Objective: To identify symptoms potentially representative of a noradrenergic symptom cluster as possible predictors of response to the selective norepinephrine reuptake inhibitor (NRI) edivoxetine when used as monotherapy or adjunctive treatment in patients with DSM-IV-TR major depressive disorder (MDD).
Methods: Pooled data from 4 adjunctive treatment trials (selective serotonin reuptake inhibitor [SSRI] + edivoxetine 6-18 mg/d vs SSRI + placebo; N = 2,066) and data from 1 monotherapy trial (edivoxetine 6-18 mg/d versus placebo; N = 495) were used to identify predictors of response related to noradrenergic symptoms using a resampling-based ensemble tree method. The trials were conducted from 2008 to 2013.
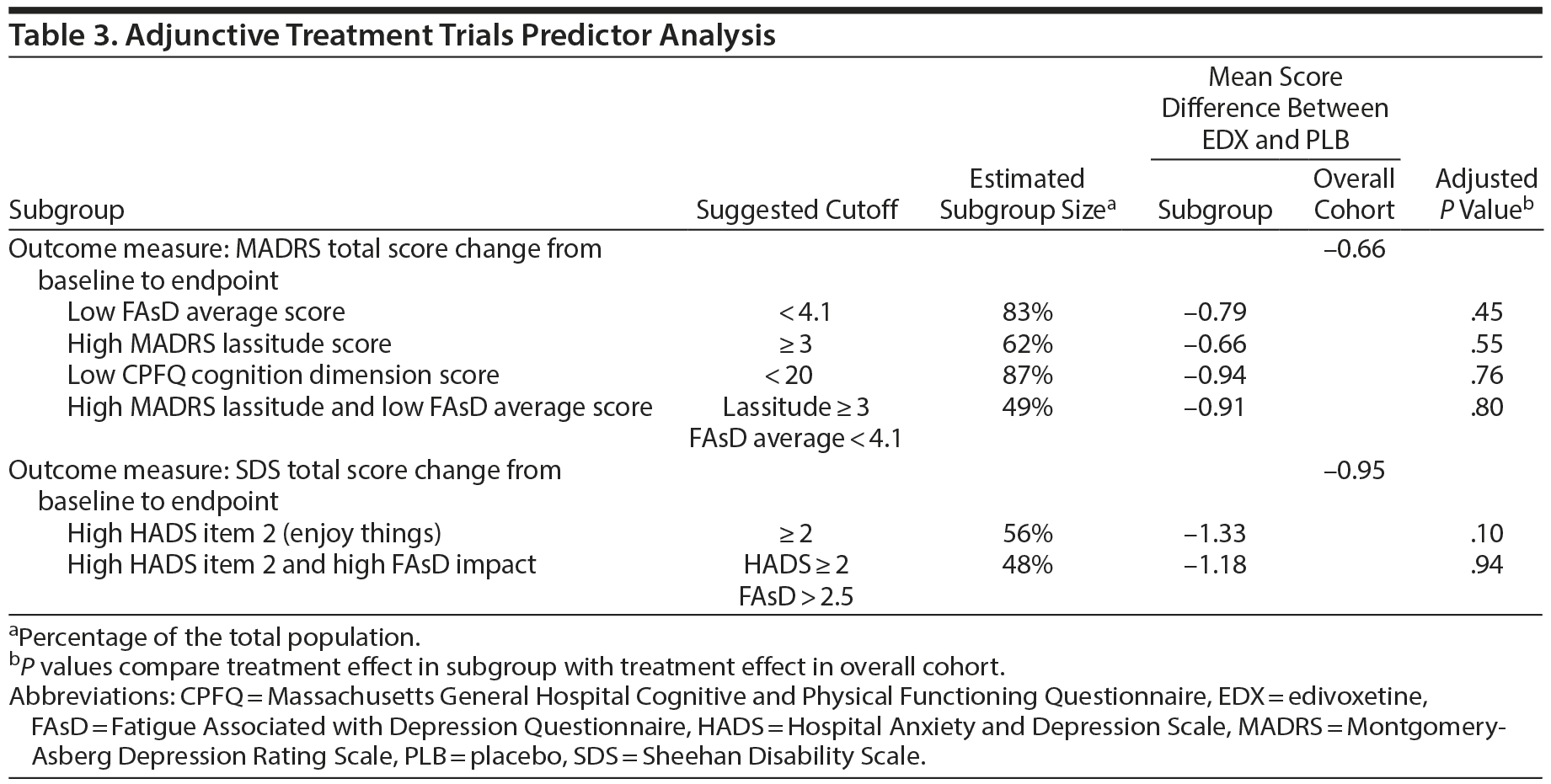
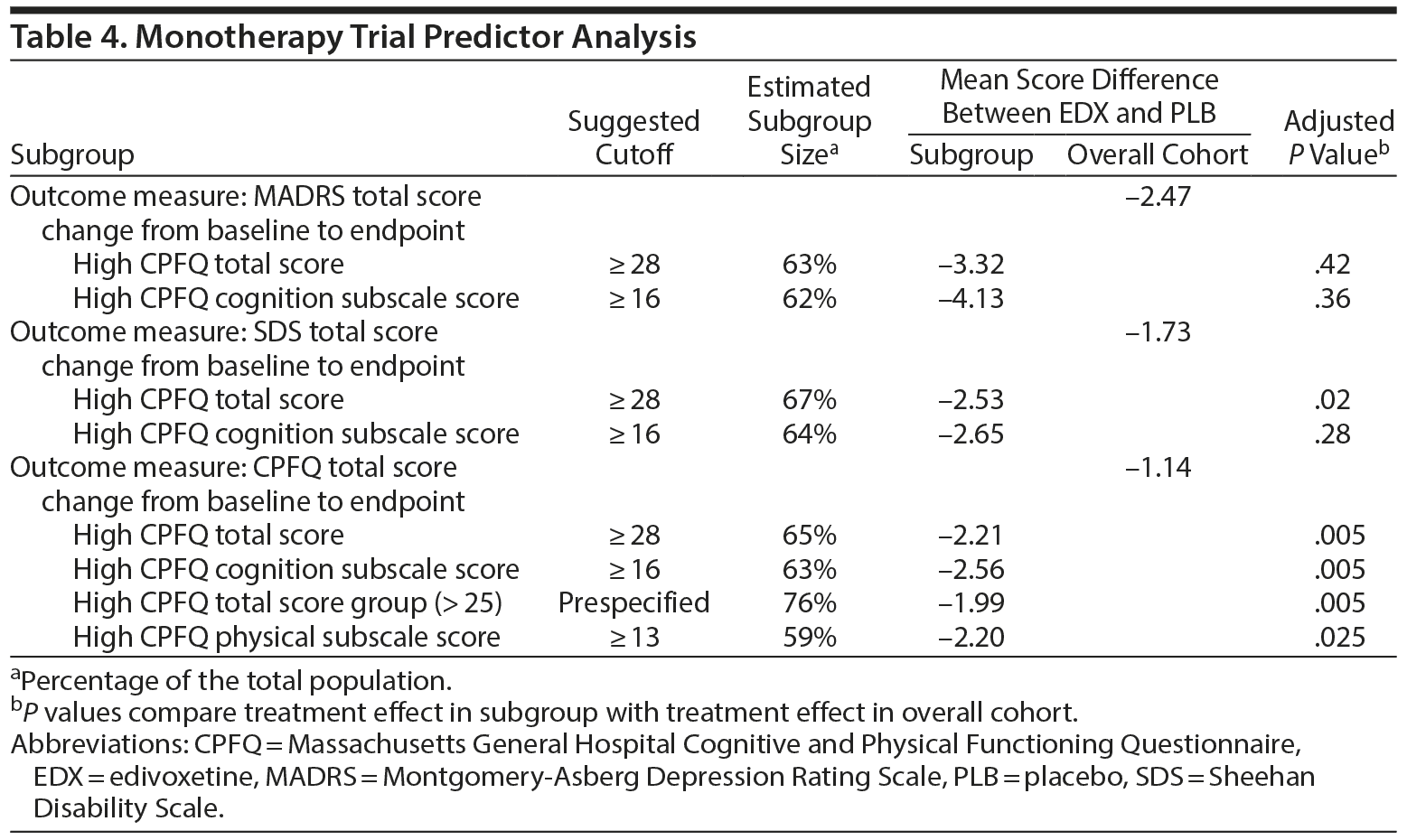
Results: In the pooled adjunctive trials, no subgroup was identified that demonstrated a greater edivoxetine-placebo treatment difference than the overall patient cohort. In the edivoxetine monotherapy trial, no subgroup showing greater mean edivoxetine-placebo differences on the Montgomery-Asberg Depression Rating Scale versus the overall patient cohort was identified; a subgroup (67%) with high b’ ‹aseline Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire (CPFQ) total score (≥ 28) showed statistically significantly (P = .02) greater mean edivoxetine-placebo differences on the Sheehan Disability Scale versus the overall patient cohort, and subgroups with baseline CPFQ total score ≥ 28 (65%), CPFQ cognition dimension score ≥ 16 (63%), or CPFQ physical dimension score ≥ 13 (59%) showed statistically significantly (P ≤ .025) greater mean edivoxetine-placebo differences on the CPFQ total score versus the overall patient cohort.
Conclusions: While we could not identify symptoms predictive of response to the selective NRI edivoxetine used as adjunctive treatment, impaired cognition and physical symptoms may predict greater improvement during monotherapy.
