and treatment response for women with antenatal (AND) and postpartum depression (PPD).
Data Sources: A systematic search was conducted to identify articles about diagnostic rates, treatment rates, adequate treatment rates, and remission rates for AND and PPD. We searched PubMed and EMBASE through March 2015.
Study Selection: Articles were included if they were in English and examined rates of detection, treatment, adequate treatment, or remission for AND or PPD.
Data Extraction and Analysis: Mean rates of diagnosis, treatment, adequate treatment, and remission were calculated and weighted based on the number of subjects in each study. Search results were dually reviewed for confirmation of study eligibility and data abstraction.
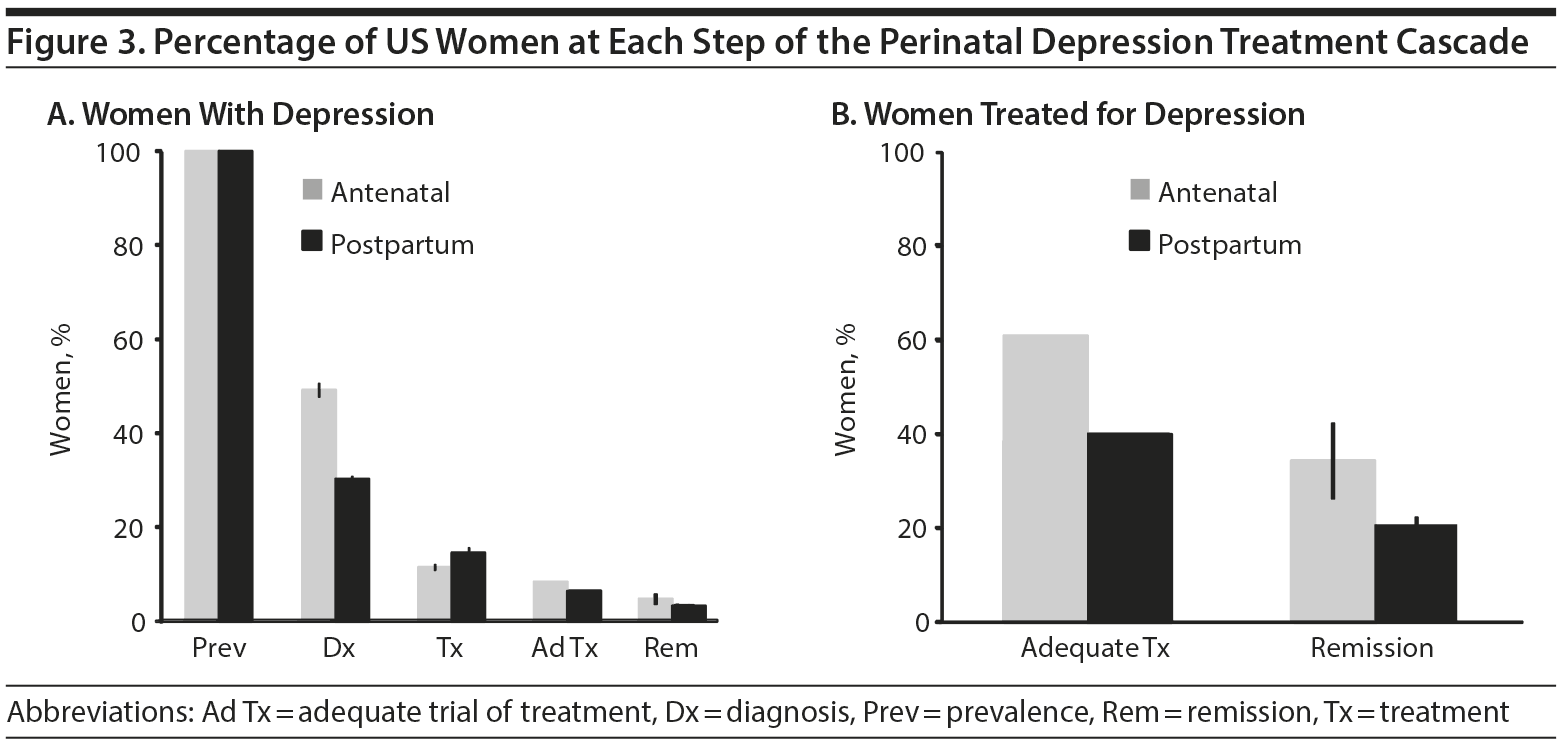
Results: Decrements occur at each branch of the cascade. Data suggest that 49.9% of women with AND and 30.8% of women with PPD are identified in clinical settings; 13.6% of women with AND and 15.8% of women with PPD receive treatment; 8.6% of women with AND and 6.3% of women with PPD receive adequate treatment; and 4.8% of women with AND and 3.2% of women with PPD achieve remission.
Conclusions: Application of the treatment cascade model suggests multiple opportunities for improving perinatal depression management, informing optimal allocation of resources, and providing adequate treatment to this underrecognized and undertreated population..
See letter by Dama et al and reply by Cox et al
This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.

The Perinatal Depression Treatment Cascade:
Baby Steps Toward Improving Outcomes

ABSTRACT
Objective: Perinatal depression is a common and costly health concern with serious implications for the mother and child. We sought to quantify the “Perinatal Depression Treatment Cascade”—the cumulative shortfalls in clinical recognition, initiation of treatment, adequacy of treatment, and treatment response for women with antenatal (AND) and postpartum depression (PPD).
Data Sources: A systematic search was conducted to identify articles about diagnostic rates, treatment rates, adequate treatment rates, and remission rates for AND and PPD. We searched PubMed and EMBASE through March 2015.
Study Selection: Articles were included if they were in English and examined rates of detection, treatment, adequate treatment, or remission for AND or PPD.
Data Extraction and Analysis: Mean rates of diagnosis, treatment, adequate treatment, and remission were calculated and weighted based on the number of subjects in each study. Search results were dually reviewed for confirmation of study eligibility and data abstraction.
Results: Decrements occur at each branch of the cascade. Data suggest that 49.9% of women with AND and 30.8% of women with PPD are identified in clinical settings; 13.6% of women with AND and 15.8% of women with PPD receive treatment; 8.6% of women with AND and 6.3% of women with PPD receive adequate treatment; and 4.8% of women with AND and 3.2% of women with PPD achieve remission.
Conclusions: Application of the treatment cascade model suggests multiple opportunities for improving perinatal depression management, informing optimal allocation of resources, and providing adequate treatment to this underrecognized and undertreated population..
J Clin Psychiatry 2016;77(9):1189-1200
dx.doi.org/10.4088/JCP.15r10174
© Copyright 2016 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of North Carolina at Chapel Hill, Chapel Hill
*Corresponding author: Elizabeth Q. Cox, MD, 101 Manning Drive, Chapel Hill, NC 27514 ([email protected]).
Perinatal depression, defined as depressive symptoms occurring either during pregnancy (antenatal depression [AND]) or 4-6 weeks after pregnancy (postpartum depression [PPD]),1,2 is a common and costly health concern with serious implications. Estimates of the prevalence of perinatal depression vary depending on the population examined and the time frame and measure used. Between 14% and 23% of women will experience AND.3 An estimated 11% of women will develop PPD within 72 hours of delivery and 16.7% within 3 months postpartum; 21.9% of women will experience a depressive disorder within the first 12 months after pregnancy.4
Both AND and PPD have been associated with significant adverse outcomes for the mother and infant. AND places the mother at risk for numerous long-term health complications, including hypertension and diabetes,5 and has been linked to maternal gestational weight retention, preeclampsia, and preterm birth, all of which increase the risk of cardiovascular disease and cause morbidity and mortality.6-8 Untreated AND has repeatedly been shown to be one of the greatest risk factors for development of PPD,3,9,10 and PPD has been deemed the greatest risk factor for maternal suicide and infanticide.11
PPD is associated with maternal difficulties in bonding with and nurturing the newborn, which affect brain development.12 PPD has also been associated with low birth weight as well as toxic stress, a dangerous form of stress response in the newborn that leads to persistently elevated cortisol levels that disrupt developing brain architecture and other organ systems, resulting in anatomic changes or physiologic dysregulations that may later impair learning and cause behavior difficulties in the child.7,13-17 For example, in children with histories of toxic stress, cortisol levels remained persistently elevated at preschool age.18
Furthermore, PPD has been associated with lactation failure, or unplanned weaning.19 The literature also demonstrates that there is an increased risk for long-term mental health problems in children of women with PPD, which is an additional encumbrance on both society and the health care system at large.12,20,21 Indeed, the 2-generational annual economic cost in the United States of not treating 1 mother with peripartum depression is $22,647.22 Data from the United States in 2008 involving an estimated 657,800 pregnancies in women with AND suggest that this cost could reach approximately 15 billion health care dollars each year.
