This work may not be copied, distributed, displayed, published, reproduced, transmitted, modified, posted, sold, licensed, or used for commercial purposes. By downloading this file, you are agreeing to the publisher’s Terms & Conditions.
Objective: Depression is often not optimally treated during pregnancy, partially because of conflicting data regarding antidepressant medication risk. This meta-analysis was conducted to determine whether antenatal antidepressant exposure is associated with congenital malformations and to assess the effect of known methodological limitations.
Data Sources: EMBASE, CINAHL, PsycINFO, and MEDLINE were searched from their start dates to June 2010. Keywords of various combinations were used, including, but not limited to depressive/mood disorder, pregnancy, antidepressant drug/agent, congenital malformation, and cardiac malformation.
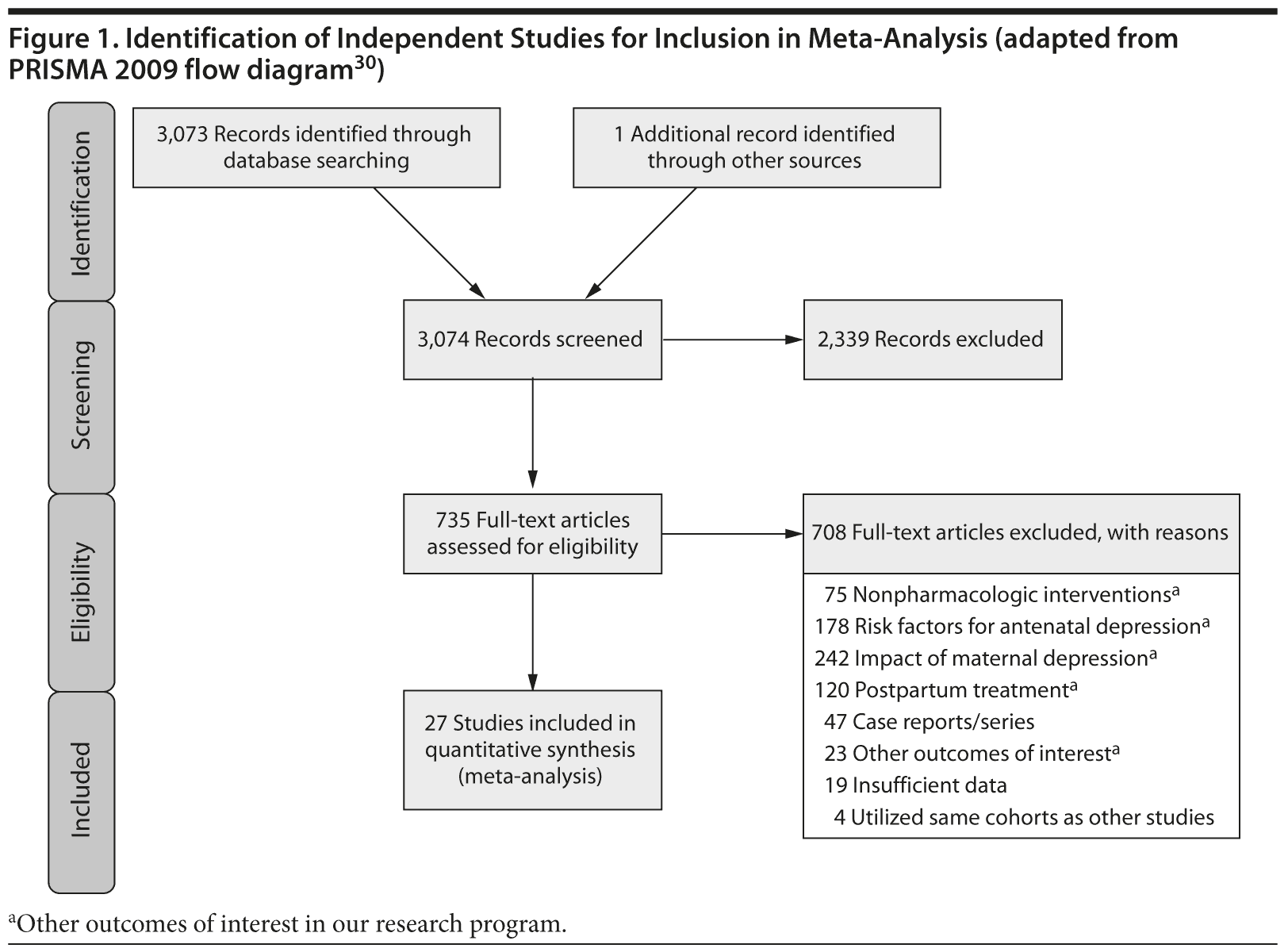
Study Selection: English language studies reporting congenital malformations associated with antidepressants were included. Of 3,074 abstracts reviewed, 735 studies were retrieved and 27 studies were included.
Data Extraction: Two reviewers working independently assessed article quality. Data on use of any antidepressant, including fluoxetine and paroxetine specifically, were extracted. Outcomes included congenital malformations, major congenital malformations, cardiovascular defects, septal heart defects (ventral septal defects and atrial septal defects), and ventral septal defects only.
Results: Nineteen studies were above quality threshold and make up the primary meta-analyses. Pooled relative risks (RRs) were derived by using random-effects methods. Antidepressant exposure was not associated with congenital malformations (RR = 0.93; 95% CI, 0.85-1.02; P = .113) or major malformations (RR = 1.07; 95% CI, 0.99-1.17; P = .095). However, increased risk for cardiovascular malformations (RR = 1.36; 95% CI, 1.08-1.71; P = .008) and septal heart defects (RR = 1.40; 95% CI, 1.10-1.77; P = .005) were found; the RR for ventral septal defects was similar to septal defects, although not significant (RR = 1.54; 95% CI, 0.71-3.33; P = .274). Pooled effects were significant for paroxetine and cardiovascular malformations (RR = 1.43; 95% CI, 1.08-1.88; P = .012). These results are contrasted with those addressing methodological limitations but are typically consistent.
Conclusions: Overall, antidepressants do not appear to be associated with an increased risk of congenital malformations, but statistical significance was found for cardiovascular malformations. Results were robust in several sensitivity analyses. Given that the RRs are marginal, they may be the result of uncontrolled confounders. Although the RRs were statistically significant, none reached clinically significant levels.
J Clin Psychiatry 2013;74(4):e293-e308
© Copyright 2013 Physicians Postgraduate Press, Inc.
Submitted: June 21, 2012; accepted November 16, 2012 (doi:10.4088/JCP.12r07966).
Corresponding author: Sophie Grigoriadis, MD, PhD, FRCPC, Women’s Mood and Anxiety Clinic: Reproductive Transitions, Department of Psychiatry, FG 29, Sunnybrook Health Sciences Centre, 2075 Bayview Ave, Toronto, ON M4N 3M5, Canada ([email protected]).

ABSTRACT
Objective: Depression is often not optimally treated during pregnancy, partially because of conflicting data regarding antidepressant medication risk. This meta-analysis was conducted to determine whether antenatal antidepressant exposure is associated with congenital malformations and to assess the effect of known methodological limitations.
Data Sources: EMBASE, CINAHL, PsycINFO, and MEDLINE were searched from their start dates to June 2010. Keywords of various combinations were used, including, but not limited to depressive/mood disorder, pregnancy, antidepressant drug/agent, congenital malformation, and cardiac malformation.
Study Selection: English language studies reporting congenital malformations associated with antidepressants were included. Of 3,074 abstracts reviewed, 735 studies were retrieved and 27 studies were included.
Data Extraction: Two reviewers working independently assessed article quality. Data on use of any antidepressant, including fluoxetine and paroxetine specifically, were extracted. Outcomes included congenital malformations, major congenital malformations, cardiovascular defects, septal heart defects (ventral septal defects and atrial septal defects), and ventral septal defects only.
Results: Nineteen studies were above quality threshold and make up the primary meta-analyses. Pooled relative risks (RRs) were derived by using random-effects methods. Antidepressant exposure was not associated with congenital malformations (RR = 0.93; 95% CI, 0.85-1.02; P = .113) or major malformations (RR = 1.07; 95% CI, 0.99-1.17; P = .095). However, increased risk for cardiovascular malformations (RR = 1.36; 95% CI, 1.08-1.71; P = .008) and septal heart defects (RR = 1.40; 95% CI, 1.10-1.77; P = .005) were found; the RR for ventral septal defects was similar to septal defects, although not significant (RR = 1.54; 95% CI, 0.71-3.33; P = .274). Pooled effects were significant for paroxetine and cardiovascular malformations (RR = 1.43; 95% CI, 1.08-1.88; P = .012). These results are contrasted with those addressing methodological limitations but are typically consistent.
Conclusions: Overall, antidepressants do not appear to be associated with an increased risk of congenital malformations, but statistical significance was found for cardiovascular malformations. Results were robust in several sensitivity analyses. Given that the RRs are marginal, they may be the result of uncontrolled confounders. Although the RRs were statistically significant, none reached clinically significant levels.
J Clin Psychiatry 2013;74(4):e293-e308
© Copyright 2013 Physicians Postgraduate Press, Inc.
Submitted: June 21, 2012; accepted November 16, 2012 (doi:10.4088/JCP.12r07966).
Corresponding author: Sophie Grigoriadis, MD, PhD, FRCPC, Women’s Mood and Anxiety Clinic: Reproductive Transitions, Department of Psychiatry, FG 29, Sunnybrook Health Sciences Centre, 2075 Bayview Ave, Toronto, ON M4N 3M5, Canada ([email protected]).
The consequences of a major depressive episode during pregnancy include effects on the mother, her infant, and family.1-3 Although treatment is essential, the use of antidepressant medication during pregnancy has been found to be lower than other times of the life cycle.4 One of the contributing factors for poor uptake of pharmacologic interventions appears to be concerns regarding the safety of antidepressant exposure for the fetus.5 For example, preliminary reports of neonatal cardiovascular malformations following paroxetine exposure in early pregnancy6 led to both the US Food and Drug Administration6a and Health Canada6b issuing advisories in 2005 warning about potential risks associated with the use of antidepressants during pregnancy. However, the warnings suggested a direct conflict between safety of the fetus and a mother’s need for antidepressant medication, perhaps without regard to the documented negative effects of untreated depression on pregnancy outcomes.3,7
The results of prior meta-analyses have been conflicting. Several have found no evidence of increased risk of major congenital malformations above the baseline8-10 rate, which has been widely cited as 1%-3% for any pregnancy in North America11-14 and less than 4% for minor congenital malformations.15 The most recent meta-analyses did report an increased risk for congenital malformations16 and cardiac malformations13,16 with paroxetine exposure specifically. Unfortunately, many individual studies have serious methodological limitations17 that were not taken into account in the previous meta-analyses. For example, raw data unadjusted for confounders were used or studies were based on convenience samples. Furthermore, it is important to note the distinction between statistical significance and clinical significance when interpreting scientific evidence for clinical practice implications. The aim of this study was to synthesize the available data on congenital malformations, including cardiac, in infants of mothers who took antidepressant medications during pregnancy. In order to address the methodological limitations of the available research, we excluded studies below a quality threshold, used adjusted data where possible, examined for the effect of exposure contamination with any antidepressant use in the control group, and examined the influence of whether or not the samples were population based or obtained by convenience.
DATA SOURCES AND STUDY SELECTION
Following the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines,18 2 professional librarians with expertise in the areas of psychopharmacology and psychiatry independently conducted literature searches. A variety of keyword combinations were utilized, such as depressive/mood disorder; pregnancy/pregnancy trimesters; tricyclic antidepressant drugs; antidepressant drug/agent; selective serotonin reuptake inhibitors, SSRIs; monoamine oxidase inhibitors; prenatal or antenatal, infant/outcomes; congenital malformation, cardiac malformation (see supplementary material for full list of keywords). Strategies were based on the subject headings specific to the individual databases searched, combined with appropriate keywords and keyword phrases, and truncated if necessary. Concepts were combined with Boolean operators (AND, OR) to either broaden “like” concepts (OR) or narrow them (AND) to ensure more than 1 concept was included in the results. The explode feature (available for all of the databases searched, with the exception of MEDLINE In-Process and Scopus) was also used to broaden like concepts. The databases used included MEDLINE (Ovid), MEDLINE In-Process (Ovid) to access current literature (keyword searching only), PsycINFO (American Psychological Association; Ovid), CINAHL (Nursing and Allied Health), EMBASE (Excerpta Medica, Elsevier; Ovid), and Scopus (Elsevier) to access current literature (keyword searching only). The databases were searched from their start date to June 30, 2010. Reference lists of reviews and meta-analyses were also searched for further articles, but none were found.
Inclusion and Exclusion Criteria
Cohort and case-control studies published in English were eligible for inclusion if they (1) reported original data and at least 1 malformation of interest, (2) reported on any pharmacologic antidepressant agent exposure (ie, selective serotonin reuptake inhibitors, tricyclic antidepressants, and monoamine oxidase inhibitors), (3) had a nonexposed comparison group of pregnant women for the antidepressant examined, and (4) provided sufficient data to calculate an effect size. Abstracts and conference proceedings were excluded, and unpublished data were not searched because the volume of potentially eligible studies would have made doing so infeasible. Outcomes of interest were identified by the research team as well as the advisory committee of key stakeholders for this program of research, which included representatives from psychiatry, family medicine, obstetrics, neonatology, public health, patient advocacy, and policy. The following outcomes were included in this meta-analysis: any congenital malformation, major congenital malformation (structural defects present at birth that have surgical, medical, or cosmetic significance or a significant effect on function or social acceptability15,19), cardiovascular malformation, septal cardiac defect (atrial septal defects or ventral septal defects), and ventral septal defects only as defined by the authors of the original publication. When more than 1 study had been published on the same cohort, we selected the study with the largest number of cases of malformations within each malformation outcome.

- Antidepressants do not appear to be associated with an increased risk of congenital malformations overall.
- Antidepressants may be associated with cardiovascular malformations.
- Clinical significance does not always follow from statistical significance, and clinicians must consider the risks of depressive illness prior to making any treatment decisions.
DATA EXTRACTION
The study was part of a larger research program developing a reference guide for physicians to assist in treatment discussions regarding antidepressant use during pregnancy with their patients. The data extraction and quality assessment processes for this program of research have been previously published.7 Two independent research assistants completed the screening for all articles by their title and abstract, and eligible articles were retrieved. Data extraction forms were completed for all eligible studies and were based on the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)20 checklist. Data extracted included source, study design, participants (sample, control, demographics, and clinical characteristics), inclusion/exclusion criteria, antidepressants examined, dosage, duration of exposure, primary and secondary outcomes, outcome assessment methods, and loss to follow-up. Authors of original publications were sent requests for raw data if not provided in the article. Eight authors were contacted and 3 replied. Of these, 1 was unable to share data because of confidentiality issues, 1 was not able to meet our timeline, and the last 1 did not respond to our request for further clarification.