Trial Registration: ClinicalTrials.gov identifiers: NCT00840034, NCT01173601, NCT01187407, NCT01185340, NCT00795821
‘ ‹
Is the Noradrenergic Symptom Cluster a Valid Construct in Adjunctive Treatment of Major Depressive Disorder?
ABSTRACT
Objective: To identify symptoms potentially representative of a noradrenergic symptom cluster as possible predictors of response to the selective norepinephrine reuptake inhibitor (NRI) edivoxetine when used as monotherapy or adjunctive treatment in patients with DSM-IV-TR major depressive disorder (MDD).
Methods: Pooled data from 4 adjunctive treatment trials (selective serotonin reuptake inhibitor [SSRI] + edivoxetine 6-18 mg/d vs SSRI + placebo; N = 2,066) and data from 1 monotherapy trial (edivoxetine 6-18 mg/d versus placebo; N = 495) were used to identify predictors of response related to noradrenergic symptoms using a resampling-based ensemble tree method. The trials were conducted from 2008 to 2013.
Results: In the pooled adjunctive trials, no subgroup was identified that demonstrated a greater edivoxetine-placebo treatment difference than the overall patient cohort. In the edivoxetine monotherapy trial, no subgroup showing greater mean edivoxetine-placebo differences on the Montgomery-Asberg Depression Rating Scale versus the overall patient cohort was identified; a subgroup (67%) with high baseline Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire (CPFQ) total score (≥ 28) showed statistically significantly (P = .02) greater mean edivoxetine-placebo differences on the Sheehan Disability Scale versus the overall patient cohort, and subgroups with baseline CPFQ total score ≥ 28 (65%), CPFQ cognition dimension score ≥ 16 (63%), or CPFQ physical dimension score ≥ 13 (59%) showed statistically significantly (P ≤ .025) greater mean edivoxetine-placebo differences on the CPFQ total score versus the overall patient cohort.