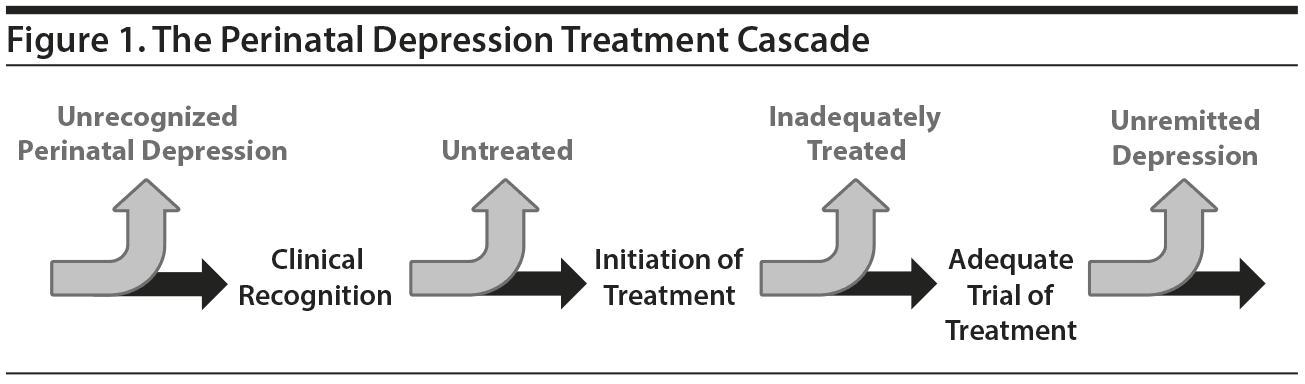
Importantly, perinatal depression is often a trigger for onset of chronic major depressive disorder, as almost 1 in 3 women still report depressive symptoms at least 4 years after childbirth.23 As with other illnesses, including major depressive disorder, a successful clinical response to perinatal depression requires a sequence of steps: the affected individual must enter the health care system, be recognized clinically, initiate treatment, receive an adequate trial of treatment, and experience remission of symptoms.24
Prior work has created a useful framework for how to consider the treatment of perinatal depression.24 We examined the “Perinatal Depression Treatment Cascade”—the cumulative shortfalls in clinical recognition, initiation of treatment, adequacy of treatment, and treatment response for women with AND and PPD in the health care system. Consideration of gaps along this continuum can help identify ways to effectively improve the identification and management of perinatal depression.
THE PERINATAL DEPRESSION TREATMENT CASCADE
The steps required for successful treatment of perinatal depression can be understood to lie along a continuum of treatment (Figure 1).24 The timing of onset of perinatal depression is an area of controversy in the field. Recently, the DSM-5 expanded the definition of perinatal depression to include onset during pregnancy (peripartum onset), in addition to within the first 4 weeks postpartum, while the ICD-10 includes up to 6 weeks postpartum (PPD).1,2 However, grouping antenatal and postpartum episodes, and not differentiating between the two, may be problematic. The means of identifying, managing, and monitoring these women over time very likely differs depending on timing of symptom onset.25
Accordingly, we reviewed the literature regarding the proportion of perinatal depression cases that are recognized clinically, the proportion of recognized cases that are then treated, the proportion of treated cases that receive an adequate trial of treatment, and the proportion of adequately treated cases that achieve full remission of symptoms. We separated AND and PPD to examine gaps that exist in the treatment cascade and determine where resources ought to be allocated so these patients may be optimally treated.
METHODS
Search Strategy
A systematic search was conducted to identify articles pertaining to the diagnostic rates, treatment rates, adequate treatment rates, and remission rates for AND and PPD. The computerized databases of PubMed and EMBASE were searched through March 2015. Broad search terms were used to ensure as many articles as possible would be identified. The search strategy used was (perinatal OR postpartum OR pregnant OR antenatal) AND depression AND (screen OR detection OR (adequate AND treatment) OR remission OR psychotherapy. Additional studies were identified through careful inspection of reference sections of the relevant articles from the initial search.

- Perinatal depression is common and costly. The Perinatal Depression Treatment Cascade was examined to formally define and evaluate treatment needs.
- 95% to 97% of women with perinatal depression are not successfully treated.
- Given the gaps that exist in this cascade, including low rates of diagnoses, treatment, adequate treatment, and symptom remission, more thoughtful allocation of resources needs to be considered at each interval.
Study Eligibility
Studies were included if they were in English and examined detection rates, treatment rates, adequate treatment rates, or remission rates for AND or PPD. In an effort to best capture a complete picture of the existing literature, all types of studies were initially evaluated, including letters to the editor, pilot studies, and open-label studies. Outcomes were operationalized as different steps along the treatment cascade. Interventions were categorized as treatment with either pharmacologic agents or psychotherapy. An adequate trial of treatment was defined as receiving at least 6 weeks of daily use of antidepressants at the recommended starting dose or more or receiving an established and accepted method of psychotherapy that lasted for at least 6 weeks. Any studies not meeting our inclusion criteria were excluded. In addition, studies that used informal means of treatment (ie, support groups) and studies that presented response to treatment but did not include calculated remission rates were excluded.
Review Process, Data Extraction, and Analysis
The literature search, study review, and data extraction were initially conducted by one individual (N.A.S.); at each step, the findings were confirmed by a second reviewer (E.Q.C., B.N.G., or S.E.M.-B.) before proceeding to the next step. Any questions that arose were resolved by discussion among all reviewers. The titles of all studies identified as a result of our search strategy were examined, and studies that clearly did not pertain to our topic of interest were eliminated. Studies clearly not meeting our inclusion criteria, based on their abstracts, were eliminated from further review. The full-text articles of the remaining studies were examined for inclusion, and data extraction was conducted if deemed appropriate. From the included studies, the full text of each article was reviewed and specific information was extracted, including study location, number of participants, time frame of the study, means of diagnosing depression, definition of remission from depression, rates of diagnosis, rates of treatment, rates of adequate treatment, rates of remission from depression, and type of treatment used.
Mean rates of diagnosis, treatment, adequate treatment, and remission were weighted based on the number of subjects in each study, and 95% confidence intervals were calculated.26 For studies that used more than one measurement tool to determine remission, the tool that gave the lower value of remission was used, as this very likely represented more stringent criteria to determine patient treatment responses. Individual remission rates were calculated for studies that used psychotherapy treatment only versus pharmacologic treatment only, as well as an overall remission rate. Given the small number of studies, we combined the results from studies using different types of psychotherapy (eg, cognitive-behavioral therapy [CBT], interpersonal therapy), although we realize that different therapeutic techniques may have different efficacies. To determine absolute numbers of individuals affected at each step of the treatment cascade, data were extracted from the National Vitals Statistic Report from 1990 to 2008.27 Values for the actual prevalence of AND and PPD were calculated based on accepted prevalence rates of 10% and 13%, respectively, of women in the United States using these data. Values for each step of the treatment cascade were then calculated using the mean rates determined above.