Quality Assessment
The Systematic Assessment of Quality in Observational Research (SAQOR)7 tool used for this investigation was developed by our team and based on previously published quality assessment tools (Downs and Black21 and the Newcastle-Ottawa Scale22) and adapted to assess study quality in this area of research. Nineteen aspects of each study under the following categories were evaluated by outcome: (1) sample, (2) control group, (3) quality of exposure/outcome measure, (4) follow-up, and (5) distorting influences. Specifically, the distorting influences category included whether analyses controlled for confounders such as depression, other psychotropic medications, smoking, alcohol, or illicit drug use. On the basis of the aforementioned criteria, we assigned a final quality rating of high, moderate, low, or very low using a modification of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system.23 Studies were then categorized as “above quality threshold” (high, moderate, or low quality) or “below quality threshold” (very low quality). Results of the data extraction and quality assessment were compared between raters for each study, with any differences resolved through consensus by the principal investigators.
Statistical Analyses
Adjusted risk estimates were preferred when these were available, and odds ratios (ORs) and relative risks (RRs) were considered as equivalent measures of risk. We calculated ORs from the raw number of cases and controls when no such risk estimate was reported. For each outcome, we calculated 1 estimate of the OR for malformations for each article and pooled these risks across studies using random-effects models.24 We added 0.5 to cells with 0 cases when calculating the OR.25 All analyses were conducted on the log scale. Visual inspection of funnel plots depicting the risk estimates (on the log scale) against their standard error and Egger regression-based test26 were used to assess publication bias. Cochrane Q and I2 were used to quantify between-study heterogeneity.27,28 I2 may be interpreted as the proportion of the total variance due to between-study heterogeneity. For studies reporting multiple exposure or control groups, these groups were combined in order to calculate a single risk estimate for each study where possible, if they were independent. For each outcome, our main analysis consisted of the pooled risk for the studies determined to be above quality threshold; further, we excluded studies that included some antidepressants in their control group (for example, when only selective serotonin reuptake inhibitor [SSRI] use was used as the exposure group but other antidepressants were not assessed and controls could have been, and in some cases were reported to have been, exposed to any antidepressants in their pregnancy) and also reran the analysis with articles that matched or adjusted their data in any way. Because of the generally low study quality, we also estimated the pooled risk excluding all convenience samples. We selected 2 specific antidepressants (fluoxetine and paroxetine) for a separate analysis of their effect on malformations where sufficient data were available, as more information was available for them compared to others. All analyses were conducted with Stata statistical software, version 10.1 (StataCorp LP)29 and similar to our other work in Ross et al.30
RESULTS
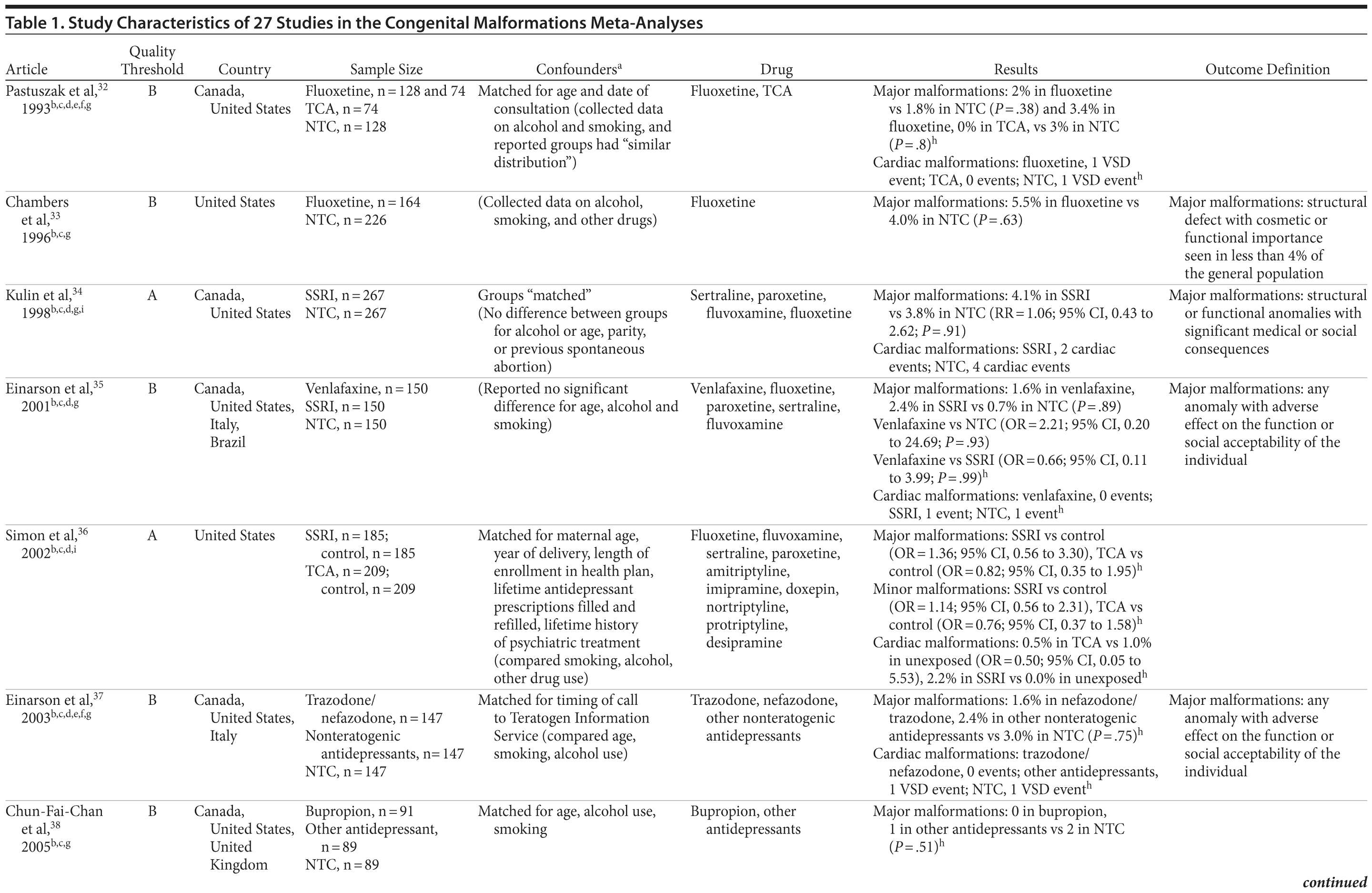
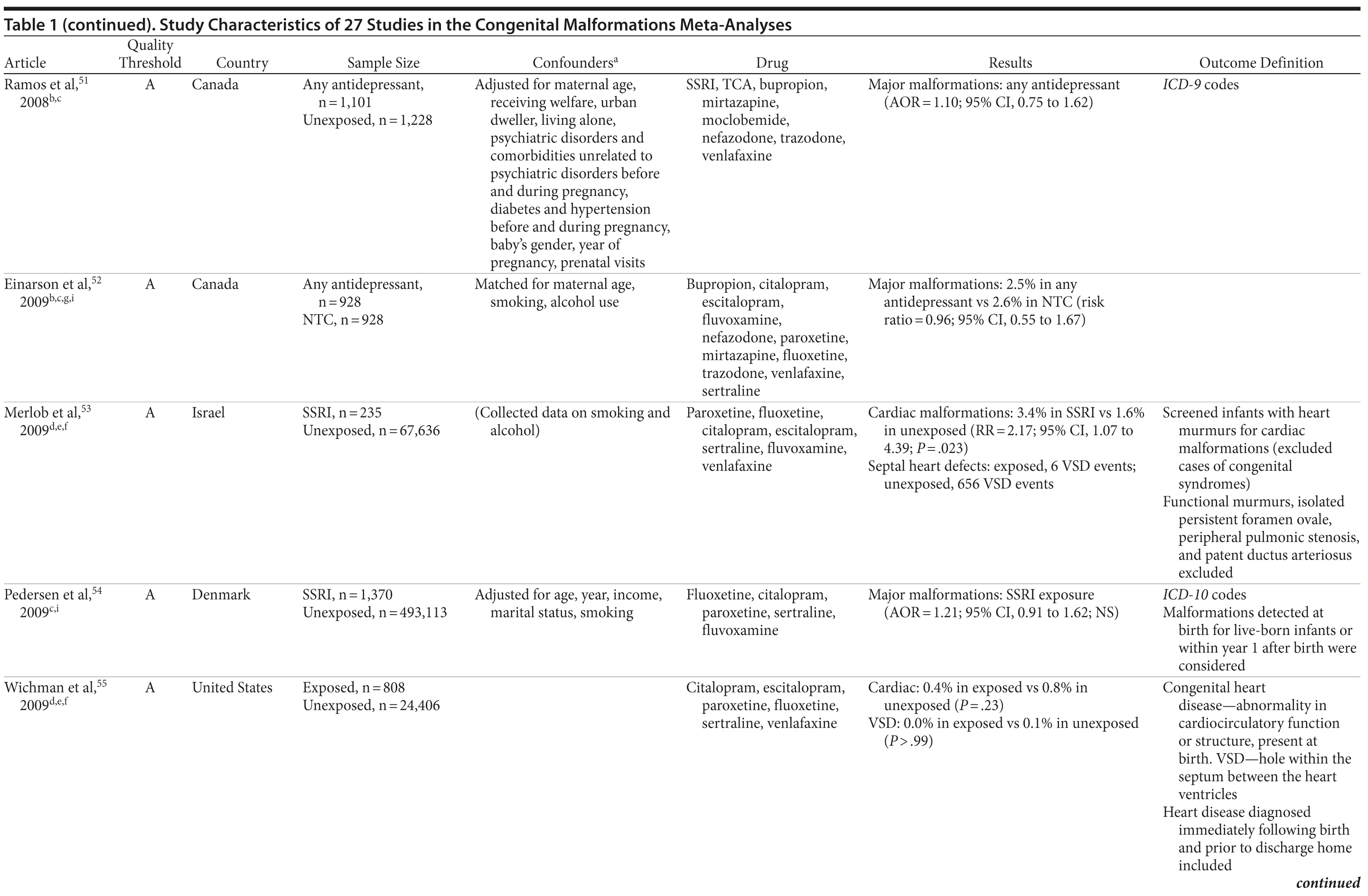
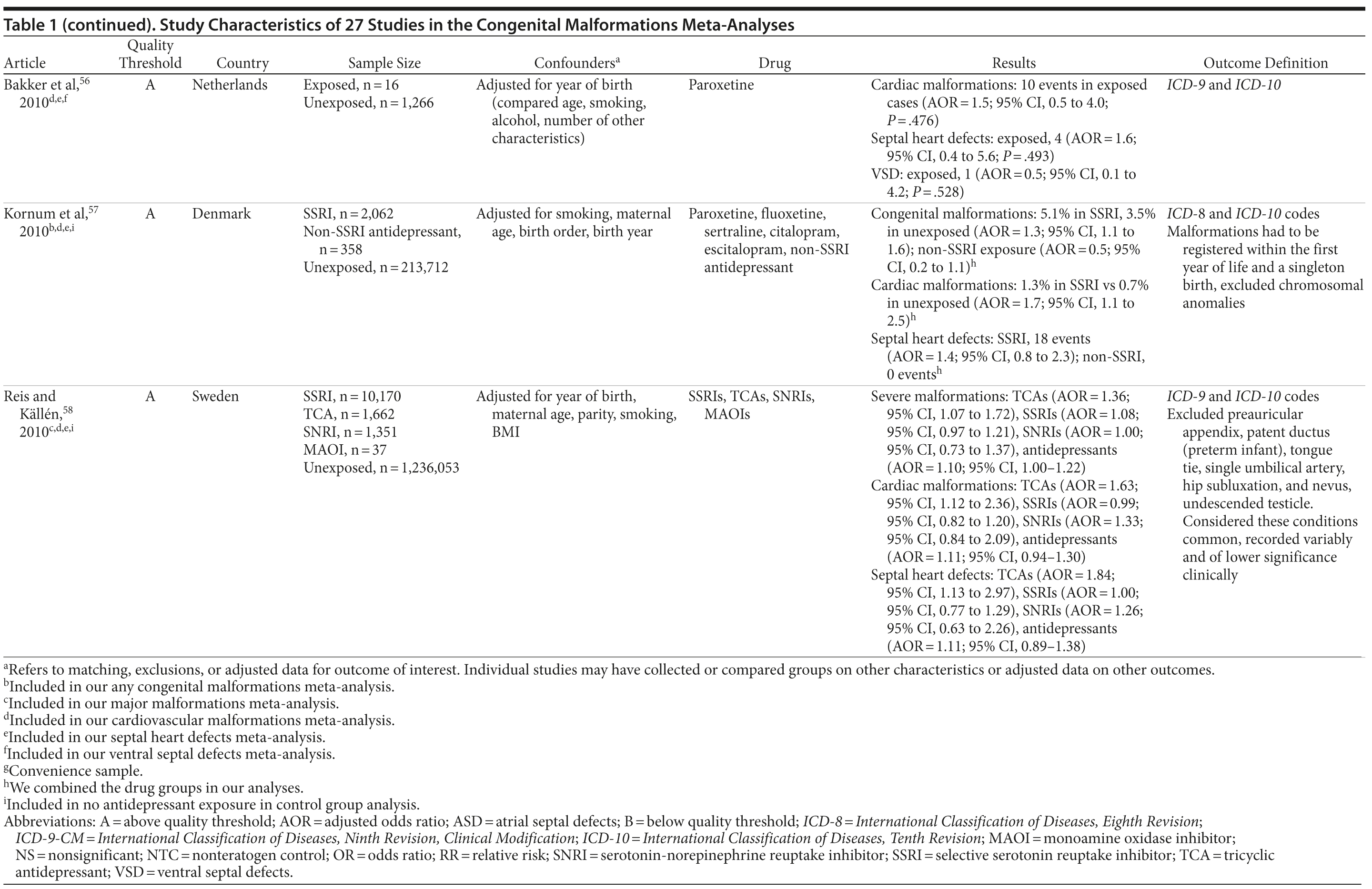
Of the 3,074 abstracts reviewed, 2,339 were excluded based on title and abstract. In total, 735 articles were retrieved and assessed for eligibility and 31 articles met the inclusion criteria (Figure 1).31 Of these, 4 articles were excluded because they were duplicate analyses of studies already included in our quantitative analysis, leaving 27 studies32-58 for a quantitative analysis (Table 1). Nineteen of the 27 studies were above the quality threshold, whereas 8 were below. Most studies reported data on more than 1 outcome (14 above quality threshold reported on any congenital malformation, 11 on any major malformation, 13 on any cardiovascular malformation, 9 on any septal cardiac defect [atrial septal defects or ventral septal defects], and 5 on ventral septal defects specifically). Eleven studies used a convenience sample, while 16 studies used either a population- or hospital-based sample. Of all studies available, 13 clearly stated that no antidepressants were used in the control group and 21 reported adjusted data or applied matching. Information on fluoxetine was available from 0 to 7 studies depending on the outcome, and 0-8 studies were available for paroxetine.