Conclusions: While we could not identify symptoms predictive of response to the selective NRI edivoxetine used as adjunctive treatment, impaired cognition and physical symptoms may predict greater improvement during monotherapy.
Trial Registration: ClinicalTrials.gov identifiers: NCT00840034, NCT01173601, NCT01187407, NCT01185340, NCT00795821
J Clin Psychiatry 2017;78(3):317-323
dx.doi.org/10.4088/JCP.15m09972
© Copyright 2016 Physicians Postgraduate Press, Inc.
aLilly Research Laboratories, Eli Lilly and Company, Indianapolis, Indiana
bUniversity of Texas Health Science Center, Houston
cDepartment of Psychiatry, University of California, San Francisco
dPsychiatry and Neurosciences Research Center, French Institute of Health and Medical Research, Paris, France
eDepartment of Psychiatry, Massachusetts General Hospital, Boston
fDr Goldberger is now affiliated with AbbVie Pharmaceuticals in Lake Bluff, Illinois, and is no longer affiliated with Eli Lilly and Company, although she was at the time this research was done and when the manuscript was accepted for publication.
*Corresponding author: Virginia L. Stauffer, PharmD, Eli Lilly and Company, Lilly Corporate Center, Indianapolis, IN 46285 ([email protected]).
Recommended medications for major depressive disorder (MDD) target 1 or more monoamine neurotransmitters (eg, serotonin, norepinephrine, dopamine).1-3 A relationship between some symptoms of MDD and individual neurotransmitter dysregulation may exist,4 and certain symptoms of depression may respond less to serotonergic than to noradrenergic antidepressants.3,5 Symptoms hypothesized to be part of the “noradrenergic symptom cluster” include decreased concentration, energy, and self-care as well as increased retardation, lassitude, and tiredness.6,7 This symptom cluster suggests an interesting hypothesis: Does adding a selective norepinephrine reuptake inhibitor (NRI) improve residual symptoms of impaired cognition and fatigue and hence improve the overall depression outcome in patients with partial response to a selective serotonin reuptake inhibitor (SSRI)?
Edivoxetine hydrochloride (hereafter, edivoxetine) is a potent and highly selective NRI.8 The efficacy of edivoxetine as adjunctive treatment for patients with MDD who were partial responders to an adequate course of treatment with an SSRI has been evaluated in one phase 29 and three phase 3 clinical studies.10 No statistically significant differences between edivoxetine and placebo were observed for the primary endpoint (mean change from baseline to endpoint in Montgomery-Asberg Depression Rating Scale [MADRS] total score) in any of the phase 3 adjunctive treatment studies.9,10 Edivoxetine monotherapy in MDD has been evaluated in 2 clinical studies, which produced mixed results. In the first study, efficacy of edivoxetine in improving depressive symptoms was not significantly different from placebo.11 In the second study, edivoxetine significantly improved depressive symptoms more than placebo.12