RESULTS
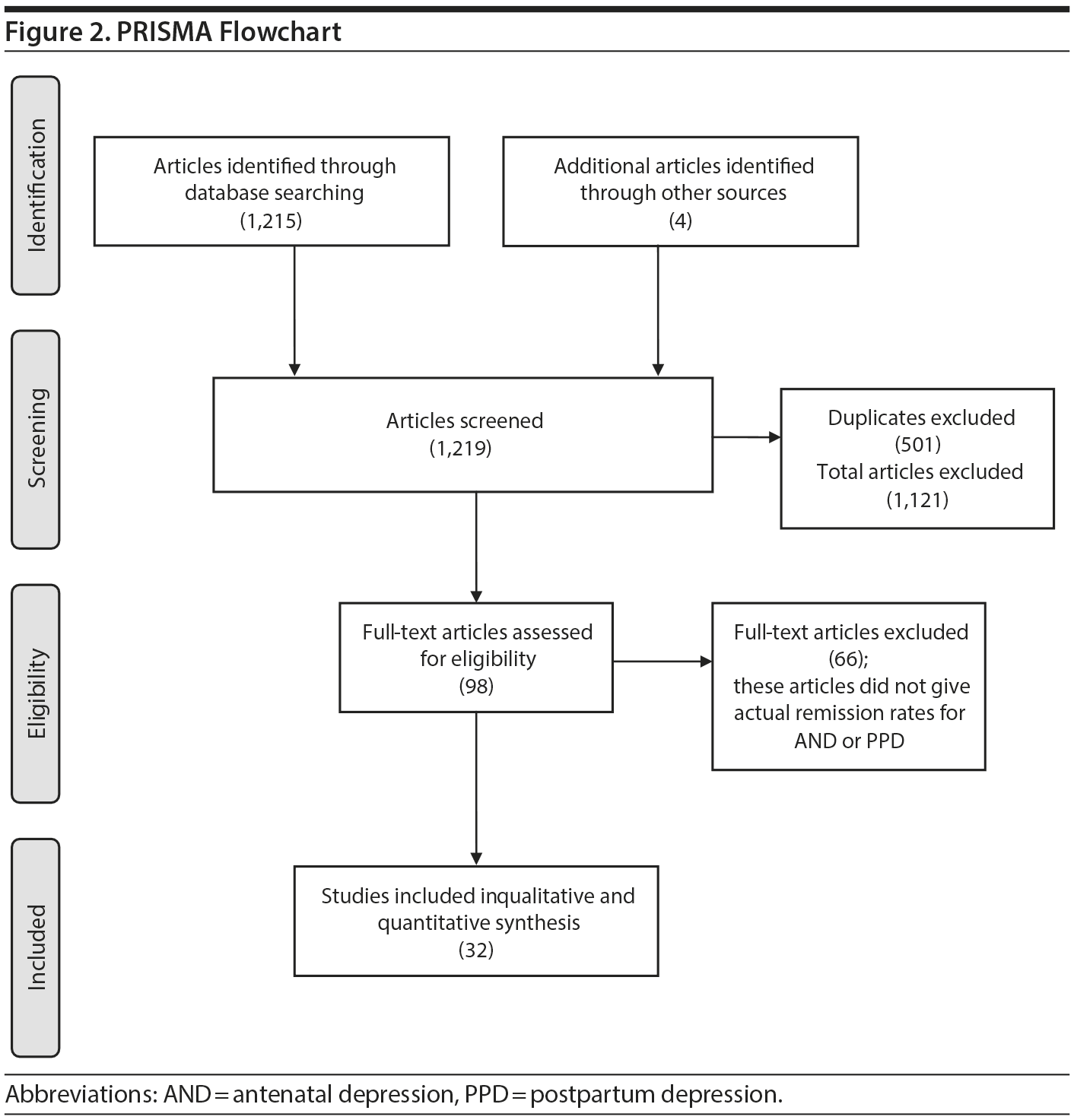
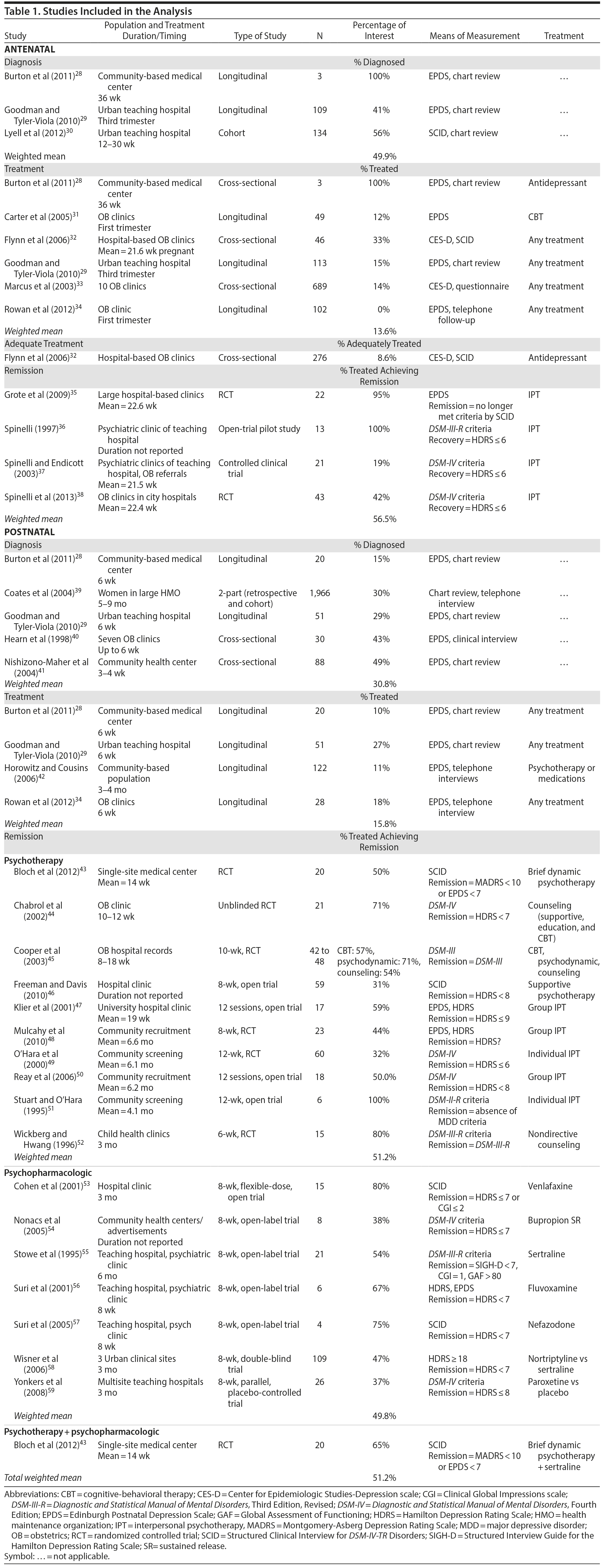
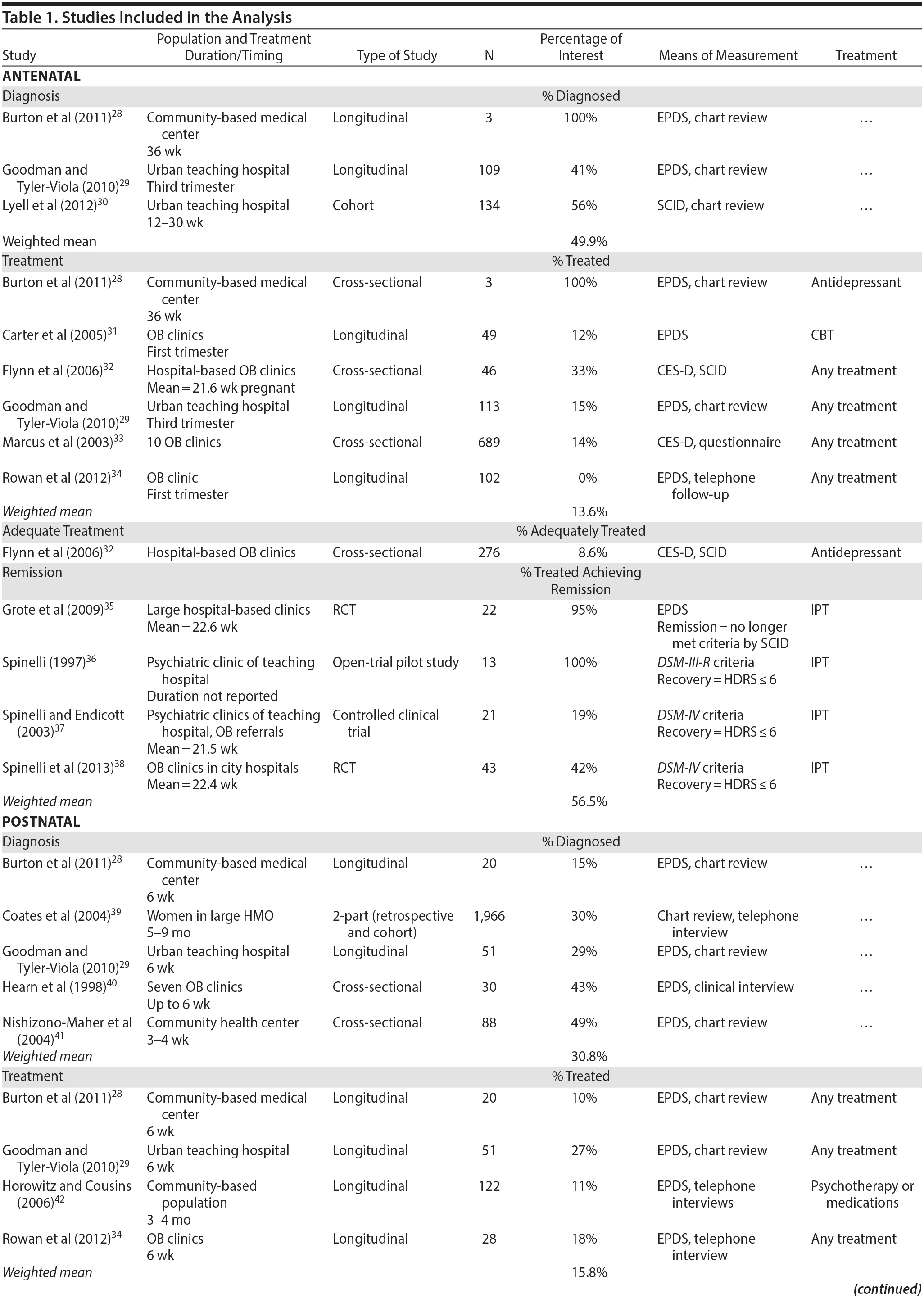
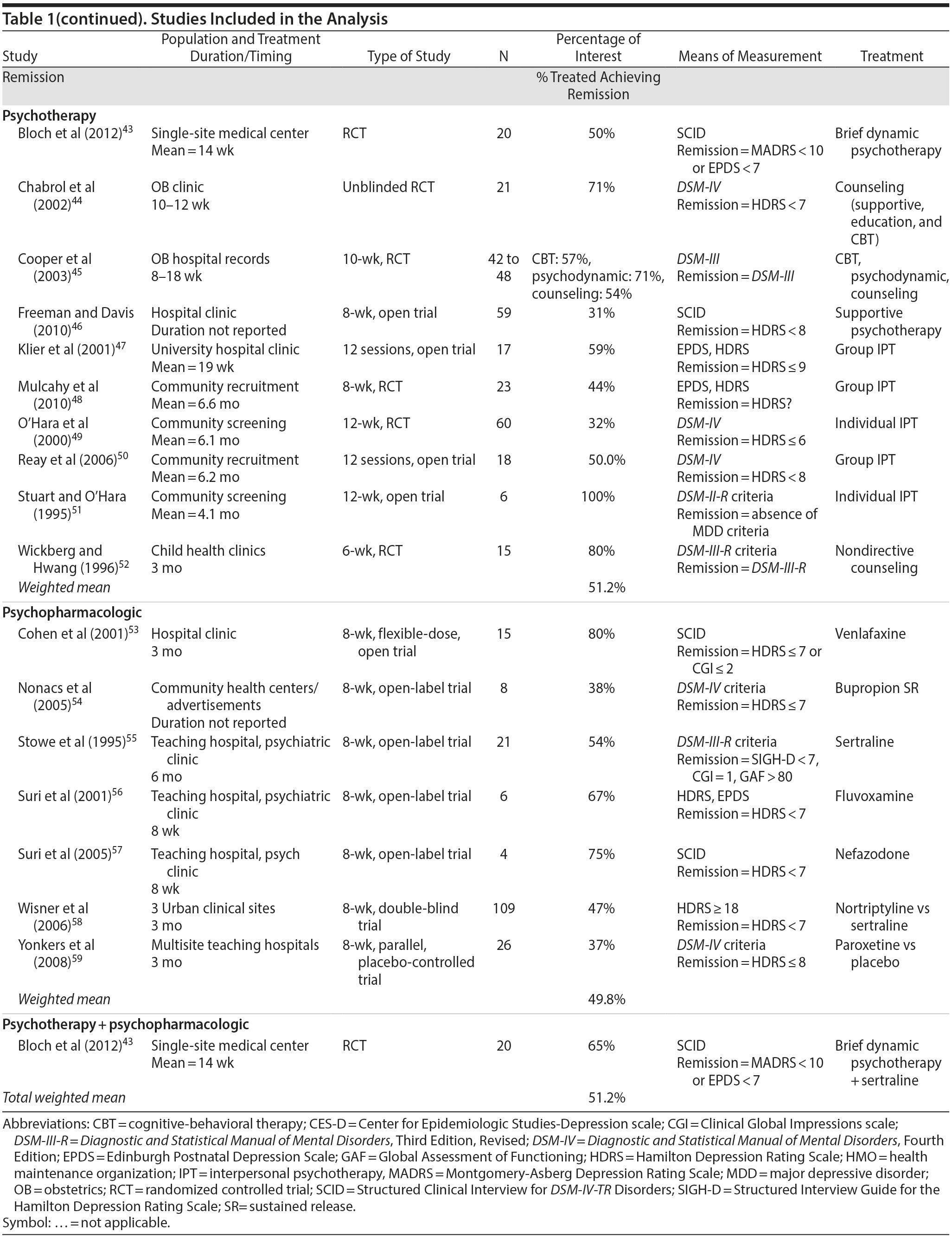
Our approach identified 1,215 articles for consideration. Four additional articles were identified from reviewing the reference sections of included articles. On the basis of our a priori inclusion criteria, we eliminated 1,121 records at the abstract level (including 501 duplicates) and identified 98 records that were examined more closely for eligibility. Of the 98 records initially included, 66 were eliminated from further evaluation because they did not give actual remission rates for AND or PPD, resulting in 32 records that were included in the analyses. From these 32 records, we extracted specific data, including the percentage of women with AND or PPD who were diagnosed with the disorder, initially treated for the disorder, and adequately treated for the disorder and who went into remission with treatment. An adequate trial of treatment was defined as at least 6 weeks of daily use of antidepressants at the recommended starting dose or greater, or an established and accepted method of psychotherapy that lasted for at least 6 weeks. All studies providing the above results were included, regardless of the quality of the study. Our approach is highlighted in PRISMA flowchart (Figure 2); included studies are highlighted in Table 1.28-59
Steps Along the Perinatal Depression Treatment Cascade
As shown in Figure 1, the Perinatal Depression Treatment Cascade consists of prevalent cases of perinatal depression (AND and PPD), which is clinically recognized and diagnosed, and followed by treatment initiation, identification of an adequate trial of treatment, and, finally, remission of symptoms. Figure 3 highlights the percentage of women in each of these categories as well as the percentage receiving an adequate trial of treatment and demonstrating remission of symptoms.
Clinical Recognition: Rate of Diagnosis
Diagnosis of AND. Three studies28-30 examined the rate of diagnosis of women with AND in the general population. One of the studies28 involved 3 women, while the other 2 studies29,30 had over 100 participants each (109 and 134, respectively). All 3 studies were longitudinal in design. Two of the studies used a screening tool (the Edinburgh Postnatal Depression Scale [EPDS])60 to identify women with AND and then used chart review to identify which of those women were given a diagnosis of depression by their clinicians. The remaining study used a structured diagnostic interview (the Structured Clinical Interview for DSM-IV-TR61) to identify women with AND and then used chart review to determine which of those women were given a diagnosis of depression by their clinicians.30 This study had the highest rate of diagnosis by clinicians. The weighted mean rate of diagnosis of AND in these studies was 49.9% (95% CI, 48.4%-51.4%) with a range of 41%-100% (Figure 3).