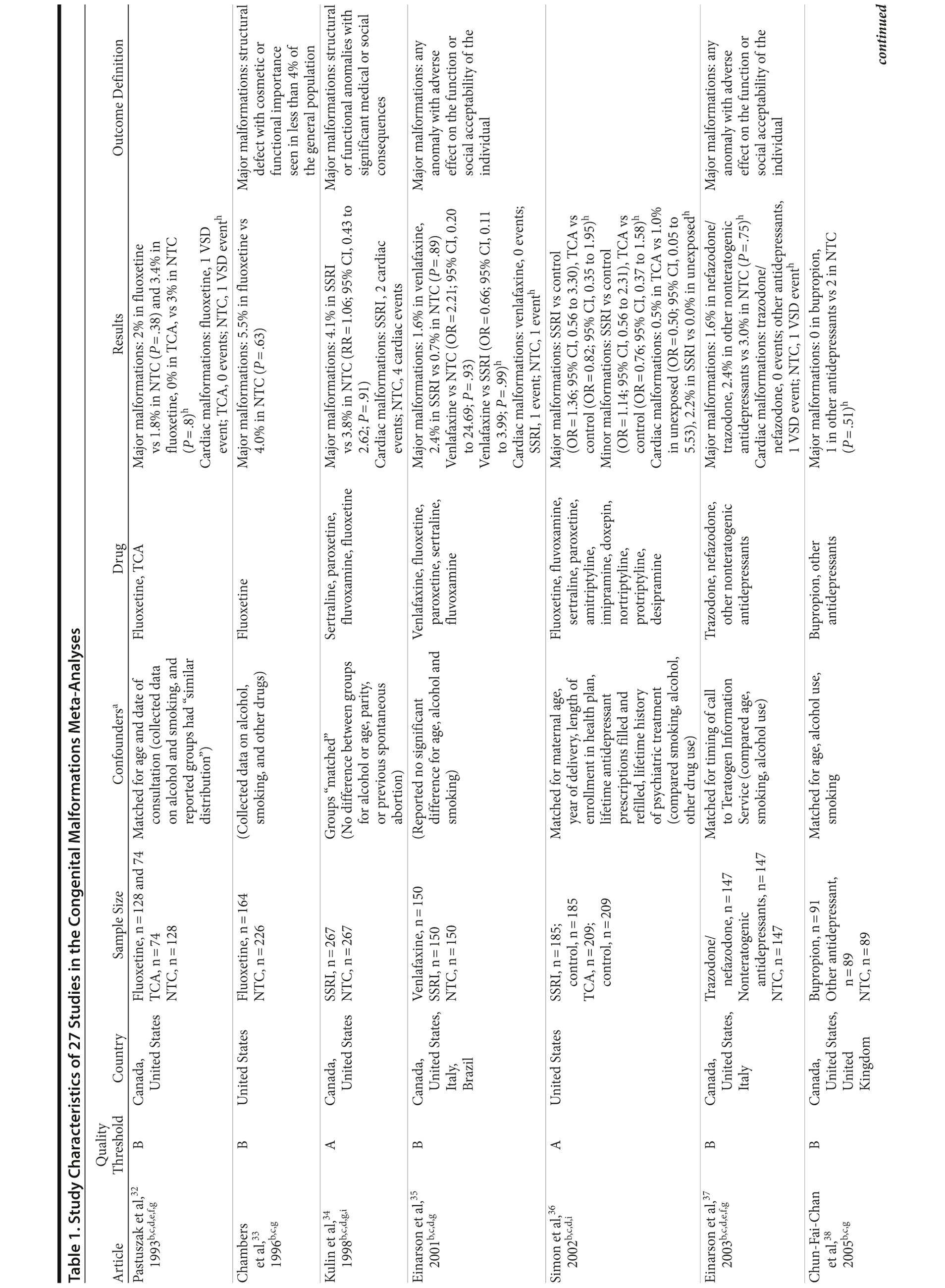
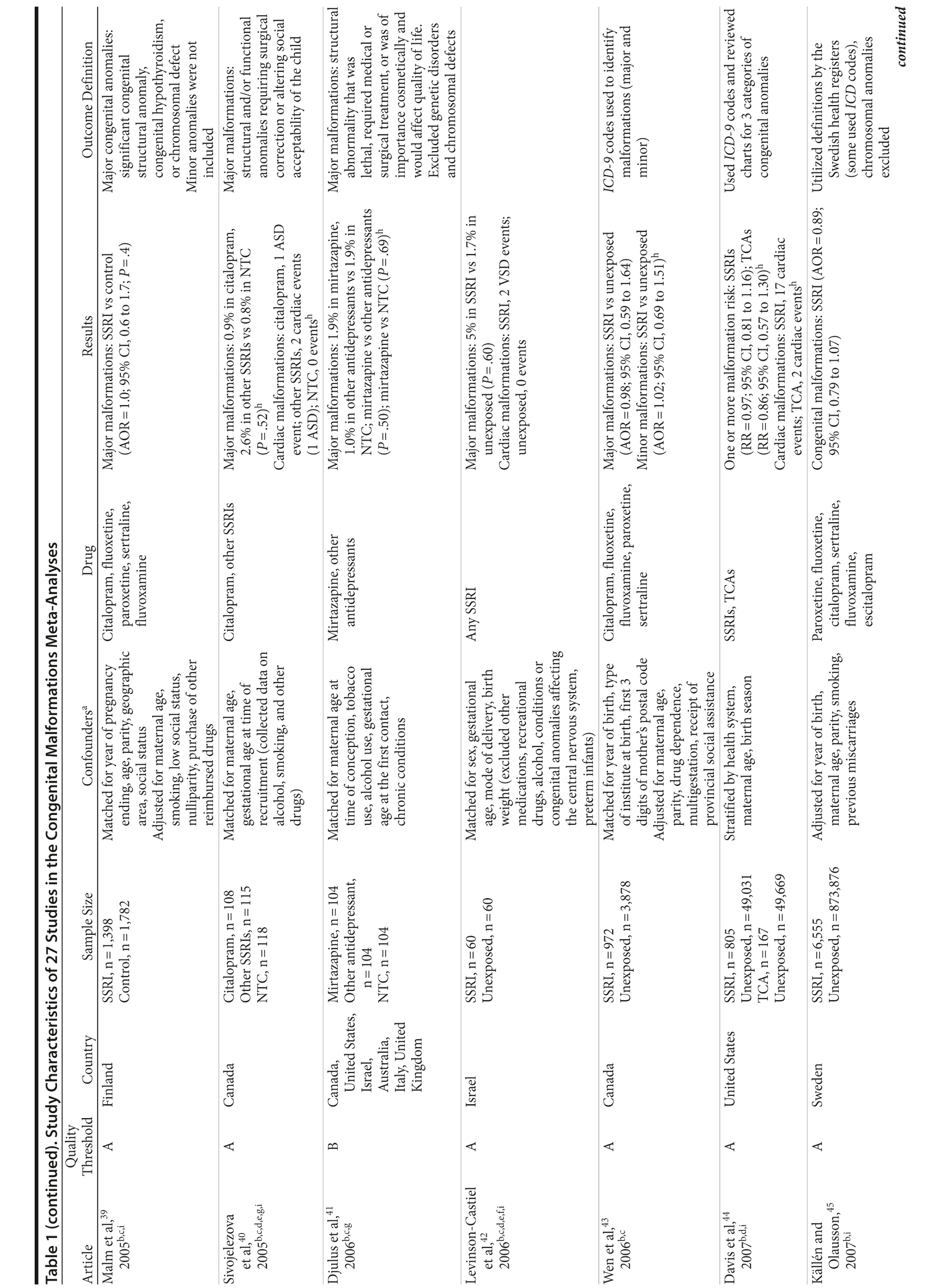
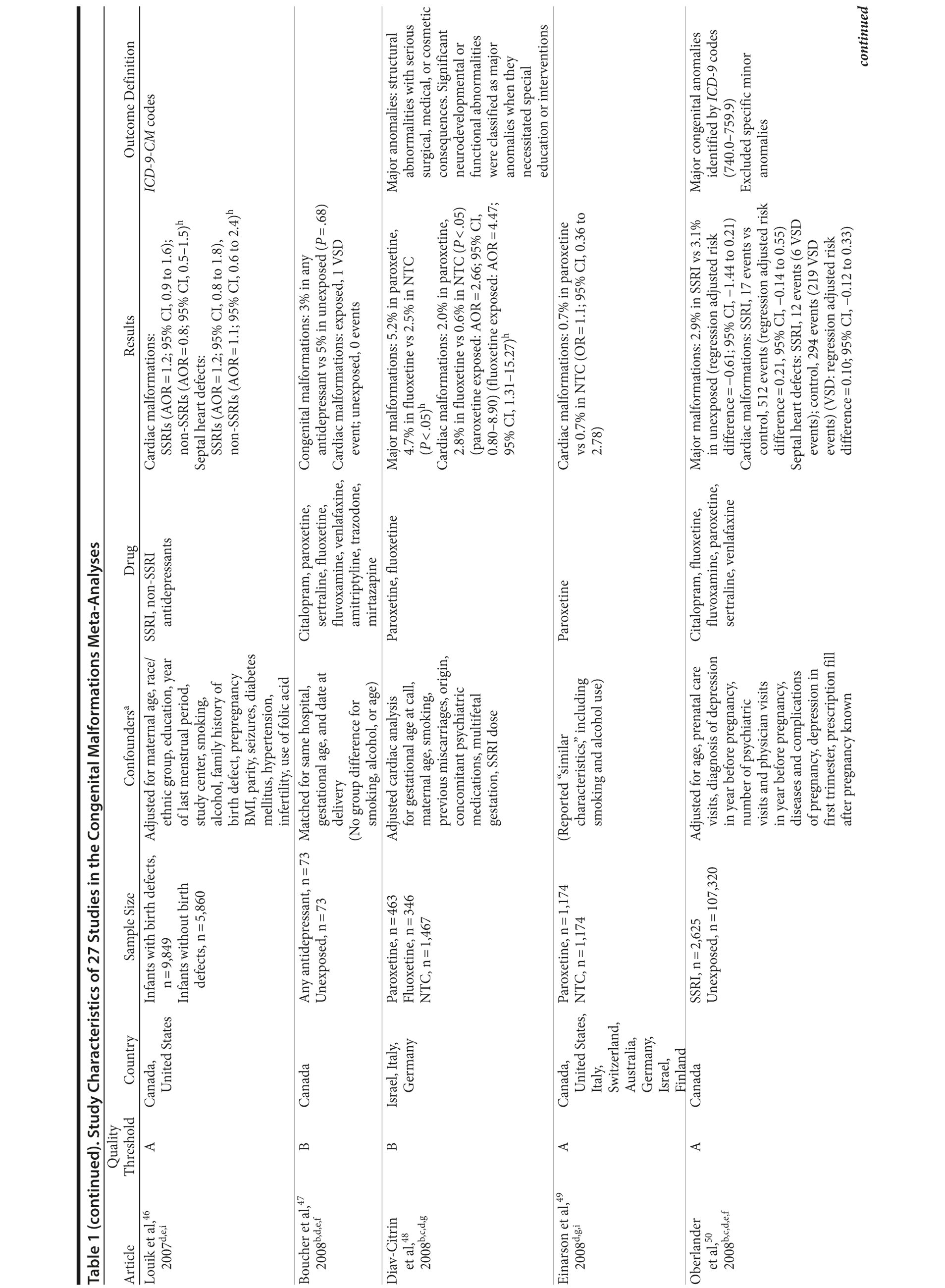
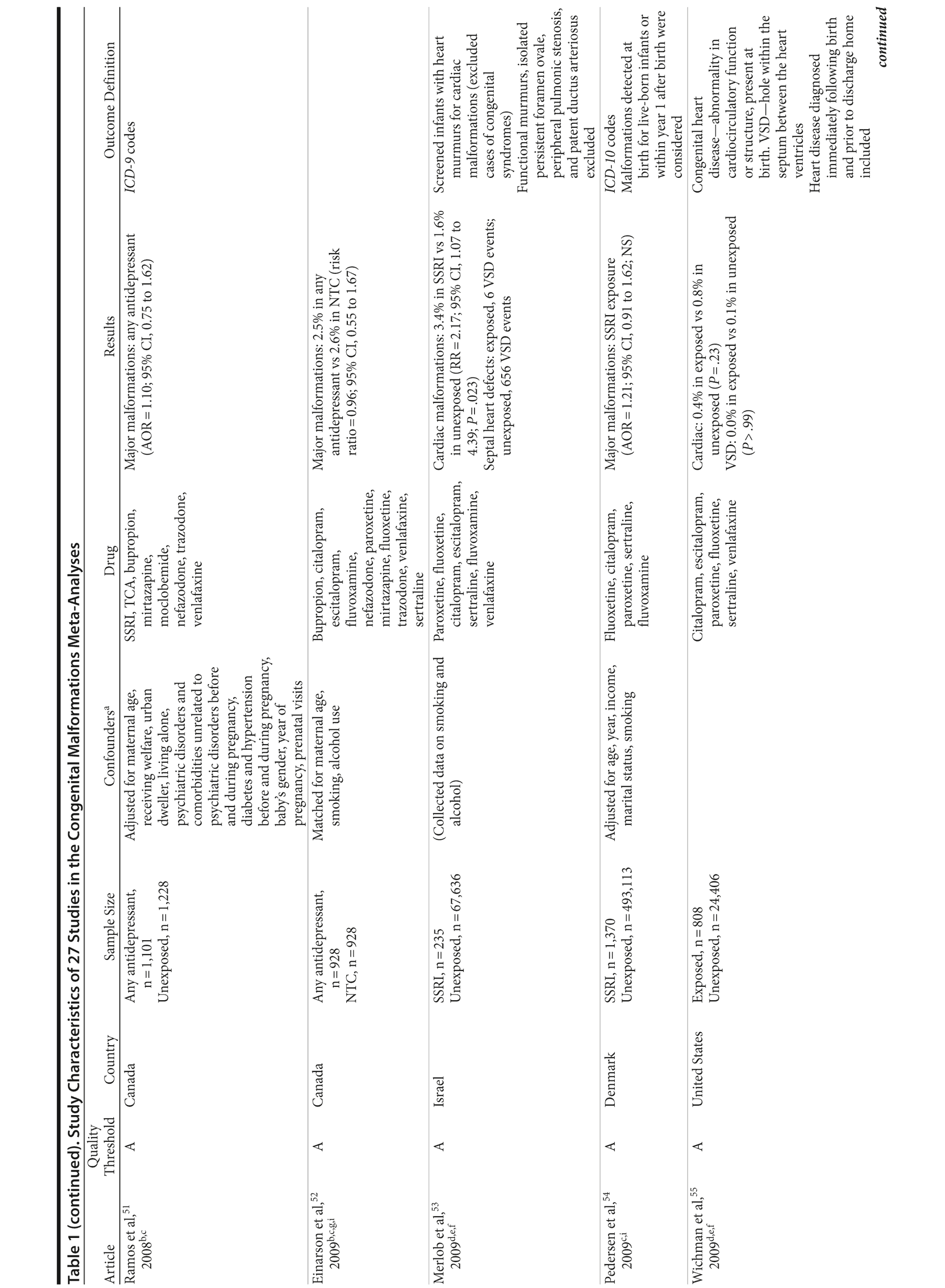
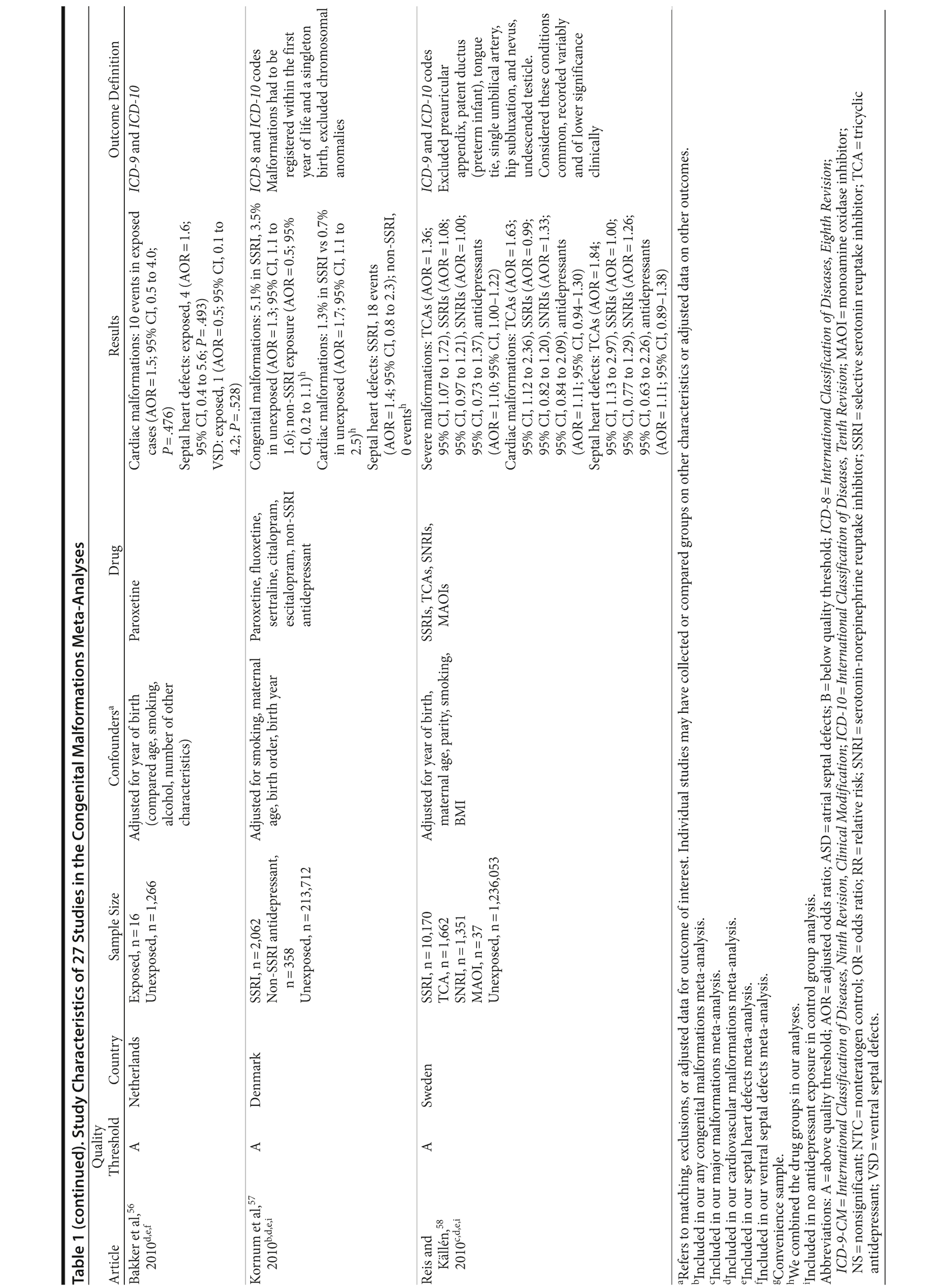
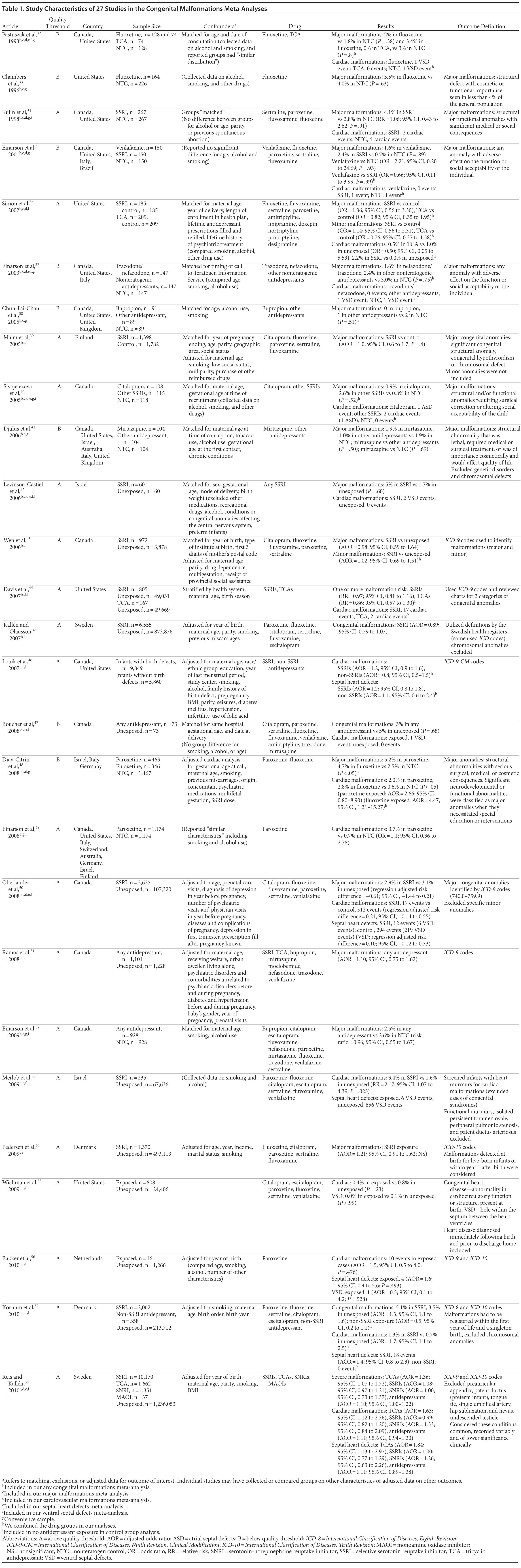
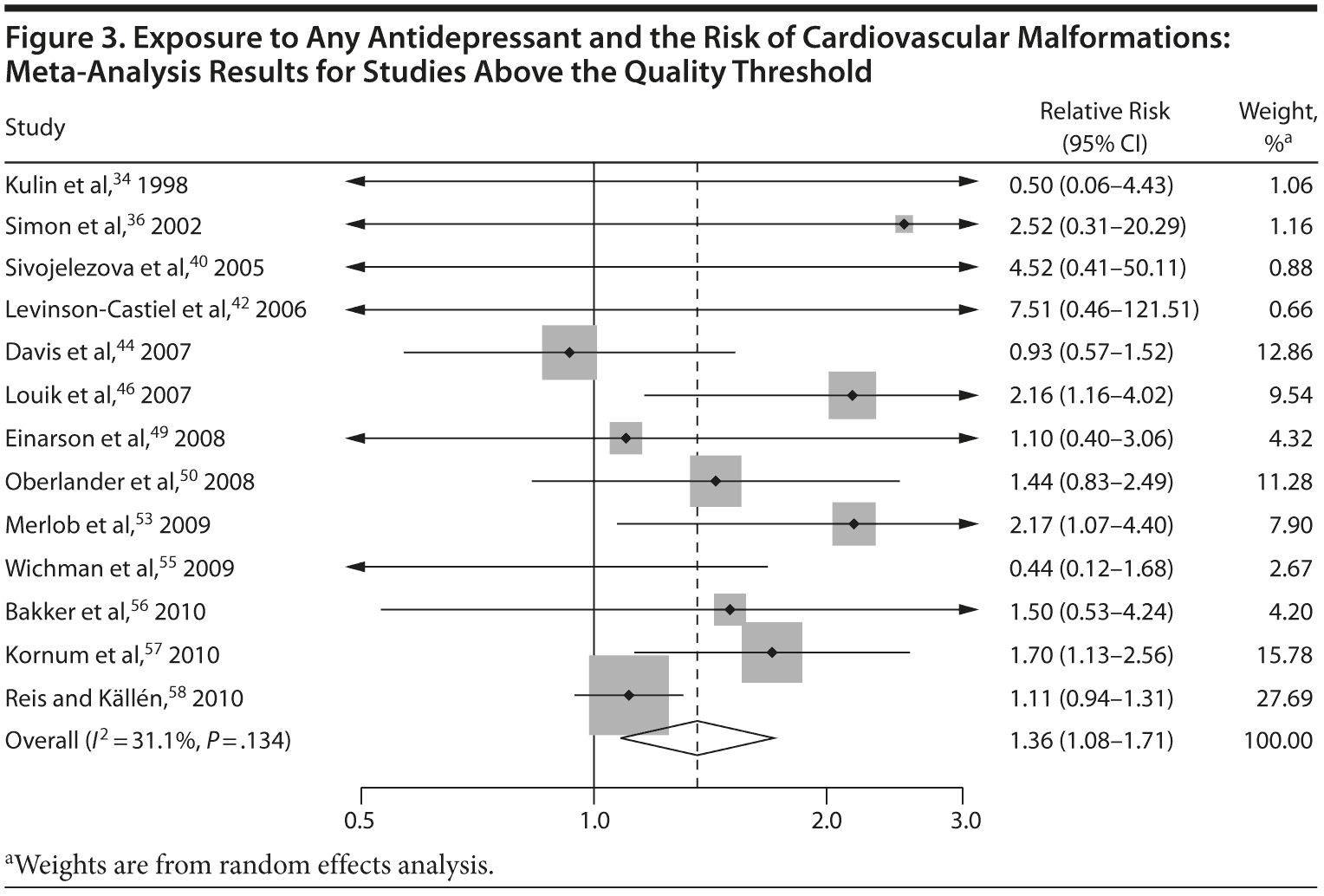
Congenital Malformations
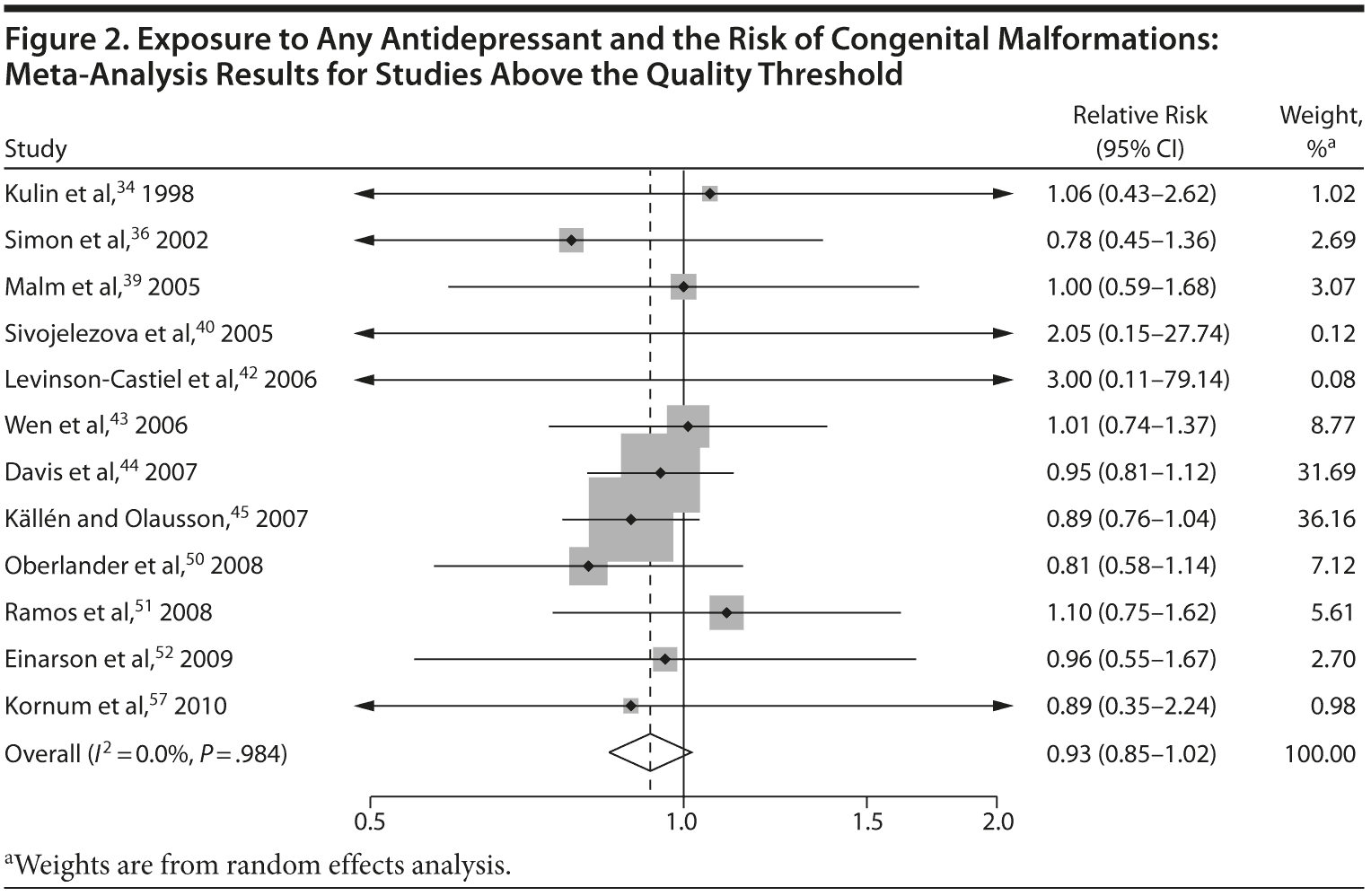
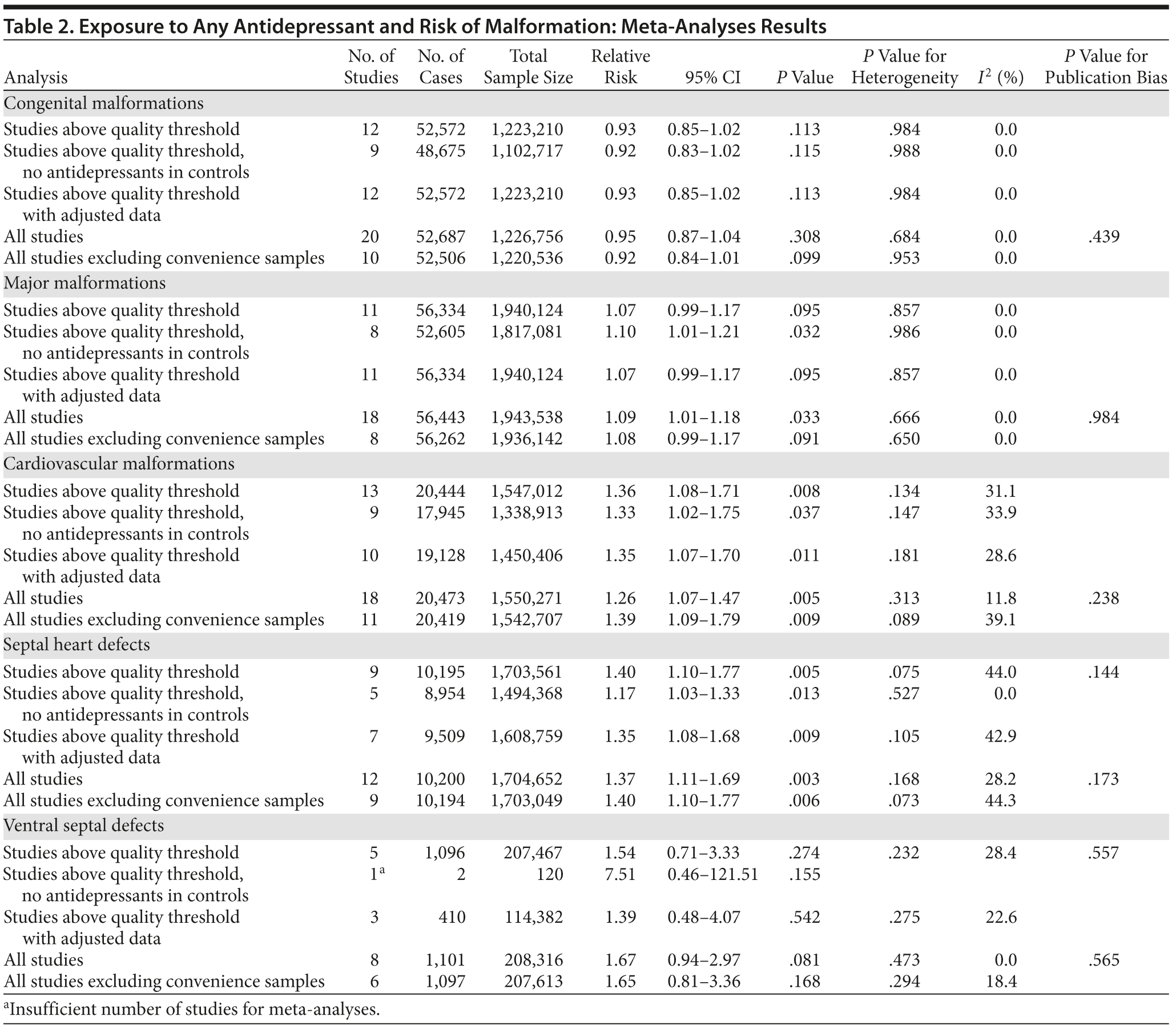
Overall, we pooled results from 12 studies[1] that were above quality threshold; the RR for congenital malformations was 0.93 (95% CI, 0.85-1.02; P = .113; Figure 2). Results were similar when studies were restricted to those studies that clearly excluded antidepressants from the control group. When studies with any adjustment were analyzed, all the studies regardless of quality, or 10 studies that did not use convenience samples (regardless of study quality), yielded similar results, with RRs ranging from 0.92 to 0.95 (Table 2). The RRs from the meta-analyses with the individual antidepressants (fluoxetine and paroxetine) were similar in magnitude (ranging from 1.02 to 1.15) and not statistically significant (Supplementary eTable 1).
Major Congenital Malformations
When only major malformations were analyzed, the pooled risk was small and not significant for the studies that were above quality threshold (RR = 1.07; 95% CI, 0.99-1.17; P = .095; Supplementary eFigure 1). Overall, the RRs for the other analyses were small, between 1.07 and 1.10 (Table 2), but statistically significant depending on the subanalysis