The objective of this post hoc analysis with prespecified hypotheses was to identify subgroups among patients diagnosed with MDD presenting primarily with symptoms of the hypothesized noradrenergic symptom cluster and to determine if those subgroups show greater improvement compared with placebo after treatment with edivoxetine either as adjunctive treatment or as monotherapy compared with the full MDD sample. To address this objective, we used an MDD dataset in which partial responders to SSRIs received adjunctive treatment with edivoxetine9,10 and a dataset in which patients with an acute episode of MDD12 received edivoxetine monotherapy.
METHODS
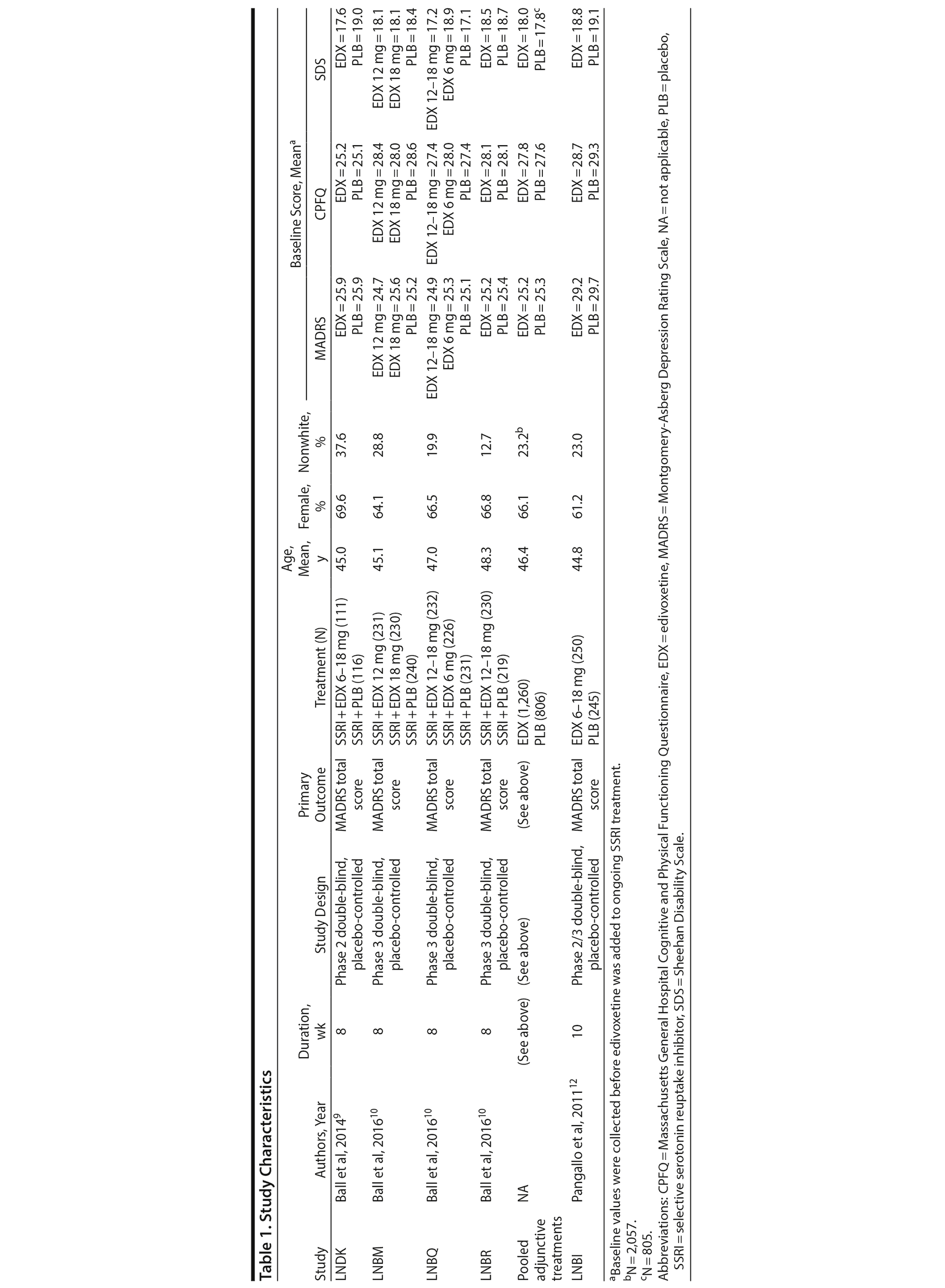
Data from 4 adjunctive treatment trials (SSRI + edivoxetine 6-18 mg/d vs SSRI + placebo; N = 2,066) and 1 monotherapy trial comparing edivoxetine 6-18 mg/d and placebo (N = 495) in adult patients with MDD (DSM-IV-TR criteria) were used to identify predictive factors of response using variables thought to be related to noradrenergic symptoms associated with MDD. The trials were conducted from 2008 to 2013. Partial treatment response to SSRIs was defined by history using investigator opinion that the patient has experienced a minimally clinically meaningful improvement with the SSRI treatment and by ≤ 75% improvement on treatment with the current SSRI at screening based on the Massachusetts General Hospital Antidepressant Treatment Response Questionnaire (MGH-ATRQ).13 Additionally, patients in all 4 adjunctive treatment studies had to have a GRID 17-Item Hamilton Depression Rating Scale (GRID-HDRS17) total score of ≥ 16 at screening. Detailed information about the included studies is available in the primary disclosures.9,10,12 Study protocols were reviewed and approved by the applicable organizational ethical review boards. Patients provided written informed consent before undergoing any study procedures, and the studies were conducted in accordance with the ethical principles of the Declaration of Helsinki, Good Clinical Practice, and applicable laws and regulations.
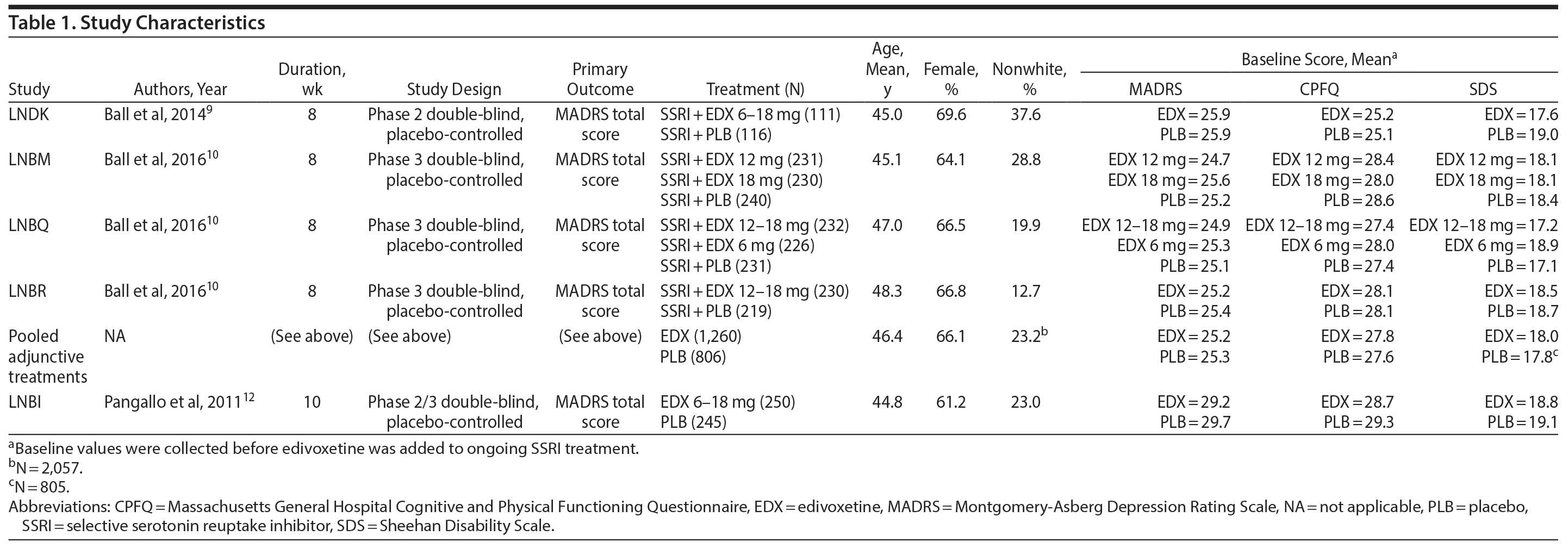
Study Characteristics
Data were pooled from 4 adjunctive treatment trials9,10 in which treatment with edivoxetine (6-18 mg/d) or placebo was added to ongoing treatment with SSRIs in adult patients with MDD who continued to present with at least moderate depressive symptoms after treatment optimization with SSRIs (Table 1). The studies were 8 weeks in duration between randomization and adjunctive treatment, utilized a placebo lead-in design of variable length, and used changes on the MADRS total score as the primary outcome measure (Table 1). Patients had a mean age of 46.4 years, and their mean ages at onset of MDD symptoms ranged from 27 to 36 years. Patients in the adjunctive treatment trials had experienced a mean of 3 to 6 prior episodes of MDD, their current MDD episodes ranged in mean lengths from 38 and 87 weeks, and their mean baseline MADRS total score ranged from 25 to 30 (Supplementary eTable 1 at PSYCHIATRIST.COM). Patients enrolled in the adjunctive treatment trials had been taking an SSRI that had been approved for MDD at a dose within the labeling guidelines for the participating country. Patients had received SSRI treatment ≥ 6 weeks before initiation of adjunctive treatment with edivoxetine, with at least the last 2 weeks (study LNDK9) or 4 weeks (studies LNBM, LNBQ, and LNBR10) of SSRI treatment at a stable, optimized dose (which could not be adjusted during the trials; Supplementary eTable 2) as determined by the investigator.