Diagnosis of PPD. Five studies28,29,39-41 examined the rate of diagnosis of women with PPD in the general population. The weighted mean diagnosis rate from these studies was 30.8% (95% CI, 30.2%-31.4%) with a range of 15%-49%. One of the studies was much larger in number than the others,39 which may have had a greater effect on the weighted mean. This study was also the only one that used chart review to first identify women with PPD, while the others used the EPDS. This study was also the only one to follow up as far out as 5-9 months after birthgiving, while all other studies looked at women 3-6 weeks after birth. Despite all these differences, the diagnosis rate in this study (30%) was near the simple mean diagnosis rate of 33%; the larger study had minimal effect on the overall weighted mean (Figure 3).
Treatment Initiation
Treatment of AND. Six studies28,29,31-34 examined the rate of treatment of women who had been diagnosed with AND. There was a wide range in the size of these studies, with total number of subjects ranging from 3 to 689. One of the medium-sized studies34 had a 0% treatment rate. Four of the studies used the EPDS to identify women with AND, while 2 used the Center for Epidemiologic Studies-Depression Scale.64 Half of the studies were longitudinal in design, while the other half were cross-sectional. None of these variables appeared to affect the treatment rates. The weighted mean rate of treatment of women with AND was 13.6% (95% CI, 11.3%-15.8%), with a range of 0%-100% (Figure 3).
Treatment of PPD. Four studies28,29,34,42 examined the rate of treatment for women with PPD. All 4 studies were longitudinal in design and all used the EPDS to identify women with PPD. Two of the studies used telephone interviews to determine how many women with PPD were treated,34,42 while 2 studies28,29 used chart review. The weighted mean rate of treatment from these studies was 15.8% (95% CI, 14.8%-16.9%), with a range of 10%-27% (Figure 3).
Adequacy of Treatment
Adequate Trial of Treatment of AND. One study32 retrospectively examined the number of “high risk” pregnant women (defined as women who were either identified through depression screening tools or clinician-identified through the use of a structured interview) who received “any treatment” for depression and those who received “adequate antidepressant treatment.” An adequate trial of treatment was defined as receiving at least 6 weeks of daily use of antidepressants at the recommended starting dose or more or receiving an established and accepted method of psychotherapy that lasted for at least 6 weeks. The researchers found that approximately 20% of the women received any treatment, and, of those that received any treatment, 43% received an adequate trial of treatment. Thus, of the whole population of women with AND, only 8.6% received an adequate trial of treatment (Figure 3).
Adequate Trial of Treatment of PPD. No studies were identified that examined the rate of women with PPD who received an adequate trial of treatment. Estimates from the general population suggest that approximately 40% of people with depression are adequately treated.24 This value is similar to the 43% value seen in the single study on AND.32 Given that 15.8% of women with PPD receive any treatment, we estimate that approximately 6.3% of all women with PPD receive an adequate trial of treatment (Figure 3).