conducted. Results were not significant for any of the analyses for paroxetine use; however, the risk associated with the use of fluoxetine was similar to the overall analyses, with slightly higher RRs between 1.20 and 1.29 (Supplementary eTable 1).
Cardiovascular Malformations
With regard to cardiovascular malformations (13 studies above quality threshold) as the disease outcome, the pooled RR was small (RR = 1.36; 95% CI, 1.08-1.71; P = .008; Figure 3) but statistically significant in all analyses, ranging from 1.26 to 1.39 (see Table 2). One study58 in particular had a strong influence on the results because of its sample size, detailed exposure measurement, and moderate study quality and was included in most subanalyses. Few studies were available for analyses stratified by medication. The risk associated with paroxetine was slightly higher (RR = 1.43; 95% CI, 1.08-1.88; P = .012; RRs between 1.43-1.47) and consistently statistically significant. The risk associated with fluoxetine was slightly lower and not statistically significant (RRs from 1.17 to 1.33; Supplementary eTable 1).
Septal Heart Defects (atrial septal defects or ventral septal defects)
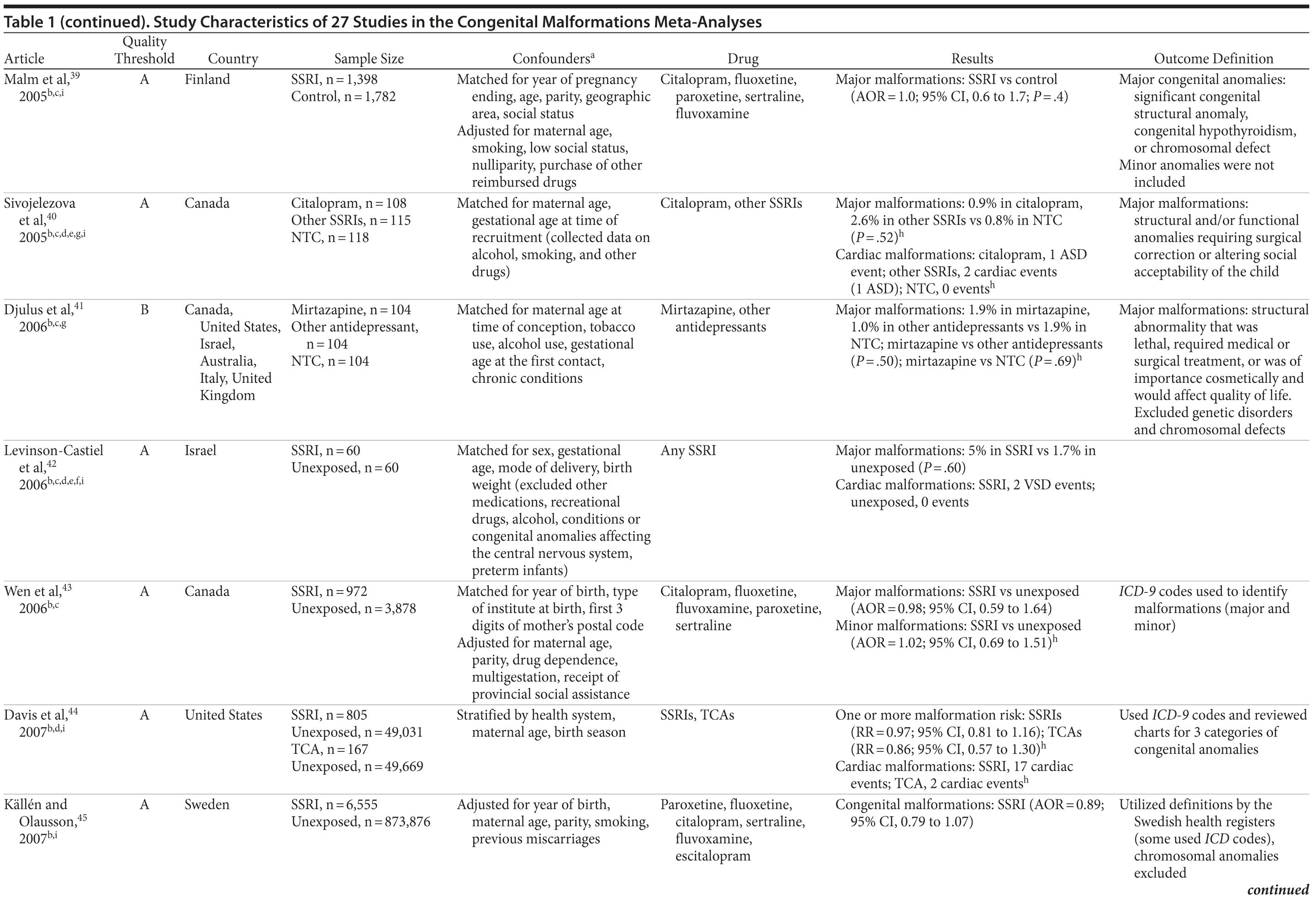
Results involving the 9 studies above quality threshold indicated a significant association between exposure to antidepressants during pregnancy and an increased risk for any septal heart defect (atrial septal defects or ventral septal defects) (RR = 1.40; 95% CI, 1.10-1.77; P = .005; Supplementary eFigure 2). In subanalyses, the RRs were similar to the findings for any cardiovascular malformation (RRs between 1.17 and 1.40; Table 2) and statistically significant. Two studies46,58 had a particularly strong influence on the pooled estimate. There was no evidence suggesting that paroxetine or fluoxetine were associated with septal defects (Supplementary eTable 1); however, only 3 and 2 studies, respectively, were available for such an analysis.
Ventral Septal Defects
Results for ventral septal defects were similar to any cardiac malformations (RR = 1.54; 95% CI, 0.71-3.33; P = .274; Supplementary eFigure 3), but they were not statistically significant; the few studies that investigated this outcome were generally small and reported very few or sometimes no case in either the exposure or the comparison group (Table 2). There were not enough studies to conduct a meta-analysis stratified by paroxetine or fluoxetine.
Publication Bias and Influential Studies
When the analyses were repeated with all identified studies regardless of study quality, we found very similar results. We did not find evidence for the presence of publication bias in any of our analyses (see Table 2). When we recalculated the pooled risk, excluding studies one by one, 1 study58 was found to have had a particular influence on the derived pooled risk. This finding was most apparent for cardiac outcomes, for which exclusion of this study resulted in a slightly higher risk. For all other analyses, the estimate was well within the pooled CIs.