- A noradrenergic symptom cluster in patients with major depressive disorder (MDD) has been described, but its impact on treatment outcome is unclear. Here, subgroups within patients diagnosed with MDD presenting primarily with symptoms of the hypothesized noradrenergic symptom cluster were identified, and their responses to treatment with edivoxetine either as adjunctive treatment or monotherapy compared with the full MDD sample was assessed.
- Adjunctive treatment with edivoxetine during ongoing treatment with selective serotonin reuptake inhibitors does not result in greater treatment response in MDD patients with noradrenergic symptoms compared to the overall MDD patient population.
- Edivoxetine monotherapy resulted in greater improvement of cognitive and physical symptoms and overall functioning in MDD patients with pronounced impairments of those functions at treatment initiation compared to the overall MDD patient sample.
Data from 1 edivoxetine monotherapy trial were available for analysis.12 In this trial, adult patients with MDD received treatment with edivoxetine 6-18 mg/d versus placebo for 10 weeks (Table 1). Data from another placebo-controlled monotherapy trial examining the efficacy and safety of edivoxetine,11 which was negative, could not be used for the current analyses because that study did not collect the needed assessments (MADRS, Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire [CPFQ],14 and Fatigue Associated with Depression Questionnaire [FAsD]).15
Assessment Scales
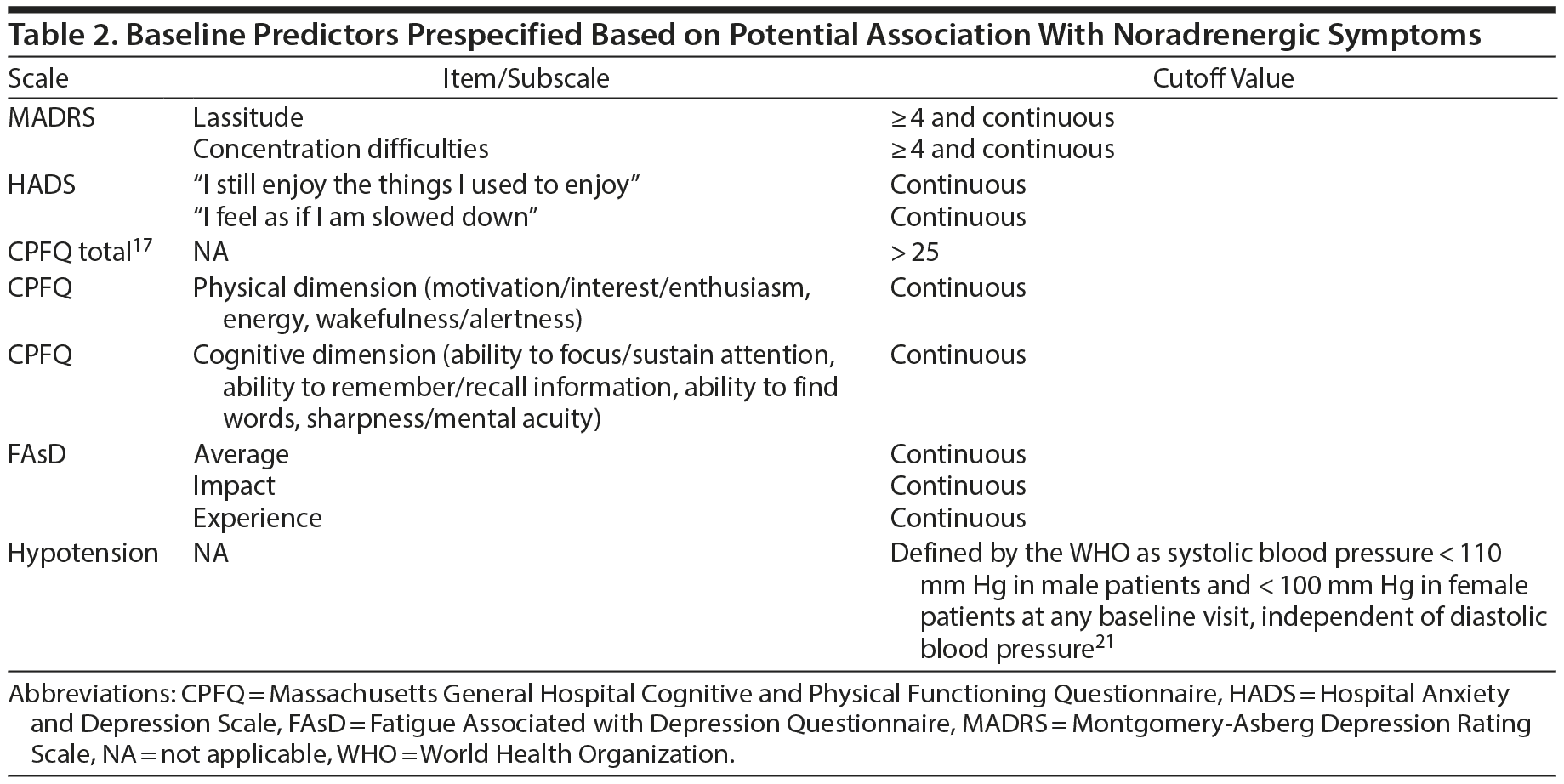
The MADRS, CPFQ, Sheehan Disability Scale (SDS), Hospital Anxiety and Depression Scale (HADS), and FAsD were used as outcome measures and/or prespecified defined predictors in our analyses.