Treatment Response: Rate of Remission
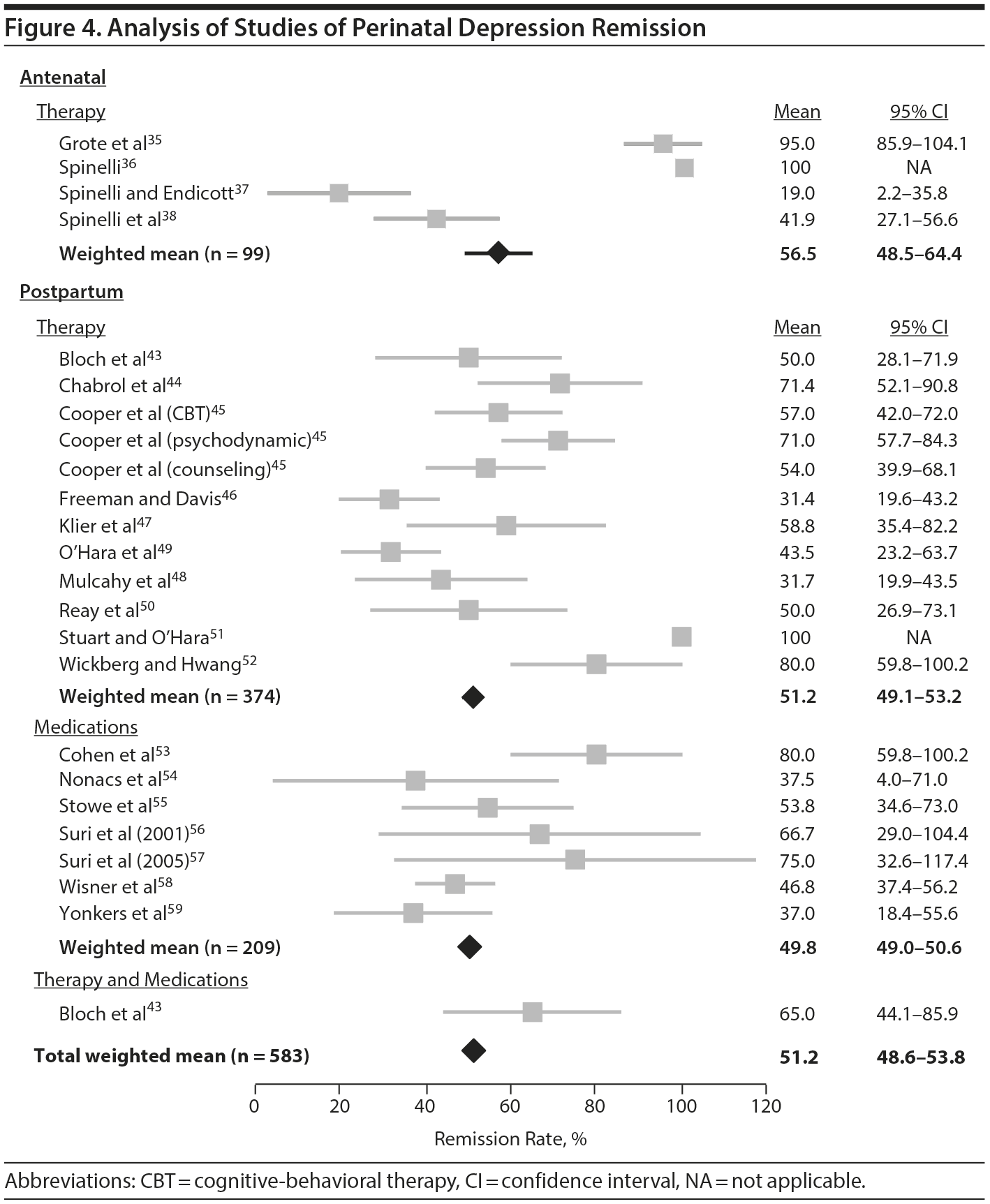
Treatment Response of AND. Four studies35-38 were identified that used psychotherapy treatment and reported remission rates for AND. No studies were identified that used pharmacologic therapy and reported remission rates for AND. The 4 studies had a weighted mean remission rate of 56.5% (95% CI, 48.5%-64.4%) and had a range of 19%-100% (Figure 4, Table 1). One of the studies36 was a 1-armed, pilot study with outcome assessors who were not blinded to treatment and had a 100% remission rate. It was included in our analysis despite the poor study design given the small number of overall studies identified. If this study were excluded, the mean remission rate would then be 43.3% (95% CI, 35.5%-51.1%). Given that only 8.6% of all women with AND receive adequate treatment, and using the weighted mean remission rate, an estimated 4.8% of all women with AND achieve remission (Figure 3).
Treatment Response of PPD. Seventeen studies43-59 examined the rate of remission of women with PPD who were adequately treated for PPD. Seven53-59 of the 17 studies used a pharmacologic intervention alone for treatment and had a weighted mean remission rate of 49.8% (95% CI, 49.0-50.6%) and a range of 38%-80% (Figure 4, Table 1). Ten43-52 of the 17 studies used a psychotherapy intervention alone for treatment and had a weighted mean remission rate of 51.2% (95% CI, 49.1%-53.3%) and a range of 31%-100% (Figure 4, Table 1). One study43 examined psychotherapy in addition to pharmacotherapy and had a remission rate of 65% in the psychotherapy + pharmacotherapy group. Overall, a variety of psychotherapy techniques were used, including brief dynamic psychotherapy, CBT, supportive psychotherapy, interpersonal psychotherapy, and nondirective counseling. Most of the studies were small in size, with only 1 pharmacologic study with more than 100 participants. Remission was defined in various ways, including based on diagnostic criteria using the DSM-IV66 or DSM-III67 as well as cutoffs on the Hamilton Depression Rating Scale (HDRS),68 the Structured Interview Guide for the Hamilton Depression Rating Scale (SIGH-D),69 the Montgomery-Asberg Depression Rating Scale (MADRS),70 and the Clinical Global Impressions scale (CGI).71 The overall weighted mean rate of remission was 51.2% of adequately treated women (95% CI, 48.6%-53.8%), with a broad range of 31%-100% (Figure 4, Table 1). Overall, given that only 6.6% of all women with PPD receive adequate treatment and using the overall weighted mean remission rate, an estimated 3.2% of all women with PPD achieve remission.
DISCUSSION: POTENTIAL AREAS TO ADDRESS THE PERINATAL DEPRESSION TREATMENT CASCADE
The framework provided offers a suggestion for potential intervention points to address the drop off between the subsequent steps.
Improving Detection: To Screen or Not to Screen?
Over 50% of women with AND or PPD are going unrecognized and undiagnosed (Figure 3). One strategy to improve outcomes would be to increase the number of women with AND or PPD who are successfully screened as a first step, which could ultimately improve treatment outcomes. Screening for perinatal depression has been mandated in several states,72 and given that the DSM-IV had previously focused solely on PPD, most screening interventions to date have been targeted during the postpartum period.73 Screening alone has been shown to have little to no impact on detection and management of PPD by clinicians,74 and without formal diagnostic evaluations or treatment implementation, screening in and of itself may not be justifiable or cost effective.75-77 As with general depression screening programs, substantial staff assistance and support are required for PPD screening programs to be successful.78 Ensuring follow-up care is paramount. During pregnancy, women have the highest contact with the health care system, making this an easier time for screening and identification; however, psychotherapy may not be available in many settings, and not everyone is comfortable treating pregnant women with psychotropic medications. Postpartum women make most contact with the health care system through their children’s pediatricians, who are largely focused on treating their identified patients—the children. However, the provision of quality care to the child should include ensuring the child is in a safe environment, with a healthy mother. Treating maternal PPD will improve child developmental outcomes and form the basis of family-centered care.
Improving Treatment Initiation
According to our estimates, approximately 85% of women with AND or PPD are not receiving treatment (Figure 3). Once these women are identified, appropriate treatments need to be available. Disease management programs, consisting of interdisciplinary care teams within health systems, have been shown to improve detection, treatment, and management of patients with MDD,79,80 as have systematic care management protocols utilizing a team-based approach with physicians and nonphysician case managers.80-82 Disease management programs have proved successful in treating depression in the non-perinatal population; therefore, these programs may prove to be equally efficacious for patients with AND and PPD.
Improving Adequacy of Treatment
Prior systematic review analyses involving depression in primary care indicate that the greatest hurdle to long-term relief from MDD is probably inadequate treatment, rather than insufficient identification.80 Our results suggest that this scenario is likely also true for AND and PPD. Only 8.6% of all women with AND and 6.6% of women with PPD receive an adequate trial of treatment, leaving over 90% of women suffering from these disorders with inadequate treatment and little relief (Figure 3). AND is the biggest risk factor for PPD, and an estimated 60%-70% of women will experience relapse when terminating antidepressant use during pregnancy.83
The American Psychiatric Association and American Congress of Obstetrics and Gynecology both recommend either psychotherapy or antidepressant medication as first-line treatment for mild-to-moderate depression.84 Medication treatment and psychotherapy are each effective treatments for perinatal depression.85-87 It is key to ensure that women are receiving adequate trials of these available and efficacious treatments.
Improving Remission: Bringing Mental Health Care to the People
According to our data, only approximately 3%-5% of women with PPD or AND are experiencing remission of their symptoms, indicating that nearly 95%-97% of these women are not successfully treated (Figure 3). Perinatal women experience numerous barriers in receiving accessible and available health care. One model that could be modified to systematically address perinatal mental health is the “Centering Pregnancy” model of care delivery. Centering Pregnancy is a group prenatal care model that was designed to address ways to improve the quality of perinatal care.88-90 A curriculum is delivered in a series of 10 group sessions over a 6-month time period with goals of improving health literacy by establishing a continuum of care.88 Areas of focus have included nutrition counseling, breastfeeding, and maternal weight gain. Mental health has not yet been addressed in this model,89 and it is possible that by adding mental health to group perinatal care models, we could improve awareness, treatment initiation, adequacy of treatment, and, ultimately, remission of symptoms for these women.