DISCUSSION
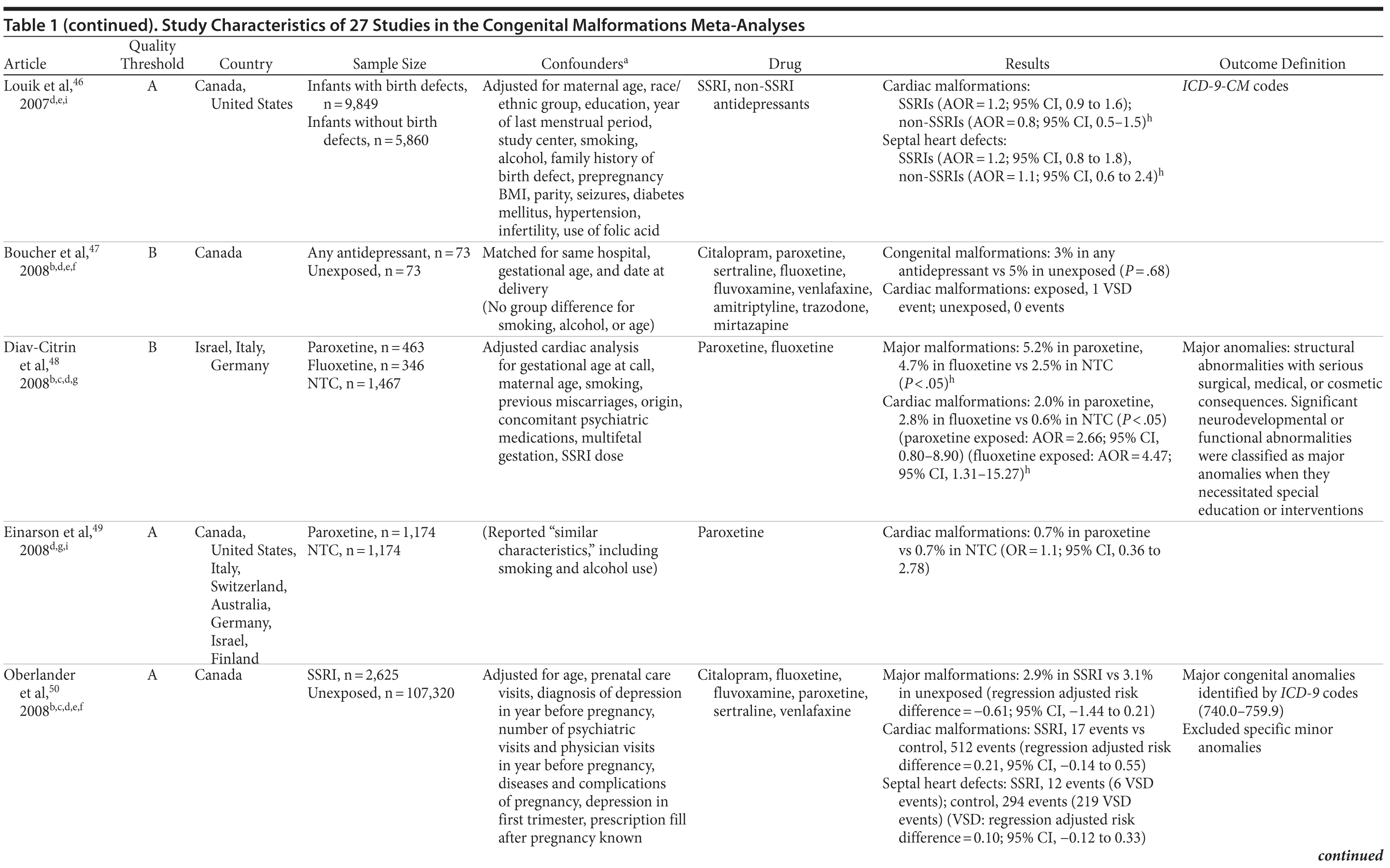
We conducted a systematic review and meta-analysis of the literature, examining associations between the use of any antidepressant and rates of overall congenital malformations, overall major congenital malformations, and, more specifically, cardiac malformations (as defined by the authors of the individual articles; see Table 1). Furthermore, on the basis of the available data for cardiac outcomes, we were then able to examine possible associations with antidepressant exposure and septal defects, as well as ventral septal defects alone. In addition to any antidepressant, we were also able to conduct analyses on 2 individual antidepressant medications (paroxetine and fluoxetine). These 2 medications were chosen on the basis of available evidence for meta-analyses on the outcomes of interest; the evidence reflects the focus of previously published literature. We found no evidence that antidepressants were associated with congenital malformations or major malformations in our primary analyses. Results were generally confirmed in several subanalyses, although there was an indication that antidepressants in general and, more specifically, fluoxetine exposure was associated with major congenital malformations. It is important to consider that these classifications of congenital and major malformations include many different malformations with different timelines of development, which can potentially confound relationships with a specific malformation, if one exists.54 It is also important to note that we were able to analyze only 2 specific antidepressants, and so we cannot draw conclusions regarding the safety of other individual antidepressant drugs that we did not examine separately. Any antidepressant exposure was found to be associated with cardiac malformations in general. More specifically, when we looked at septal heart defects, we also found any antidepressant exposure to be associated. However, it is important to consider that statistical significance is not equivalent to clinical significance. There were only a few studies with which to further examine categorizations of cardiac malformations, and these studies had a small number of cases. We were able to analyze only ventral septal defects as a subcategory of septal defects, and these analyses were not significant, although the RRs were similar. Further research of high methodological quality is needed to confirm the results presented here.
There may be different associations between specific antidepressants and malformations. For example, antidepressants as a whole were associated with risk for any cardiac malformation, as was paroxetine, yet fluoxetine was not. Regardless of whether a class or individual effect exists, it is important to note that, overall, these results are largely reassuring, as even the nonsignificant meta-analyses did not return an RR higher than 1.67, with the highest significant one being 1.47, which is below the 2-fold increase benchmark that has been cited for clinical significance in this field.52 The findings also are relieving, as depression during pregnancy is common, with rates as high as 20% being reported,59 and 1.8%-2.1% of pregnancies having reported exposure to an SSRI,60,61 among the most frequently used of the antidepressants in pregnancy.62
As far as we are aware, our study is the first meta-analysis to report a small increase in major malformations with fluoxetine, the oldest of the SSRIs in use. Note, however, that this was not a consistent finding, as our main analysis, which was with the higher quality studies, was not significant, although it was reduced to 4 studies. Several meta-analyses previously did report an increased risk for cardiovascular malformations with paroxetine (ie, Bar-Oz et al,13 Wurst et al16) but, unlike Wurst et al,16 we did not find an increased risk for congenital malformations with this drug. Ours is the first meta-analysis, to our knowledge, to specifically examine the type of malformation implicated, and our results suggest that septal heart defects are associated with antidepressant exposure. However, although paroxetine was associated with cardiovascular malformations in general, it was not associated with septal heart defects in our analysis. Isolated ventricular malformations are a common type of congenital cardiovascular malformation, with an incidence rate of approximately 2%-5%.63 These are typically small muscular defects, though they can be large or complex, and are often asymptomatic and/or close spontaneously early on and thus are frequently not detected.63 Atrial septal defects, on the other hand, are less common63 and are approximately 7% of congenital cardiac lesions.64 As there are more severe and less severe types, some will require surgical intervention in infancy to avoid negative outcomes,65 while others close spontaneously.63 In contrast to the majority of ventricular septal defects that do close by the first year, it is often the atrial septal defects that are not diagnosed early and present in adulthood.63 Previous research suggested that the inclusion of common reversible heart defects such as the ventral ones may inflate any existing association found with antidepressant exposure.13,49,52,63 We attempted to differentiate between atrial septal defects and ventral septal defects, but there were not enough studies. Although our ventral septal defects analysis was not significant, the RRs were in a similar range to the overall septal defects analysis, which was significant. Note that the CIs were wide for the ventral septal defects-only analyses, and, thus, perhaps the results are biased. Sources of uncontrolled bias leading to the marginal increased risk for septal defects may include detection bias.13 Women with depression may be more likely to have an ultrasound and echocardiogram in pregnancy and after birth. They may also have an increased use of health care services due to the depression itself or due to the publicity surrounding the risks of congenital malformations with SSRIs. Furthermore, many more women with depression may request diagnostic testing of their infants compared to healthy mothers.13 All of the aforementioned potential sources of bias could increase the chance of false detection of an increased risk for septal defects and SSRI exposure. More research is certainly needed that focuses on these types of malformations, with the goal of determining whether an association with the type of cardiovascular effect exists, if it is dependent on a particular drug, or if indeed it is a class effect.
Strengths and Limitations
Perinatal researchers and clinicians have poor evidence-based information on effective treatments for perinatal women, as conducting the evidence-based gold standard, the randomized controlled trial, especially with pharmacological interventions, has ethical barriers among others.66 Our reliance on observational studies and registries67,68 may propagate biases, as they have methodological limitations; thus, we keenly await new studies for replication and to confirm previous results. One of the strengths of our analysis was our attention to study quality. We limited our analyses to studies determined to be above a certain threshold of quality, according to the SAQOR, excluding studies below that threshold. Further, we ran subanalyses to examine whether limiting the data to those studies that (1) confirmed no antidepressant use in the control group, (2) had any adjustments made to the data, or (3) did not use convenience sampling among all the studies influenced any of the study results. We found few differences associated with study quality, suggesting that the findings presented in our analysis are quite robust. One exception was our analysis for major congenital malformations. While the pooled risk measure was not statistically significant in our main analysis using only studies above our quality threshold, the pooled estimates became statistically significant in 2 subanalyses. Regardless of the statistical significance, however, it is important to note that the RRs increased from 1.07 to only 1.10. As such, a very small and only marginally significant effect was present, even in these secondary analyses. Thus, largely, differences were small when examining all available data versus studies above our quality threshold. This is noteworthy given the known methodological flaws in these data as well as our concerns with our inability to make definitive conclusions based on previous research.
A further strength of our work rests on the fact that we used adjusted or matched data where possible in our meta-analyses, as well as running subanalyses for studies with any adjustments. Research in the area has been criticized for not taking into account “non-iatrogenic confounders” in pregnancy.17 Indeed, many studies either did not adjust for any confounders, including depression, or applied matching in very small samples, which makes it difficult to assess whether such matching was successful. However, the most influential studies were also the large ones, and thus heavily influenced the results; these studies did adjust for several potential confounders.45,46,50,57,58 Thus, most of our pooled risk estimates were heavily influenced by studies that did use adjusted data, including our subanalyses. Although this is relieving to a certain extent, these studies used data from population-based registries; they provide better evidence than that provided by data derived from convenience samples, but they are not randomized controlled trials, which provide the best evidence. The absence of evidence from randomized controlled trials highlights the importance for more research. There seems to be a clear need for more studies of high methodological quality that clearly separate antidepressant exposure across intervention and control groups and accurately adjust for the potential confounding effect of other risk factors for malformations.
Our study was limited by the quality of the articles available to be synthesized in our meta-analyses. Often, the specific outcomes examined were not precisely operationalized by authors of the original studies. For example, many studies reported to be examining “malformations,” but did not always specify whether they were major or minor. Studies often did not control for or assess if other psychotropic medications, in addition to antidepressants (ie, hypnotics), were used, the dose, or the timing of use during the pregnancy. We have noted that 1 of the most influential studies was Reis and Källén,58 and, although it was above our quality threshold, it did not control for hypnotics or benzodiazepines. The size and study quality of the articles from the Swedish registries dominated the pooled effects, yet we did not actually have any knowledge of how many women took the medication as prescribed. A recent study by Källén et al69 found that prescription data do not always accurately reflect use. Moreover, in another review70 of how exposure to medication is classified in research utilizing administrative data, the authors concluded that there is tremendous uncertainty about the exposure. As fundamental factors, such as the dose of medication, timing of use during the perinatal period, duration of time the medication was taken, and the way in which women who discontinue using the medication at any point in pregnancy are dealt with in studies that use administrative data, the risk estimates may not be accurate.70 Few studies included any information on diagnosis; that is, whether the medication was actually prescribed for depression. Antidepressants are also indicated for anxiety disorders, which are increasingly recognized as common in pregnancy13,71; these disorders themselves are also associated with adverse birth outcomes, including lower birth weight,72 placental abruption,73 and decreased fetal activity.74 Finally, we were not able to control for the effects of depression itself, and it is possible that the disease itself may be associated with malformations, perhaps via its association with inadequate prenatal care.59 Malnourishment overall (poor diet quality) and vitamin deficiencies (such as folic acid) are associated with various congenital malformations, including neural tube defects, congenital heart defects, and orofacial clefts.75,76 Depressed women are also more likely to smoke,77 and not all studies controlled for smoking despite the known association of smoking with untoward effects.78 Although our analysis was limited to what we deemed to be above quality threshold, and meta-analysis pools data so that conclusions can be reached with higher sample sizes, the original study limitations cannot be entirely ignored.67
The data on the effects of in utero antidepressant exposure have grown recently, and it is now time to pay closer attention to study design in our interpretation of these findings.17,69,70 It will be possible for future research to operationalize the precise malformations to be investigated and not group all. Expanding upon proposed future designs,17 the ideal study, aside from a sufficiently powered multisite randomized controlled trial, would examine the effects within 1 diagnostic group (ie, unipolar major depression of moderate to severe symptomatology), excluding or controlling for known confounders (ie, other medications, smoking or alcohol use), with similar timed exposure (ie, within the first month of the first trimester) and compared to (a) euthymic women without a history of depression and any current medication use and to (b) depressed women of similar severity who are not treated with antidepressant medication. Such a study would indeed be challenging to conduct, as the ethics of randomization into the treatment versus no medication treatment arm can be debated as well as the potential effects of nonmedication treatment on outcome; however, it would be able to provide many answers.
Implications
The decision to use antidepressants during pregnancy must take into account the severity of the illness, whether there is a risk for suicide in particular, and the likelihood of relapse if medication is discontinued.2 This is especially important to consider in light of a small baseline risk for malformations in the general population, with a small increase in risk potentially associated with antidepressant exposure for some outcomes where the absolute risk remains low. Moreover, it still remains to be determined whether some antidepressants are safer to use than others, as we were able to examine only 2 individual antidepressants in our meta-analyses (paroxetine and fluoxetine), or whether a class effect does indeed exist. We did not find a risk for cardiac malformations with the use of fluoxetine, for example, but we did find an increased risk with the use of paroxetine. Note, however, that although it was significant, the RR was still below 1.5 in all subanalyses conducted. While we were able to examine only the drugs for which the most data were available, future studies should specifically examine sertraline and citalopram, as data are also accumulating for these commonly used drugs. Potential risks of antidepressant treatment must be considered in light of other evidence. As many as 68% of women recruited from psychiatric settings who discontinued antidepressant use during pregnancy have been found to relapse,62 leaving both the woman and her infant vulnerable to the potential effects of untreated perinatal depression. Data derived from obstetric clinics did not demonstrate a clear link between risk of a depressive episode and the discontinuation of antidepressant medication during pregnancy.79 However, the study did demonstrate that women with more severe illness (greater than 4 depressive episodes prior to pregnancy) are at high risk for recurrence, even after accounting for the use of antidepressant medication. Treatment decisions must weigh the effects of untreated maternal depression (both in the immediate and long term) for a mother and fetus against the potential adverse effects of antidepressant exposure on the fetus. Moreover, untreated depression can have tremendous impacts on quality of life and experience of pregnancy; these factors must also be taken into account when making treatment decisions.
SUMMARY OF RESULTS AND IMPORTANT CONSIDERATIONS
Results
Any antidepressant medication exposure
Significantly associated with:
- Cardiovascular malformations (RR = 1.36)
- Septal heart defects (atrial septal defects and ventral septal defects) (RR = 1.40)
Not significantly associated with:
- Congenital malformations (RR = 0.93)
- Major malformations (RR = 1.07)
Significant when all studies included (RR = 1.09), but RR similar to the nonsignificant RR of higher quality studies only
- Ventral septal defects (RR = 1.54)
Able to be analyzed only for exposure to any antidepressant
Paroxetine exposure
Significantly associated with:
- Cardiovascular malformations (RR = 1.43)
Not significantly associated with:
- Congenital malformations (RR = 1.03)
- Major malformations (RR = 1.11)
- Septal heart defects (RR = 0.97)
Fluoxetine exposure
Not significantly associated with:
- Congenital malformations (RR = 1.10)
- Major malformations (RR = 1.20)
Significant when all studies included (RR = 1.25), but RR similar to the nonsignificant RR of higher quality studies only
- Cardiovascular malformations (RR = 1.17)
- Septal heart defects (RR = 1.18)
Considerations
- Congenital malformations and major malformations categories include a variety of malformations with different gestational timelines of development. The grouping of all malformations together may cause confounding of potential associations if they exist.