Consistent with the primary outcome assessment in the individual studies, baseline-to-endpoint change in MADRS total score was used as the primary global outcome measure. As a secondary global outcome measure, baseline-to-endpoint change in SDS total score was utilized. Dimensional outcome was assessed using baseline-to-endpoint change in CPFQ total score.
The MADRS16 is a clinician-rated 10-item depression assessment scale, with each item rated on a scale of 0 (not present) to 6 (extremely bad). The MADRS total score is the sum of items 1 through 10, and the total score ranges from 0 to 60.16
The CPFQ14 is a patient-rated scale assessing the patient’s cognitive and physical symptoms over the previous month with 7 questions: (a) “How has your motivation/interest/enthusiasm been over the past month?” (b) “How has your wakefulness/alertness been over the past month?” (c) “How has your energy been over the past month?” (d) “How has your ability to focus/sustain attention been over the past month?” (e) “How has your ability to remember/recall information been over the past month?” (f) “How has your ability to find words been over the past month?” and (g) “How has your sharpness/mental acuity been over the past month?” The physical dimension subscale consists of questions a, b, and c, while the cognitive dimension subscale includes questions d, e, f, and g. Each question is rated on a 6-point scale (1 = greater than normal; 2 = normal; 6 = totally absent) and the total score ranges from 7 to 42.14,17
The SDS18 is a patient-rated scale assessing functional impairment in 3 domains over the prior week: work/school, social life/leisure activities, and family life/home responsibilities. Patients rate their impairment in those 3 domains with a 10-point visual analog scale (0 = not at all; 10 = extremely) resulting in a total score ranging from 0 (unimpaired) to 30 (highly impaired).18,19
The HADS20 is a patient-rated scale assessing both anxiety and depression with 2 independent subscales that include 14 items (7 items for each subscale) rated on a 4-point scale (0 to 3). Both the HADS anxiety and depression subscale scores range from 0 to 21, and higher scores indicate greater illness severity.20
The FAsD15 is a patient-rated 13-item scale assessing fatigue in patients with MDD over the prior week. The FAsD experience subscale score is based on 6 items assessed with a 5-point Likert scale, with responses ranging from “never” to “always,” and the impact subscale score is based on 7 items (5 items are rated on a 5-point Likert scale, with responses ranging from “not at all” to “very much,” and 2 items require yes/no responses).15
Several patient baseline characteristics and disease severity measures were prespecified as potential predictors before subgroup identification analysis based on their potential associations with noradrenergic symptoms (Table 2).
Statistical Analyses
Included in the analyses were all randomized patients with baseline and at least 1 postbaseline assessment on the outcome variable, as well as baseline predictor variables available. For each outcome variable (MADRS total score, SDS total score, and CPFQ total score), a detection tool using a resampling-based ensemble tree method22,23 was applied to search and identify patient subgroups with larger drug-placebo treatment effects compared with the overall population based on the specified predictor variables in Table 2. In this process, 400 subsamples, each having the size of 50% of the full sample, were randomly generated via sampling without replacement. Within each subsample, a recursive partitioning24,25 algorithm was first applied to the drug-treated arm to produce an up-to 2-level decision tree, which used binary split(s) based on predictor variables to classify subjects into subgroups with differential treatment responses. The minimum subgroup size allowed was 50 patients. The subgroup showing better treatment outcome on drug treatment was extracted and compared to the outcome from the corresponding subgroup in the placebo arm. The subgroup was detected as a preliminary candidate if the observed drug-placebo relative treatment effect was significant and larger than the relative treatment effect in the overall subsample. For each detected preliminary subgroup in a subsample, the drug-placebo difference of this subgroup in the complement subsample (remaining 50% subjects not used in preliminary detection) was also obtained to confirm the direction of treatment effect. The above detection process was repeated for all 400 random subsamples, and the most frequently detected and confirmed subgroups became top candidates. Similar subgroups were aggregated over different subsamples, and the averaged drug-placebo differences in complement subsamples provided a bias-adjusted estimate of relative treatment effect. Finally, a permutation test was also conducted to assess the statistical significance of the treatment effect in the detected subgroups against the treatment effect in the overall sample, and the reported P values were adjusted for multiple comparisons.