Furthermore, a collaborative, multidisciplinary approach among psychiatry, obstetrics and gynecology (OB/GYN), and pediatrics may help address this public health care problem. In a group of depressed women in an OB/GYN setting, those who received collaborative care experienced a greater satisfaction with patient care, demonstrated an improvement in functioning, were more likely to have their depressive symptoms decreased by 50% at 12 months out from treatment, were more likely to attend at least 4 or more mental health specialty visits, and were more likely to receive an adequate dose of an antidepressant.91 Furthermore, the women within that cohort who had no insurance or public coverage benefited the most from the collaborative care model.92 Two studies94,95 have examined collaborative care in specifically treating PPD. In the stepped care intervention program, women enrolled in collaborative care had better understanding of their diagnosis of PPD, as well as actual receipt of treatment.95 In the TRIPPD (Translating Research Into Practice for Postpartum Depression) study,94 28 practices were randomized to either usual care or an intervention utilizing collaborative care with tools for PPD screening, diagnosis, initiation of treatment, and follow-up within the practice; women who were enrolled in collaborative care were more likely to receive treatment for PPD and had lower levels of depressive symptoms at 6 and 12 months postpartum. Alternative methods of reaching out to patients and bringing mental health care directly to the consumer are also promising. A manualized telephone-based peer support program reaching out to patients in their home postpartum may also prove to be efficacious.96
While universal screening for AND or PPD could become standard of care, providers will need to know what to do with a positive result, and collaborative care models, as well as innovative programs such as telephone-based peer support programs, may provide appropriate instructions for how to access care and proceed with treatment. Limitations of such models may include political and cultural fit, cost, level of resources required, necessary expertise needed, and ease of adoption.89,97
CONCLUSIONS
In our review, we found similarly low rates of diagnosis, treatment, and remission for both AND and PPD. While improvements are being made in identifying and screening women for perinatal depression, management remains grossly inadequate: nearly 50%-70% of women go undetected, approximately 85% go untreated, 91%-93% are not adequately treated, and 95%-97% continue to suffer from symptoms without remission. Our data show greater challenges for PPD, which has lower diagnostic, adequate treatment, and remission rates than AND (30.8%, 6.3%, and 3.2%, vs 49.9%, 8.6%, and 4.8%, respectively). In 2008, there were 6,578,000 pregnancies and 4,248,000 births in the United States.27 Using estimates of prevalence of 10% for AND and 13% for PPD, our data suggest that 657,800 women developed AND and 552,240 women developed PPD that year. Of these women, only 31,772 women with AND and 17,893 women with PPD achieved remission, leaving 626,028 women with uncontrolled AND and 534,347 women with uncontrolled PPD.
Our study does have several limitations. We completed a strategic, systematic search; however, we did not complete a formal systematic review (and therefore had no formal means for quality assessment of included studies). It is possible that some relevant studies were missed; however, in general, the literature available in this area is minimal. Additionally, we extrapolated data for adequate trials of treatment from non-perinatal populations due to lack of available data for perinatal women. However, given the lack of data for the perinatal populations, these results may actually overestimate actual rates, making the actual proportion of patients who remit even lower than our calculated rates. As more data become available in this area, further analyses of rates will be needed.
Results from the existing mandated screening programs for perinatal depression indicate that screening these women alone is insufficient.76 The educational campaign and screening used were not associated with improved treatment initiation, follow-up, or continued care in the Medicaid population of New Jersey.98 In another study reviewing 54 mandated screening programs, only 8 of these programs had clearly stated both their interventions and outcomes in their reports.99 Given that these diseases have validated, efficacious treatments, these findings highlight the need for more thoughtful allocation of our limited resources so that patients may be optimally treated. Once a patient has a positive screen for depression, referral for a diagnostic assessment and proper treatment must be initiated.77 Indeed, screening in combination with additional interventions has been associated with superior rates of both detection and treatment in perinatal care settings.100 Further mechanisms must be put into place for such programs to succeed, such as monitoring that screening is actually occurring or providing incentives to providers to screen individuals and follow-up on patient care.
Our proposed treatment cascade identifies multiple steps along the perinatal management continuum at which increased effort could substantially improve outcomes. Consistent with the United States Preventive Services Task Force recommendation that adults should be screened for depression when staff-assisted depression care supports are in place to systematically assure accurate diagnosis, effective treatment, and follow-up,101 a model for the perinatal population that addresses each one of these steps by providing evidence-based support could very likely produce substantially beneficial effects. Indeed, a collaborative care model is associated with better results, as compared to non-integrative models, by adequately treating patients and improving patient satisfaction with care.91 At each branch of the treatment cascade, improvements must be considered in order to optimally allocate resources and provide sufficient treatment to patients with perinatal depression. Ultimately, a combination of system-level enhancements for the identification and treatment of perinatal depression is necessary to substantially improve depression outcomes and quality of life among pregnant and postpartum women with depression.
Submitted: June 15, 2015; accepted January 20, 2016.
Drug names: bupropion SR (Wellbutrin, Aplenzin, and others), fluvoxamine (Luvox and others), nortriptyline (Pamelor and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others).
Disclosure of off-label usage: The authors have determined that, to the best of their knowledge, bupropion, fluvoxamine, nortriptyline, paroxetine, sertraline, venlafaxine, and nefazodone are not approved by the US Food and Drug Administration for the treatment of perinatal depression.
Financial disclosure: Dr Meltzer-Brody has received grant/research support from Sage Therapeutics. Drs Cox, Sowa, and Gaynes have no personal affiliations or financial relationships with any commercial interest to disclose relative to the article.
Funding/support: Support was provided by the following grants: RO1—1R01MH104468-01 awarded to Dr Meltzer-Brody and grants from the National Institutes of Health (UNC Clinical and Translational Science Award, UL1 TR001111) and the Agency for Healthcare Research and Quality (“Topic Refinement and Large Systematic Review Strategies to Improve Mental Health Care”) awarded to Dr Gaynes.
Role of the sponsor: Sponsors had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Previous presentation: Data were presented by Dr Cox at Department of Psychiatry Grand Rounds at UNC Hospital; June 10, 2015; Chapel Hill, North Carolina ▪ 2nd Biennial Perinatal Mental Health Conference at Northwestern Hospital; November 4, 2015; Chicago, Illinois.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. ICD-10 Classifications of Mental and Behavioural Disorder: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Arlington, VA: American Psychiatric Publishing; 2013.
3. Gaynes BN, Gavin N, Meltzer-Brody S, et al. Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ). 2005;(119):1-8. PubMed
4. Elisei S, Lucarini E, Murgia N, et al. Perinatal depression: a study of prevalence and of risk and protective factors. Psychiatr Danub. 2013;25(suppl 2):S258-S262. PubMed
5. AHRQ. Breastfeeding and Maternal and Infant Health Outcomes in Developed Countries. Rockville, MD: Department of Health and Human Services; 2007.
6. Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry. 2013;74(4):e321-e341. PubMed doi:10.4088/JCP.12r07968
7. Herring SJ, Rich-Edwards JW, Oken E, et al. Association of postpartum depression with weight retention 1 year after childbirth. Obesity (Silver Spring). 2008;16(6):1296-1301. PubMed doi:10.1038/oby.2008.71
8. Qiu C, Williams MA, Calderon-Margalit R, et al. Preeclampsia risk in relation to maternal mood and anxiety disorders diagnosed before or during early pregnancy. Am J Hypertens. 2009;22(4):397-402. PubMed doi:10.1038/ajh.2008.366
9. Meltzer-Brody S, Bledsoe-Mansori SE, Johnson N, et al. A prospective study of perinatal depression and trauma history in pregnant minority adolescents. Am J Obstet Gynecol. 2013;208(3):e1-e7. PubMed doi:10.1016/j.ajog.2012.12.020
10. Milgrom J, Gemmill AW, Bilszta JL, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108(1-2):147-157. PubMed doi:10.1016/j.jad.2007.10.014
11. Lindahl V, Pearson JL, Colpe L. Prevalence of suicidality during pregnancy and the postpartum. Arch Women Ment Health. 2005;8(2):77-87. PubMed doi:10.1007/s00737-005-0080-1
12. Earls MF; Committee on Psychosocial Aspects of Child and Family Health AAP. Incorporating recognition and management of perinatal and postpartum depression into pediatric practice. Pediatrics. 2010;126(5):1032-1039. PubMed doi:10.1542/peds.2010-2348
13. Garner AS, Shonkoff JP, Siegel BS, et al; Committee on Psychosocial Aspects of Child and Family Health; Committee on Early Childhood Adoption, and Dependent Care; Section on Developmental and Behavioral Pediatrics. Early childhood adversity, toxic stress, and the role of the pediatrician: translating developmental science into lifelong health. Pediatrics. 2012;139(1):e224-e231. PubMed doi:10.1542/peds.2011-2662
14. National Scientific Council on the Developing Child. Excessive Stress Disrupts the Architecture of the Developing Brain: Working Paper 3. Cambridge, MA: Center on the Developing Child at Harvard University; 2005.
15. National Scientific Council on the Developing Child. Early Experiences Can Alter Gene Expression and Affect Long-Term Development: Working Paper #10. Cambridge, MA: Center on the Developing Child at Harvard University; 2005.