- Results seem robust, but uncontrolled confounders cannot be excluded. For example, we cannot be sure the medication exposure from data derived from administrative sources accurately reflects use.
- Because of limited data, we were able to examine only septal defects (atrial septal defects and ventral septal defects) and then ventral septal defects, but not atrial septal defects alone.
- Although the ventral septal defects analysis was not significant, the RRs were similar to the RRs found in the septal defects analysis, which was significant, suggesting that a significant association may indeed exist for ventral septal defects, which may be evident with more research.
- Alternatively, including common reversible heart defects, such as ventral septal defects, could increase the chance of finding an association.
- There may be detection bias leading to a false detection of an association for antidepressant drugs and septal defects.
- On the basis of available data, we were able to analyze only 2 individual medications (paroxetine and fluoxetine), and so it was not possible to determine if there was a class effect or an individual drug effect or to determine the safety of other individual antidepressant drugs.
- This bias in the available data on individual antidepressant medications also reflects previous research interests and foci.
- Few studies included any information on diagnosis. The effects of a major depressive episode must also be considered.
- Although there were statistically significant results, one must consider clinical significance.
- None of the significant RRs found were above 1.47, and the clinically significant benchmark for this field has been cited as being a 2-fold increase.
Drug names: bupropion (Wellbutrin, Aplenzin, and others), citalopram (Celexa and others), desipramine (Norpramin and others), doxepin (Silenor and others), escitalopram (Lexapro and others), fluoxetine (Prozac and others), fluvoxamine (Luvox and others), imipramine (Tofranil and others), mirtazapine (Remeron and others), nortriptyline (Pamelor, Aventyl, and others), paroxetine (Paxil, Pexeva, and others), protriptyline (Vivactil and others), sertraline (Zoloft and others), trazodone (Oleptro and others), venlafaxine (Effexor and others).
Author affiliations: Department of Psychiatry, Women’s College Hospital, Toronto (Dr Grigoriadis and Mss VonderPorten and Mamisashvili); Women’s College Research Institute, Toronto, (Drs Grigoriadis, Ross, and Dennis); Department of Psychiatry, University Health Network, and Clinical Trials Resource Centre, Toronto General Research Institute (Dr Grigoriadis); Department of Psychiatry, Sunnybrook Health Sciences Centre, Toronto (Drs Grigoriadis and Cheung and Mss VonderPorten and Mamisashvili); Sunnybrook Research Institute, Toronto (Drs Grigoriadis and Cheung); Department of Psychiatry (Drs Grigoriadis, Rehm, Dennis, Cheung, and Ross), Dalla Lana School of Public Health (Drs Roerecke and Rehm), Faculty of Medicine (Drs Grigoriadis, Rehm, Dennis, Cheung, and Ross), Faculty of Nursing (Dr Dennis), Departments of Pediatrics, Pharmacology, Pharmacy, and Medical Genetics (Dr Koren), and Institute of Medical Sciences (Dr Steiner), University of Toronto, Toronto; Social and Epidemiological Research Department, Centre for Addiction and Mental Health, Toronto (Drs Ross, Roerecke, and Rehm); Motherisk Program, The Hospital for Sick Children, Toronto; and Departments of Medicine, Pediatrics and Physiology/Pharmacology, University of Western Ontario, London (Dr Koren); Departments of Psychiatry and Behavioural Neurosciences and Obstetrics and Gynecology, McMaster University; Women’s Health Concerns Clinic, St Joseph’s Healthcare, Hamilton (Dr Steiner); Healthy Child Development Program, Ontario College of Family Physicians, Toronto; York Central Hospital, Richmond Hill; and Markham Stouffville Hospital, Markham (Dr Mousmanis); and Epidemiological Research Unit, Technische Universitפt Dresden, Klinische Psychologie & Psychotherapie, Dresden, Germany (Dr Rehm). Dr Grigoriadis is now with the Department of Psychiatry, Sunnybrook Health Sciences Centre, and Sunnybrook Research Institute, Toronto. Mss VonderPorten and Mamisashvili are with the Department of Psychiatry, Sunnybrook Health Sciences Centre, Toronto, Ontario, Canada.
Author contributions: Conception and design: Drs Grigoriadis and Ross; data analysis and interpretation: Drs Grigoriadis, Ross, Roerecke, Rehm, Dennis, Koren, Steiner, Mousmanis, and Cheung and Mss VonderPorten and Mamisashvili; drafting or revision of the manuscript: Drs Grigoriadis, Roerecke, Rehm, Ross, Dennis, Koren, Steiner, Mousmanis, and Cheung and Mss VonderPorten and Mamisashvili; and approval of the final version of the manuscript for publication: Drs Grigoriadis, Ross, Roerecke, Rehm, Dennis, Koren, Steiner, Mousmanis, and Cheung and Mss VonderPorten and Mamisashvili. Drs Grigoriadis and Ross had full access to all of the data and take responsibility for the integrity of the data and the accuracy of the data analysis.
Potential conflicts of interest: In the last 5 years, Dr Grigoriadis has received honoraria as a consultant and a member of an advisory committee or for lectures from Wyeth, GlaxoSmithKline, Pfizer, Servier, Eli Lilly Canada, and Lundbeck; and has received research grant support from the Canadian Institutes of Health Research (CIHR), Ontario Ministry of Health, Ontario Mental Health Foundation, and CR Younger Foundation. Dr Steiner has been a consultant to AstraZeneca, Azevan, Servier, Bayer Canada, and Lundbeck; has received grant/research support from CIHR, Pfizer, Eli Lilly, and Lundbeck; and has received honoraria from the Society for Women’s Health Research and AstraZeneca. Drs Roerecke, Rehm, Dennis, Koren, Mousmanis, Cheung, and Ross and Mss VonderPorten and Mamisashvili have no financial disclosures to report.
Funding/support: Funding was provided by a Research Syntheses grant from the CIHR, KRS-83127, and the Ontario Ministry of Health and Long-Term Care (Drug Innovation Fund), grant # 2008-005. Dr Grigoriadis is supported by a New Investigator Award in Women’s Health Research from CIHR in partnership with the Ontario Women’s Health Council. Dr Ross has a new investigator award from CIHR and the Ontario Women’s Health Council, award NOW-84656. In addition, support to the Centre for Addiction and Mental Health for salary of scientists and infrastructure has been provided by the Ontario Ministry of Health and Long-Term Care.
Disclaimer: The views expressed here do not necessarily reflect those of the Ministry of Health and Long-Term Care.
Previous presentations: Preliminary results of this study were presented at the ▪ Motherisk Update 2012 (paper title: The effect of antidepressant medications on mothers and babies—an updated systematic analysis); May 23, 2012; Toronto, Ontario, Canada ▪ Canadian Psychiatric Association Conference (paper title: Evidence-based discussion guide for antidepressant treatment in depressed pregnant and postpartum women); October 13-15, 2011; Vancouver, British Columbia, Canada ▪ Canadian Institutes for Health Research, Innovations in Gender, Sex and Health Research Conference; (paper titles: (1) Effects of in utero exposure to antidepressant medication during pregnancy: a meta-analysis and (2) Short-term and long-term impact of untreated maternal depression on the child); November 22-23, 2010; Toronto, Ontario, Canada ▪ Canadian Psychiatric Association Conference; (paper title: Outcomes associated with antidepressant medication use during pregnancy: a meta-analysis); September 23-26, 2010; Toronto, Ontario, Canada ▪ 36th Annual Meeting of the North American Society for Psychosocial Obstetrics and Gynecology; (poster title: Preliminary results from the Reproductive Life Stages Algorithm Project: use of antidepressant medication during pregnancy and lactation: development of an evidence-based decision tool); February 10-13, 2010; Richmond, Virginia ▪ Organization for the Study of Sex Differences 3rd Annual Meeting; (poster title: An evidence-based algorithm to quantify risk-benefit decision-making for use of antidepressant medication during pregnancy and lactation); June 4-6, 2009; Toronto, Ontario, Canada ▪ 35th Annual Meeting of the North American Society for Psychosocial Obstetrics and Gynecology; (poster title: Use of antidepressant medication during pregnancy and lactation: development of an evidence-based decision tool); February 4-7, 2009; New Haven, Connecticut ▪ Canadian College of Neuropsychopharmacology Annual Meeting; (poster, title: An evidence-based algorithm to quantify risk-benefit decision-making for use of antidepressant medication during pregnancy and lactation. Research in progress: current state of knowledge); June 6-9, 2008; Toronto, Ontario, Canada.
Acknowledgments: The authors are very grateful for the advisory committee members, who provided valuable insights and lent their time and expertise to this project. The authors would like to thank Hiltrud Dawson, RN (Health Nexus, Toronto, Canada); Michael Dunn, MD (Sunnybrook Health Sciences Centre, Toronto, Canada); Adrienne Einarson, RN (The Hospital for Sick Children, Toronto, Canada); Alicja Fishell, MD (Women’s College Hospital, Toronto, Canada); Jasmine Gandhi, MD (Ottawa Hospital, Ottawa, Canada); Jan Kasperski, RN, MHSc (Ontario College of Family Physicians, Toronto, Canada); Sidney Kennedy, MD (University Health Network, Toronto, Canada); Diane Meschino, MD (Women’s College Hospital, Toronto, Canada); Irena Nulman, MD, PhD (The Hospital for Sick Children, Toronto, Canada); Sagar Parikh, MD (University Health Network, Toronto, Canada); Paula Ravitz, MD (Mount Sinai Hospital, Toronto, Canada); Sarah Romans, MD (University of Otago, Wellington, New Zealand); Simone Vigod, MD (Women’s College Hospital, Toronto, Canada); Jennifer Blake, MD (Sunnybrook Health Sciences Centre, Toronto, Canada); and Karen Wade, RN, MScN (Toronto Public Health, Toronto, Canada). The authors would also like to thank Lindsay Witton, MSW (Women’s College Hospital, Toronto, Canada); Maura O’ Keefe, MSW (Women’s College Hospital, Toronto, Canada); and Svetlana Emelianova, MD (North York General Hospital, North York, Canada), for providing their feedback during many research meetings. Sheila Lacroix, MLS (Centre for Addiction and Mental Health, Toronto, Canada), and Ani Orchanian-Cheff, MISt (University Health Network, Toronto, Ontario), devised search strategies and completed literature search. The authors would also like to thank Alex Kiss, PhD (Sunnybrook Health Sciences Centre, Toronto, Canada), for performing preliminary meta-analyses; Allison Eady, BA (Women’s College Hospital, Toronto, Canada), Jenna McKay, BA (Women’s College Hospital, Toronto, Canada), and Kimberly Radford, BA (Women’s College Hospital, Toronto, Canada), for assisting with data extraction and the quality assessment processes. Dr Kennedy has received research funding or honoraria in the past from AstraZeneca, Biovail, Eli Lilly, GlaxoSmithKline, Janssen-Ortho, Lundbeck, Pfizer, St Jude Medical, and Servier. Ms Einarson has conducted research funded by an unrestricted grant from Eli Lilly Canada. Dr Nulman has held a CIHR Collaborative grant with Duchesnay. Dr Parikh has had education or research grants or served on advisory boards or as a speaker for AstraZeneca, Apotex, Biovail, Bristol-Myers Squibb, CIHR, Canadian Network for Mood and Anxiety Treatments, Canadian Psychiatric Association, GlaxoSmithKline, Genpharm, Janssen, Eli Lilly, Lundbeck, Novartis, Pfizer, and Wyeth. There were no conflicts of interest or relevant financial disclosures reported by any of the other acknowledged individuals.
Supplementary material: See accompanying pages.
REFERENCES
1. ACOG Committee on Practice Bulletins-Obstetrics. ACOG Practice Bulletin: clinical management guidelines for obstetrician-gynecologists number 92, April 2008 (replaces practice bulletin number 87, November 2007). Use of psychiatric medications during pregnancy and lactation. Obstet Gynecol. 2008;111(4):1001-1020. PubMed
2. Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403-413. PubMed doi:10.1016/j.genhosppsych.2009.04.003
3. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry. 2010;67(10):1012-1024. PubMed doi:10.1001/archgenpsychiatry.2010.111
4. Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10):1515-1520. PubMed doi:10.1176/appi.ajp.2007.06111893
5. Bonari L, Koren G, Einarson TR, et al. Use of antidepressants by pregnant women: evaluation of perception of risk, efficacy of evidence based counseling and determinants of decision making. Arch Women Ment Health. 2005;8(4):214-220. PubMed doi:10.1007/s00737-005-0094-8
6. Epidemiology study: final report on bupropion and other antidepressants, including paroxetine, in pregnancy and the occurrence of cardiovascular and major congenital malformations. GlaxoSmithKline Web site. http://www.gsk-clinicalstudyregister.com/result_detail.jsp?protocolId=113694_3&studyId=D9F4ACBC-25C0-4F26-9266-BBC8044508F5&compound=bupropion. Accessed January 29, 2013.
6a. Public health advisory: paroxetine. US Food and Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm051731.htm. Updated January 27, 2010. Accessed January 31, 2013.
6b. Paxil (paroxetine) and possible increased risk of birth defects. Health Canada Web site. http://www.hc-sc.gc.ca/dhp-mps/medeff/advisories-avis/public/_2005/paxil_3_pa-ap-eng.php. Updated October 7, 2005. Accessed January 31, 2013.