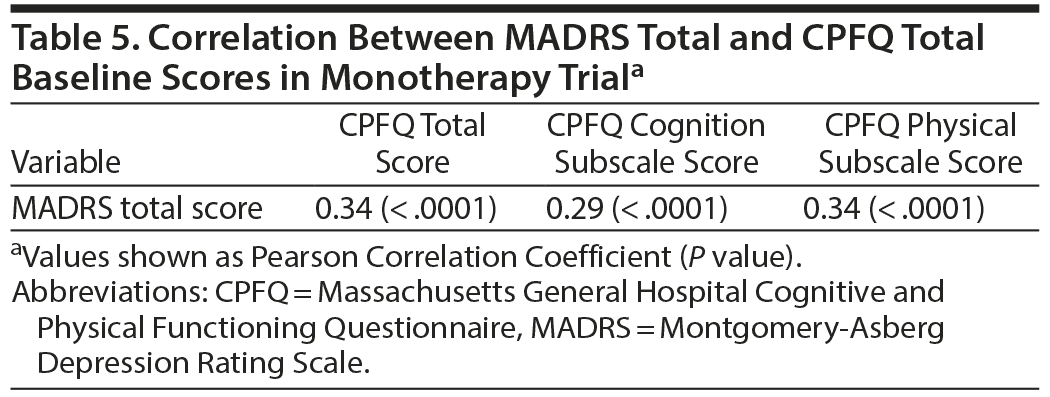
To assess the interdependence of the assessment scales used as outcome measures and predictors, Pearson correlation coefficients of baseline scores were examined and an analysis of covariance (ANCOVA) was conducted on the MADRS total score changes with baseline MADRS score, treatment, baseline CPFQ score group, and treatment-by-CPFQ group interaction terms in the model.
RESULTS
Adjunctive Treatment Trials
Using baseline-to-endpoint MADRS total score, SDS total score, or CPFQ total score changes, none of the tested predictors identified a subgroup with a significantly greater and clinically meaningful response to edivoxetine versus placebo compared with the overall study cohort (Table 3).
Monotherapy Trial
Testing of the prespecified predictors in the monotherapy trial dataset revealed subgroups defined by baseline CPFQ scores (Table 4). The difference between edivoxetine and placebo in MADRS total score change was greater in the identified subgroups with higher baseline severity as indicated by CPFQ scores versus the overall study cohort, but did not reach statistical significance. Treatment differences in SDS total score and CPFQ total score changes as outcome measures were statistically significantly greater in the identified CPFQ subgroups (Table 4).
Relationship of Baseline MADRS Total and Baseline CPFQ Scores in Monotherapy Trial
To assess the interdependence of the assessment scales used as outcome measures and predictors, Pearson correlation coefficients of baseline scores were examined (Table 5). Correlations between baseline MADRS total score and CPFQ total or subscale scores were weak, with coefficients ranging from 0.29 to 0.34. Hence, the patient group with higher severity in cognitive and physical impairment as assessed with CPFQ is semi-independent of the patient group with higher depression severity as assessed with the MADRS at baseline. Further, an ANCOVA was conducted on the MADRS total score changes with baseline MADRS score, treatment, baseline CPFQ score group, and treatment-by-CPFQ group interaction terms in the model. The treatment-by-CPFQ group interaction was statistically significant (P = .002 for CPFQ cognitive score; P = .024 for CPFQ total score) after adjusting for baseline MADRS score as covariate, with larger edivoxetine advantage over placebo observed in patients with higher CPFQ severity at baseline.
DISCUSSION
On the basis of differences in the mechanisms of action between SSRIs and NRIs, it should theoretically be possible to identify a MDD patient subgroup with noradrenergic symptoms receiving additional benefits from adding an NRI to its existing SSRI treatment. However, this was not the case in the current post hoc analyses—no prespecified patient subgroup within the noradrenergic symptom cluster was identified for which adjunctive treatment with edivoxetine resulted in greater improvement on the assessed outcome measures versus placebo compared with the overall patient cohort. In the overall pooled sample, edivoxetine and placebo did not significantly differ on the primary outcome measure (MADRS total score).9,10 The 4 included studies were comparable. Additionally, the total sample size in the pooled analyses should be sufficient to identify a subgroup with noradrenergic symptoms should such a subgroup exist.
However, a patient subgroup with hypothesized noradrenergic-responsive symptoms was identified in a monotherapy trial. This subgroup had higher baseline CPFQ total and cognitive dimension scores. Symptom improvement, as reflected by SDS total score and CPFQ total score changes, during treatment with edivoxetine versus placebo was statistically significantly greater than improvement in the overall patient cohort. Between-treatment differences in MADRS total score changes were also greater in the patient subgroup with higher baseline CPFQ score, but did not reach statistical significance. However, when baseline MADRS severity was accounted for in the ANCOVA, drug-placebo treatment differences on the MADRS were significantly greater in the high CPFQ group than in overall sample. These findings regarding the effect of baseline CPFQ severity on treatment difference in depression outcome appeared to be independent of baseline MADRS total score symptom severity, as indicated by low correlations between MADRS total scores and CPFQ total and domain scores at baseline and in the ANCOVA, an analysis of CPFQ severity effect on MADRS mean change adjusting for baseline MADRS score as covariate. Similar findings were reported for vortioxetine (10 and 20 mg/d) in a post hoc analysis of data from a placebo-controlled monotherapy trial.17 In patients with MDD with a baseline CPFQ score > 25, treatment with vortioxetine statistically significantly (P < .05) improved endpoint cognitive symptoms (CPFQ cognitive dimension score). Although vortioxetine also improved overall depressive symptoms in this subpopulation, path analysis demonstrated that the improvement in cognitive symptoms was a direct effect and not solely due to improvement of depressive symptoms.17 Cognitive symptoms might be a better predictor of noradrenergic response than other symptoms thought to be norepinephrine-related.