16. Dawson G, Ashman S. On the origins of a vulnerability to depression: the influence of the early social environment on the development of psychobiological systems related to risk for affective disorder. In: Nelson C, ed. The Effects of Adversity on Neurobehavioral Development: Minnesota Symposium on Child Psychology. Mahwah, NJ: Lawrence Erlbaum & Assoc; 2000:245-280.
17. Shonkoff JP, Garner AS, Siegel BS, et al; Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232-e246. PubMed doi:10.1542/peds.2011-2663
18. Ashman SB, Dawson G, Panagiotides H, et al. Stress hormone levels of children of depressed mothers. Dev Psychopathol. 2002;14(2):333-349. PubMed doi:10.1017/S0954579402002080
19. Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123(4):e736-e751. PubMed doi:10.1542/peds.2008-1629
20. Agnafors S, Sydsjö G, Dekeyser L, et al. Symptoms of depression postpartum and 12 years later-associations to child mental health at 12 years of age. Matern Child Health J. 2013;17(3):405-414. PubMed doi:10.1007/s10995-012-0985-z
21. Essex MJ, Klein MH, Cho E, et al. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biol Psychiatry. 2002;52(8):776-784. PubMed doi:10.1016/S0006-3223(02)01553-6
22. Diaz J, Chase R. The cost of untreated maternal depression, brief. Wilder Research Web site. http://www.wilder.org/Wilder-Research/Publications/Studies/Cost%20of%20
Untreated%20Maternal%20Depression/The%20Cost%20of%20Untreated%20Maternal
%20Depression,%20Brief.pdf. Updated 2010. Accessibility verified July 27, 2016
23. Woolhouse H, Gartland D, Mensah F, et al. Maternal depression from early pregnancy to 4 years postpartum in a prospective pregnancy cohort study: implications for primary health care. BJOG. 2015;122(3):312-321. 10.1111/1471-0528.12837 PubMed
24. Pence BW, O’ Donnell JK, Gaynes BN. The depression treatment cascade in primary care: a public health perspective. Curr Psychiatry Rep. 2012;14(4):328-335. PubMed doi:10.1007/s11920-012-0274-y
25. Putnam K, Blackmore-Robertson E, Payne J, et al; Postpartum Depression: Action Towards Causes and Treatment (PACT) Consortium. Heterogeneity of postpartum depression: a latent class analysis. Lancet Psychiatry. 2015;2(1):59-67. PubMed doi:10.1016/S2215-0366(14)00055-8
26. Sowa NA, Cholera R, Pence BW, et al. Perinatal depression in HIV-infected African women: a systematic review. J Clin Psychiatry. 2015;76(10):1385-1396. PubMed doi:10.4088/JCP.14r09186
27. Ventura SJ, Curtin SC, Abma JC, et al. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep. 2012;60(7):1-21. PubMed
28. Burton A, Patel S, Kaminsky L, et al. Depression in pregnancy: time of screening and access to psychiatric care. J Matern Fetal Neonatal Med. 2011;24(11):1321-1324. PubMed doi:10.3109/14767058.2010.547234
29. Goodman JH, Tyer-Viola L. Detection, treatment, and referral of perinatal depression and anxiety by obstetrical providers. J Womens Health (Larchmt). 2010;19(3):477-490. PubMed doi:10.1089/jwh.2008.1352
30. Lyell DJ, Chambers AS, Steidtmann D, et al. Antenatal identification of major depressive disorder: a cohort study. Am J Obstet Gynecol. 2012;207(6):e1-e6. PubMed doi:10.1016/j.ajog.2012.09.030
31. Carter FA, Carter JD, Luty SE, et al. Screening and treatment for depression during pregnancy: a cautionary note. Aust N Z J Psychiatry. 2005;39(4):255-261. PubMed doi:10.1080/j.1440-1614.2005.01562.x
32. Flynn HA, Blow FC, Marcus SM. Rates and predictors of depression treatment among pregnant women in hospital-affiliated obstetrics practices. Gen Hosp Psychiatry. 2006;28(4):289-295. PubMed doi:10.1016/j.genhosppsych.2006.04.002
33. Marcus SM, Flynn HA, Blow FC, et al. Depressive symptoms among pregnant women screened in obstetrics settings. J Womens Health (Larchmt). 2003;12(4):373-380. PubMed doi:10.1089/154099903765448880
34. Rowan P, Greisinger A, Brehm B, et al. Outcomes from implementing systematic antepartum depression screening in obstetrics. Arch Women Ment Health. 2012;15(2):115-120. PubMed doi:10.1007/s00737-012-0262-6
35. Grote NK, Swartz HA, Geibel SL, et al. A randomized controlled trial of culturally relevant, brief interpersonal psychotherapy for perinatal depression. Psychiatr Serv. 2009;60(3):313-321. PubMed doi:10.1176/ps.2009.60.3.313
36. Spinelli MG. Interpersonal psychotherapy for depressed antepartum women: a pilot study. Am J Psychiatry. 1997;154(7):1028-1030. PubMed doi:10.1176/ajp.154.7.1028
37. Spinelli MG, Endicott J. Controlled clinical trial of interpersonal psychotherapy versus parenting education program for depressed pregnant women. Am J Psychiatry. 2003;160(3):555-562. PubMed doi:10.1176/appi.ajp.160.3.555
38. Spinelli MG, Endicott J, Leon AC, et al. A controlled clinical treatment trial of interpersonal psychotherapy for depressed pregnant women at 3 New York City sites. J Clin Psychiatry. 2013;74(4):393-399. PubMed doi:10.4088/JCP.12m07909
39. Coates AO, Schaefer CA, Alexander JL. Detection of postpartum depression and anxiety in a large health plan. J Behav Health Serv Res. 2004;31(2):117-133. PubMed doi:10.1007/BF02287376
40. Hearn G, Iliff A, Jones I, et al. Postnatal depression in the community. Br J Gen Pract. 1998;48(428):1064-1066. PubMed
41. Nishizono-Maher A, Kishimoto J, Yoshida H, et al. The role of self-report questionnaire in the screening of postnatal depression- a community sample survey in central Tokyo. Soc Psychiatry Psychiatr Epidemiol. 2004;39(3):185-190. PubMed doi:10.1007/s00127-004-0727-7
42. Horowitz JA, Cousins A. Postpartum depression treatment rates for at-risk women. Nurs Res. 2006;55(2 suppl):S23-S27. PubMed doi:10.1097/00006199-200603001-00005
43. Bloch M, Meiboom H, Lorberblatt M, et al. The effect of sertraline add-on to brief dynamic psychotherapy for the treatment of postpartum depression: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(2):235-241. PubMed doi:10.4088/JCP.11m07117
44. Chabrol H, Teissedre F, Saint-Jean M, et al. Prevention and treatment of post-partum depression: a controlled randomized study on women at risk. Psychol Med. 2002;32(6):1039-1047. PubMed doi:10.1017/S0033291702006062
45. Cooper PJ, Murray L, Wilson A, et al. Controlled trial of the short- and long-term effect of psychological treatment of post-partum depression, I: impact on maternal mood. Br J Psychiatry. 2003;182(5):412-419. PubMed doi:10.1192/bjp.182.5.412
46. Freeman MP, Davis MF. Supportive psychotherapy for perinatal depression: preliminary data for adherence and response. Depress Anxiety. 2010;27(1):39-45. PubMed doi:10.1002/da.20596
47. Klier CM, Muzik M, Rosenblum KL, et al. Interpersonal psychotherapy adapted for the group setting in the treatment of postpartum depression. J Psychother Pract Res. 2001;10(2):124-131. PubMed
48. Mulcahy R, Reay RE, Wilkinson RB, et al. A randomised control trial for the effectiveness of group Interpersonal Psychotherapy for postnatal depression. Arch Women Ment Health. 2010;13(2):125-139. PubMed doi:10.1007/s00737-009-0101-6
49. O’ Hara MW, Stuart S, Gorman LL, et al. Efficacy of interpersonal psychotherapy for postpartum depression. Arch Gen Psychiatry. 2000;57(11):1039-1045. PubMed doi:10.1001/archpsyc.57.11.1039
50. Reay R, Fisher Y, Robertson M, et al. Group interpersonal psychotherapy for postnatal depression: a pilot study. Arch Women Ment Health. 2006;9(1):31-39. PubMed doi:10.1007/s00737-005-0104-x
51. Stuart S, O’ Hara MW. Treatment of postpartum depression with interpersonal psychotherapy. Arch Gen Psychiatry. 1995;52(1):75-76. PubMed doi:10.1001/archpsyc.1995.03950130075009
52. Wickberg B, Hwang CP. Counselling of postnatal depression: a controlled study on a population based Swedish sample. J Affect Disord. 1996;39(3):209-216. PubMed doi:10.1016/0165-0327(96)00034-1
53. Cohen LS, Viguera AC, Bouffard SM, et al. Venlafaxine in the treatment of postpartum depression. J Clin Psychiatry. 2001;62(8):592-596. PubMed doi:10.4088/JCP.v62n0803
54. Nonacs RM, Soares CN, Viguera AC, et al. Bupropion SR for the treatment of postpartum depression: a pilot study. Int J Neuropsychopharmacol. 2005;8(3):445-449. PubMed doi:10.1017/S1461145705005079
55. Stowe Z, Casarella J, Landry J, et al. Sertraline in the treatment of women with postpartum major depression. Depression. 1995;3(1-2):49-55. doi:10.1002/depr.3050030109
56. Suri R, Burt VK, Altshuler LL, et al. Fluvoxamine for postpartum depression. Am J Psychiatry. 2001;158(10):1739-1740. PubMed doi:10.1176/appi.ajp.158.10.1739
57. Suri R, Burt VK, Altshuler LL. Nefazodone for the treatment of postpartum depression. Arch Women Ment Health. 2005;8(1):55-56. PubMed doi:10.1007/s00737-005-0071-2
58. Wisner KL, Hanusa BH, Perel JM, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. J Clin Psychopharmacol. 2006;26(4):353-360. PubMed doi:10.1097/01.jcp.0000227706.56870.dd
59. Yonkers KA, Lin H, Howell HB, et al. Pharmacologic treatment of postpartum women with new-onset major depressive disorder: a randomized controlled trial with paroxetine. J Clin Psychiatry. 2008;69(4):659-665. PubMed doi:10.4088/JCP.v69n0420
60. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150(6):782-786. PubMed doi:10.1192/bjp.150.6.782
61. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Patient Edition (SCID-I/P). New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002.