7. Ross LE, Grigoriadis S, Mamisashvili L, et al. Quality assessment of observational studies in psychiatry: an example from perinatal psychiatric research. Int J Methods Psychiatr Res. 2011;20(4):224-234. PubMed doi:10.1002/mpr.356
8. Addis A, Koren G. Safety of fluoxetine during the first trimester of pregnancy: a meta-analytical review of epidemiological studies. Psychol Med. 2000;30(1):89-94. PubMed doi:10.1017/S0033291799001270
9. Einarson TR, Einarson A. Newer antidepressants in pregnancy and rates of major malformations: a meta-analysis of prospective comparative studies. Pharmacoepidemiol Drug Saf. 2005;14(12):823-827. PubMed doi:10.1002/pds.1084
10. Rahimi R, Nikfar S, Abdollahi M. Pregnancy outcomes following exposure to serotonin reuptake inhibitors: a meta-analysis of clinical trials. Reprod Toxicol. 2006;22(4):571-575. PubMed doi:10.1016/j.reprotox.2006.03.019
11. Kalter H. Five-decade international trends in the relation of perinatal mortality and congenital malformations: stillbirth and neonatal death compared. Int J Epidemiol. 1991;20(1):173-179. PubMed doi:10.1093/ije/20.1.173
12. Viguera AC, Cohen LS, Baldessarini RJ, et al. Managing bipolar disorder during pregnancy: weighing the risks and benefits. Can J Psychiatry. 2002;47(5):426-436. PubMed
13. Bar-Oz B, Einarson T, Einarson A, et al. Paroxetine and congenital malformations: meta-analysis and consideration of potential confounding factors. Clin Ther. 2007;29(5):918-926. PubMed doi:10.1016/j.clinthera.2007.05.003
14. Kalter H. New challenges. In: Teratology in the Twentieth Century Plus Ten. 1st ed. New York, NY: Springer; 2010:53-55. doi:10.1007/978-90-481-8820-8_5
15. Adam M, Hudgins L. The importance of minor anomalies in the evaluation of the newborn. Neoreviews. 2003;4(4):e99-e104. doi:10.1542/neo.4-4-e99
16. Wurst KE, Poole C, Ephross SA, et al. First trimester paroxetine use and the prevalence of congenital, specifically cardiac, defects: a meta-analysis of epidemiological studies. Birth Defects Res A Clin Mol Teratol. 2010;88(3):159-170. PubMed doi:10.1002/bdra.20627
17. Gentile S. Selective serotonin reuptake inhibitor exposure during early pregnancy and the risk of birth defects. Acta Psychiatr Scand. 2011;123(4):266-275. PubMed doi:10.1111/j.1600-0447.2011.01673.x
18. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008-2012. PubMed doi:10.1001/jama.283.15.2008
19. American College of Medical Genetics. Evaluation of the newborn with single or multiple congenital anomalies New York State Department of Health Web site. http://www.healthboard.com/websites/Detailed/14196.html Updated 1999. Accessed December 12, 2012.
20. von Elm E, Altman DG, Egger M, et al; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453-1457. PubMed doi:10.1016/S0140-6736(07)61602-X
21. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. PubMed doi:10.1136/jech.52.6.377
22. Wells GA, Shea B, O’ Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Department of Epidemiology and Community Medicine, University of Ottawa, Canada. Ottawa Hospital Research Institute Web site. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm Accessed December 12, 2012.
23. Guyatt GH, Oxman AD, Vist GE, et al; GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. PubMed doi:10.1136/bmj.39489.470347.AD
24. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. PubMed doi:10.1016/0197-2456(86)90046-2
25. Schlesselman JJ. Case-Control Studies: Design, Conduct, Analysis. New York, NY: Oxford University Press; 1982.
26. Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. PubMed doi:10.1136/bmj.315.7109.629
27. Cochran W. The combination of estimates from different experiments. Biometrics. 1954;10(1):101-129. doi:10.2307/3001666
28. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539-1558. PubMed doi:10.1002/sim.1186
29. Stata Corporation Stata Statistical Software. College Station, TX: Stata Corporation; 2008.
30. Ross LE, Grigoriadis S, Mamisashvili L et al. Selected pregnancy and delivery outcomes after exposure to antidepressant medication: a systematic review and meta-analysis. JAMA Psychiatry. Published online ahead of print February 27, 2013. doi:10.1001/jamapsychiatry.2013.684
31. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873-880. PubMed
32. Pastuszak A, Schick-Boschetto B, Zuber C, et al. Pregnancy outcome following first-trimester exposure to fluoxetine (Prozac). JAMA. 1993;269(17):2246-2248. PubMed doi:10.1001/jama.1993.03500170076037
33. Chambers CD, Johnson KA, Dick LM, et al. Birth outcomes in pregnant women taking fluoxetine. N Engl J Med. 1996;335(14):1010-1015. PubMed doi:10.1056/NEJM199610033351402
34. Kulin NA, Pastuszak A, Sage SR, et al. Pregnancy outcome following maternal use of the new selective serotonin reuptake inhibitors: a prospective controlled multicenter study. JAMA. 1998;279(8):609-610. PubMed doi:10.1001/jama.279.8.609
35. Einarson A, Fatoye B, Sarkar M, et al. Pregnancy outcome following gestational exposure to venlafaxine: a multicenter prospective controlled study. Am J Psychiatry. 2001;158(10):1728-1730. PubMed doi:10.1176/appi.ajp.158.10.1728
36. Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry. 2002;159(12):2055-2061. PubMed doi:10.1176/appi.ajp.159.12.2055
37. Einarson A, Bonari L, Voyer-Lavigne S, et al. A multicentre prospective controlled study to determine the safety of trazodone and nefazodone use during pregnancy. Can J Psychiatry. 2003;48(2):106-110. PubMed
38. Chun-Fai-Chan B, Koren G, Fayez I, et al. Pregnancy outcome of women exposed to bupropion during pregnancy: a prospective comparative study. Am J Obstet Gynecol. 2005;192(3):932-936. PubMed doi:10.1016/j.ajog.2004.09.027
39. Malm H, Klaukka T, Neuvonen PJ. Risks associated with selective serotonin reuptake inhibitors in pregnancy. Obstet Gynecol. 2005;106(6):1289-1296. PubMed doi:10.1097/01.AOG.0000187302.61812.53
40. Sivojelezova A, Shuhaiber S, Sarkissian L, et al. Citalopram use in pregnancy: prospective comparative evaluation of pregnancy and fetal outcome. Am J Obstet Gynecol. 2005;193(6):2004-2009. PubMed doi:10.1016/j.ajog.2005.05.012
41. Djulus J, Koren G, Einarson TR, et al. Exposure to mirtazapine during pregnancy: a prospective, comparative study of birth outcomes. J Clin Psychiatry. 2006;67(8):1280-1284. PubMed doi:10.4088/JCP.v67n0817
42. Levinson-Castiel R, Merlob P, Linder N, et al. Neonatal abstinence syndrome after in utero exposure to selective serotonin reuptake inhibitors in term infants. Arch Pediatr Adolesc Med. 2006;160(2):173-176. PubMed doi:10.1001/archpedi.160.2.173
43. Wen SW, Yang Q, Garner P, et al. Selective serotonin reuptake inhibitors and adverse pregnancy outcomes. Am J Obstet Gynecol. 2006;194(4):961-966. PubMed doi:10.1016/j.ajog.2006.02.019
44. Davis RL, Rubanowice D, McPhillips H, et al; HMO Research Network Center for Education, Research in Therapeutics. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug Saf. 2007;16(10):1086-1094. PubMed doi:10.1002/pds.1462
45. Kפllén BA, Otterblad Olausson P. Maternal use of selective serotonin re-uptake inhibitors in early pregnancy and infant congenital malformations. Birth Defects Res A Clin Mol Teratol. 2007;79(4):301-308. PubMed doi:10.1002/bdra.20327
46. Louik C, Lin AE, Werler MM, et al. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. N Engl J Med. 2007;356(26):2675-2683. PubMed doi:10.1056/NEJMoa067407
47. Boucher N, Bairam A, Beaulac-Baillargeon L. A new look at the neonate’s clinical presentation after in utero exposure to antidepressants in late pregnancy. J Clin Psychopharmacol. 2008;28(3):334-339. PubMed doi:10.1097/JCP.0b013e318173aa2e
48. Diav-Citrin O, Shechtman S, Weinbaum D, et al. Paroxetine and fluoxetine in pregnancy: a prospective, multicentre, controlled, observational study. Br J Clin Pharmacol. 2008;66(5):695-705. PubMed
49. Einarson A, Pistelli A, DeSantis M, et al. Evaluation of the risk of congenital cardiovascular defects associated with use of paroxetine during pregnancy. Am J Psychiatry. 2008;165(6):749-752. PubMed doi:10.1176/appi.ajp.2007.07060879
50. Oberlander TF, Warburton W, Misri S, et al. Major congenital malformations following prenatal exposure to serotonin reuptake inhibitors and benzodiazepines using population-based health data. Birth Defects Res B Dev Reprod Toxicol. 2008;83(1):68-76. PubMed doi:10.1002/bdrb.20144
51. Ramos E, St-André M, Rey E, et al. Duration of antidepressant use during pregnancy and risk of major congenital malformations. Br J Psychiatry. 2008;192(5):344-350. PubMed doi:10.1192/bjp.bp.107.042523
52. Einarson A, Choi J, Einarson TR, et al. Incidence of major malformations in infants following antidepressant exposure in pregnancy: results of a large prospective cohort study. Can J Psychiatry. 2009;54(4):242-246. PubMed
53. Merlob P, Birk E, Sirota L, et al. Are selective serotonin reuptake inhibitors cardiac teratogens? echocardiographic screening of newborns with persistent heart murmur. Birth Defects Res A Clin Mol Teratol. 2009;85(10):837-841. PubMed doi:10.1002/bdra.20615
54. Pedersen LH, Henriksen TB, Vestergaard M, et al. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ. 2009;339:b3569. PubMed doi:10.1136/bmj.b3569
55. Wichman CL, Moore KM, Lang TR, et al. Congenital heart disease associated with selective serotonin reuptake inhibitor use during pregnancy. Mayo Clin Proc. 2009;84(1):23-27. PubMed doi:10.4065/84.1.23
56. Bakker MK, Kerstjens-Frederikse WS, Buys CHCM, et al. First-trimester use of paroxetine and congenital heart defects: a population-based case-control study. Birth Defects Res A Clin Mol Teratol. 2010;88(2):94-100. PubMed
57. Kornum JB, Nielsen RB, Pedersen L, et al. Use of selective serotonin-reuptake inhibitors during early pregnancy and risk of congenital malformations: updated analysis. Clin Epidemiol. 2010;2:29-36. PubMed
58. Reis M, Kפllén B. Delivery outcome after maternal use of antidepressant drugs in pregnancy: an update using Swedish data. Psychol Med. 2010;40(10):1723-1733. PubMed doi:10.1017/S0033291709992194
59. Bonari L, Pinto N, Ahn E, et al. Perinatal risks of untreated depression during pregnancy. Can J Psychiatry. 2004;49(11):726-735. PubMed
60. Ververs T, Kaasenbrood H, Visser G, et al. Prevalence and patterns of antidepressant drug use during pregnancy. Eur J Clin Pharmacol. 2006;62(10):863-870. PubMed doi:10.1007/s00228-006-0177-0
61. Bakker MK, Kölling P, van den Berg PB, et al. Increase in use of selective serotonin reuptake inhibitors in pregnancy during the last decade, a population-based cohort study from the Netherlands. Br J Clin Pharmacol. 2008;65(4):600-606. PubMed doi:10.1111/j.1365-2125.2007.03048.x
62. Cohen LS, Altshuler LL, Harlow BL, et al. Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. (corrected) (published erratum appears in JAMA Jul 12;296(2) JAMA. 2006;295(5):499-507. PubMed doi:10.1001/jama.295.5.499
63. Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890-1900. PubMed doi:10.1016/S0735-1097(02)01886-7
64. Hoffman JIE. Incidence of congenital heart disease 1: postnatal incidence. Pediatr Cardiol. 1995;16(3):103-113. PubMed doi:10.1007/BF00801907
65. Lammers A, Hager A, Eicken A, et al. Need for closure of secundum atrial septal defect in infancy. J Thorac Cardiovasc Surg. 2005;129(6):1353-1357. PubMed doi:10.1016/j.jtcvs.2004.10.007
66. Brandon AR. Ethical barriers to perinatal mental health research and evidence-based treatment: an empirical study. AJOB Primary Research. 2011;2(1):2-12. doi:10.1080/21507716.2011.561517
67. Jüni P, Witschi A, Bloch R, et al. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282(11):1054-1060. PubMed doi:10.1001/jama.282.11.1054
68. Mallen C, Peat G, Croft P. Quality assessment of observational studies is not commonplace in systematic reviews. J Clin Epidemiol. 2006;59(8):765-769. PubMed doi:10.1016/j.jclinepi.2005.12.010
69. Kפllén B, Nilsson E, Olausson PO. Antidepressant use during pregnancy: comparison of data obtained from a prescription register and from antenatal care records. Eur J Clin Pharmacol. 2011;67(8):839-845. PubMed doi:10.1007/s00228-011-1021-8
70. Grzeskowiak LE, Gilbert AL, Morrison JL. Exposed or not exposed? exploring exposure classification in studies using administrative data to investigate outcomes following medication use during pregnancy. Eur J Clin Pharmacol. 2012;68(5):459-467. PubMed doi:10.1007/s00228-011-1154-9
71. Grigoriadis S, de Camps Meschino D, Barrons E, et al. Mood and anxiety disorders in a sample of Canadian perinatal women referred for psychiatric care. Arch Women Ment Health. 2011;14(4):325-333. PubMed doi:10.1007/s00737-011-0223-5
72. Hernández-Mart×nez C, Val VA, Murphy M, et al. Relation between positive and negative maternal emotional states and obstetrical outcomes. Women Health. 2011;51(2):124-135. PubMed doi:10.1080/03630242.2010.550991
73. de Paz NC, Sanchez SE, Huaman LE, et al. Risk of placental abruption in relation to maternal depressive, anxiety and stress symptoms. J Affect Disord. 2011;130(1-2):280-284. PubMed doi:10.1016/j.jad.2010.07.024
74. Groome LJ, Swiber MJ, Bentz LS, et al. Maternal anxiety during pregnancy: effect on fetal behavior at 38 to 40 weeks of gestation. J Dev Behav Pediatr. 1995;16(6):391-396. PubMed
75. Czeizel AE. Periconceptional folic acid and multivitamin supplementation for the prevention of neural tube defects and other congenital abnormalities. Birth Defects Res A Clin Mol Teratol. 2009;85(4):260-268. PubMed doi:10.1002/bdra.20563
76. Carmichael SL, Yang W, Feldkamp ML, et al; National Birth Defects Prevention Study. Reduced risks of neural tube defects and orofacial clefts with higher diet quality. Arch Pediatr Adolesc Med. 2012;166(2):121-126. PubMed doi:10.1001/archpediatrics.2011.185
77. Pratt LA, Brody DJ. Depression and Smoking in the US Household Population Aged 20 and Over, 2005-2008. NCHS Data Brief, No 34. Hyattsville, MD: National Center for Health Statistics; 2010.
78. Einarson A, Riordan S. Smoking in pregnancy and lactation: a review of risks and cessation strategies. Eur J Clin Pharmacol. 2009;65(4):325-330. PubMed doi:10.1007/s00228-008-0609-0
79. Yonkers KA, Gotman N, Smith MV, et al. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy? Epidemiology. 2011;22(6):848-854. PubMed
Editor’s Note: We encourage authors to submit papers for consideration as a part of our Focus on Women’s Mental Health section. Please contact Marlene P. Freeman, MD, at [email protected].
This PDF is free for all visitors!