62. Evins GG, Theofrastous JP, Galvin SL. Postpartum depression: a comparison of screening and routine clinical evaluation. Am J Obstet Gynecol. 2000;182(5):1080-1082. PubMed doi:10.1067/mob.2000.105409
63. Leung SS, Leung C, Lam TH, et al. Outcome of a postnatal depression screening programme using the Edinburgh Postnatal Depression Scale: a randomized controlled trial. J Public Health (Oxf). 2011;33(2):292-301. PubMed doi:10.1093/pubmed/fdq075
64. Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385-401. doi:10.1177/014662167700100306
65. Chabrol H, Teissedre F, Saint-Jean M, et al. Detection, prevention and treatment of postpartum depression: a controlled study of 859 patients. Encephale. 2002;28(1):65-70. PubMed
66. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
67. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Third Edition. Washington, DC: American Psychiatric Association; 1980.
68. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
69. Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45(8):742-747. PubMed doi:10.1001/archpsyc.1988.01800320058007
70. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382-389. PubMed doi:10.1192/bjp.134.4.382
71. Guy W. Clinical Global Impressions. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Rockville, MD: National Institute of Mental Health; 1976.
72. US legislative action. Postpartum Support International Web site. http://www.postpartum.net/professionals/legislation/. 2012. Accessed June 18, 2014.
73. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Washington, DC: American Psychological Association; 1994.
74. Gilbody S, Sheldon T, House A. Screening and case-finding instruments for depression: a meta-analysis. CMAJ. 2008;178(8):997-1003. PubMed doi:10.1503/cmaj.070281
75. Paulden M, Palmer S, Hewitt C, et al. Screening for postnatal depression in primary care: cost effectiveness analysis. BMJ. 2009;339:b5203. PubMed doi:10.1136/bmj.b5203
76. Thombs BD, Arthurs E, Coronado-Montoya S, et al. Depression screening and patient outcomes in pregnancy or postpartum: a systematic review. J Psychosom Res. 2014;76(6):433-446. PubMed doi:10.1016/j.jpsychores.2014.01.006
77. Chaudron LH, Wisner KL. Perinatal depression screening: let’s not throw the baby out with the bath water! J Psychosom Res. 2014;76(6):489-491. PubMed doi:10.1016/j.jpsychores.2014.03.011
78. O’ Connor EA, Whitlock EP, Beil TL, et al. Screening for depression in adult patients in primary care settings: a systematic evidence review. Ann Intern Med. 2009;151(11):793-803. PubMed doi:10.7326/0003-4819-151-11-200912010-00007
79. Neumeyer-Gromen A, Lampert T, Stark K, et al. Disease management programs for depression: a systematic review and meta-analysis of randomized controlled trials. Med Care. 2004;42(12):1211-1221. PubMed doi:10.1097/00005650-200412000-00008
80. Badamgarav E, Weingarten SR, Henning JM, et al. Effectiveness of disease management programs in depression: a systematic review. Am J Psychiatry. 2003;160(12):2080-2090. PubMed doi:10.1176/appi.ajp.160.12.2080
81. Solberg LI, Trangle MA, Wineman AP. Follow-up and follow-through of depressed patients in primary care: the critical missing components of quality care. J Am Board Fam Pract. 2005;18(6):520-527. PubMed doi:10.3122/jabfm.18.6.520
82. Gensichen J, Beyer M, Muth C, et al. Case management to improve major depression in primary health care: a systematic review. Psychol Med. 2006;36(1):7-14. PubMed doi:10.1017/S0033291705005568
83. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA. 2006;295(5):499-507. PubMed doi:10.1001/jama.295.5.499
84. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114(3):703-713. PubMed doi:10.1097/AOG.0b013e3181ba0632
85. Wisner KL, Zarin DA, Holmboe ES, et al. Risk-benefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157(12):1933-1940. PubMed doi:10.1176/appi.ajp.157.12.1933
86. Kim DR, O’ Reardon JP, Epperson CN. Guidelines for the management of depression during pregnancy. Curr Psychiatry Rep. 2010;12(4):279-281. PubMed doi:10.1007/s11920-010-0114-x
87. Claridge AM. Efficacy of systemically oriented psychotherapies in the treatment of perinatal depression: a meta-analysis. Arch Women Ment Health. 2014;17(1):3-15. PubMed doi:10.1007/s00737-013-0391-6
88. Hale N, Picklesimer AH, Billings DL, et al. The impact of Centering Pregnancy Group Prenatal Care on postpartum family planning. Am J Obstet Gynecol. 2014;210(1):e1-e7. PubMed doi:10.1016/j.ajog.2013.09.001
89. Xaverius PK, Grady MA. Centering pregnancy in Missouri: a system level analysis. ScientificWorldJournal. 2014;2014:285386. PubMed doi:10.1155/2014/285386
90. Picklesimer AH, Billings D, Hale N, et al. The effect of CenteringPregnancy group prenatal care on preterm birth in a low-income population. Am J Obstet Gynecol. 2012;206(5):e1-e7. PubMed doi:10.1016/j.ajog.2012.01.040
91. Melville JL, Reed SD, Russo J, et al. Improving care for depression in obstetrics and gynecology: a randomized controlled trial. Obstet Gynecol. 2014;123(6):1237-1246. PubMed doi:10.1097/AOG.0000000000000231
92. Katon W, Russo J, Reed SD, et al. A randomized trial of collaborative depression care in obstetrics and gynecology clinics: socioeconomic disadvantage and treatment response. Am J Psychiatry. 2015;172(1):32-40. PubMed doi:10.1176/appi.ajp.2014.14020258
93. Dennis CL, Bloomberg LS. Psychosocial interventions for the treatment of perinatal depression. Best Pract Res Clin Obstet Gynaecol. 2014;28(1):97-111. PubMed doi:10.1016/j.bpobgyn.2013.08.008
94. Yawn BP, Dietrich AJ, Wollan P, et al; TRIPPD practices. TRIPPD: a practice-based network effectiveness study of postpartum depression screening and management. Ann Fam Med. 2012;10(4):320-329. PubMed doi:10.1370/afm.1418
95. Gjerdingen D, Crow S, McGovern P, et al. Stepped care treatment of postpartum depression: impact on treatment, health, and work outcomes. J Am Board Fam Med. 2009;22(5):473-482. PubMed doi:10.3122/jabfm.2009.05.080192
96. Dennis CL. The process of developing and implementing a telephone-based peer support program for postpartum depression: evidence from two randomized controlled trials. Trials. 2014;15(131):131. PubMed doi:10.1186/1745-6215-15-131
97. Rogers E. Diffusion of Innovations. New York, NY; The Free Press; 1995.
98. Kozhimannil KB, Adams AS, Soumerai SB, et al. New Jersey’s efforts to improve postpartum depression care did not change treatment patterns for women on Medicaid. Health Aff (Millwood). 2011;30(2):293-301. PubMed doi:10.1377/hlthaff.2009.1075
99. Yawn BP, Olson AL, Bertram S, et al. Postpartum depression: screening, diagnosis and management programs 2000 through 2010. Depress Res Treat. 2012;2012:363964. PubMed doi:10.1155/2012/363964
100. Byatt N, Levin LL, Ziedonis D, et al. Enhancing participation in depression care in outpatient perinatal care settings: a systematic review. Obstet Gynecol. 2015;126(5):1048-1058. PubMed doi:10.1097/AOG.0000000000001067
101. US Preventive Services Task Force. Screening for depression in adults: US preventive services task force recommendation statement. Ann Intern Med. 2009;151(11):784-792. PubMed doi:10.7326/0003-4819-151-11-200912010-00006
This PDF is free for all visitors!