The extent of overlap between primarily serotonergic and primarily noradrenergic symptoms is unknown, and this hypothesis has not been fully tested a priori. Studies comparing selective noradrenergic agents with selective serotonergic agents have not shown clear differences among the symptoms that are treated by either compound class.26 The observed differences between findings with monotherapy or adjunctive therapy may be explained by the crosstalk between norepinephrine and serotonin systems and its implications for antidepressant response. For example, Blier27 has suggested that SSRIs do affect the norepinephrine system via the inhibitory projections of serotonin neurons on norepinephrine neurons. Therefore, patients who are already on a stable dose of an SSRI before receiving adjunctive treatment with an NRI might not have as much potential for improvement during treatment with the NRI compared with patients who receive NRI monotherapy. In addition, enough overlap in SSRI and NRI mechanisms of action may exist that not enough difference remains to improve symptoms in this adjunctive treatment paradigm. Both SSRIs28 and NRIs29 decrease noradrenergic neuron firing, suggesting that both substance classes may have common effects on pathways involved in MDD pathophysiology. These findings suggest that the addition of norepinephrine to a system previously desensitized with ongoing serotonergic reuptake inhibition may not have the same effect on depressive symptoms compared with parallel activation of serotonergic and noradrenergic pathways.30-32 This previous desensitization might be part of the reason why adjunctive treatment trials with edivoxetine also did not show statistically significant separation from placebo in the overall patient samples, while NRIs were effective in some monotherapy trials (eg, edivoxetine12 and reboxetine33,34).
Greater improvements in SDS total scores in the identified CPFQ subgroups compared with the overall patient cohort might indicate better social functioning of this group after treatment with an NRI. These observations are consistent with findings by Dubini and colleagues,35 who reported a significant effect of reboxetine on social functioning in patients with MDD.
Our results suggest a need for more specific scales to assess specific symptom dimensions in patients with MDD. While the MADRS and the HDRS provide reliable assessments of patients’ overall depression severity, specialized scales like the CPFQ provide greater sensitivity when examining cognition and physical symptoms in patients with MDD.
The interpretation of our results is limited by the post hoc design of the analyses, the pooled dataset, and variability in edivoxetine doses among individuals. However, the individual pooled studies used very similar study designs and included comparable patient populations, and our hypotheses were prespecified. Additionally, for edivoxetine monotherapy, only data from a trial with positive results could be included in the current analyses. Prospective studies are warranted to further explore targeted treatment of patients with MDD who primarily present with symptoms representative of a noradrenergic symptom cluster. Finally, we used the resampling-based ensemble tree method because it can search for subgroups with larger treatment contrasts and provide bias-adjusted estimates as well as multiplicity adjustment. Other statistical methods, such as utilizing cluster analysis techniques, can also be adopted for subgroup identification—additional studies are warranted testing and comparing the results of other appropriate analysis approaches. One asset of the current analyses is the large sample size for the pooled adjunctive treatment dataset. The presented findings are informative for the development of compounds influencing noradrenergic signaling pathways.
CONCLUSIONS
We could not identify a subgroup of patients with MDD who had hypothesized noradrenergic symptoms more responsive to adjunctive treatment with the selective NRI edivoxetine while receiving ongoing treatment with an SSRI compared with the full patient sample. However, patients with greater cognitive impairment and physical symptoms showed greater improvements of cognitive and physical symptoms and overall functioning during edivoxetine monotherapy as compared to placebo.
Submitted: March 18, 2015; accepted October 29, 2015.
Online first: September 27, 2016.
Drug names: vortioxetine (Trintellix).
Potential conflicts of interest: Drs Stauffer and Peng are full-time employees and shareholders of Eli Lilly and Company. Dr Goldberger is a full-time employee and shareholder of AbbVie Pharmaceuticals. Dr Marangell is a shareholder of Eli Lilly and Company. Dr Nelson has been, during the past 12 months, an advisor or consultant to Bristol Myers Squibb, Corcept, Eli Lilly, Genentech, Lundbeck, Otsuka, Janssen, Sunovion, and Pfizer; has received honoraria from Bristol Myers Squibb (Canada), Otsuka (Asia), and Genentech; has received research support from the National Institute of Mental Health and Avid; and owns stock in Atosssa. Dr Gorwood received, during the last 5 years, research grants from Eli Lilly and Servier and fees for presentations at congresses or participation in scientific boards from AstraZeneca, Biocodex, Bristol-Myers-Squibb, Janssen, Lilly, Lundbeck, Naurex, Otsuka, Roche, Sanofi Pasteur MSD, and Servier; and has served as a consultant for Lilly, Servier, and Janssen-Cilag. For a comprehensive list of lifetime disclosures of Dr Fava, see http://mghcme.org/faculty/faculty-detail/maurizio_fava.
Funding/support: Eli Lilly and Company funded this study.
Role of the sponsor: Eli Lilly and Company sponsored the design, conduct, interpretation, and reporting of this study.
Acknowledgments: The authors thank Alexandra Heinloth, MD, and Angela Lorio, BS, both full-time employees of inVentiv Health Clinical, LLC, for writing and editorial assistance. Eli Lilly and Company contracted inVentiv Health Clinical, LLC, for writing and editorial services.
Supplementary material: Available at PSYCHIATRIST.COM.
REFERENCES
1. Connolly KR, Thase ME. Emerging drugs for major depressive disorder. Expert Opin Emerg Drugs. 2012;17(1):105-126. PubMed doi:10.1517/14728214.2012.660146
2. Blier P, El Mansari M. Serotonin and beyond: therapeutics for major depression. Philos Trans R Soc Lond B Biol Sci. 2013;368(1615):20120536. PubMed doi:10.1098/rstb.2012.0536
3. Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatr Dis Treat. 2011;7(suppl 1):9-13. PubMed
4. Blier P. Neurotransmitter targeting in the treatment of depression. J Clin Psychiatry. 2013;74(suppl 2):19-24. PubMed doi:10.4088/JCP.12084su1c.04
5. Nutt D, Demyttenaere K, Janka Z, et al. The other face of depression, reduced positive affect: the role of catecholamines in causation and cure. J Psychopharmacol. 2007;21(5):461-471. PubMed doi:10.1177/0269881106069938
6. Blier P, Briley M. The noradrenergic symptom cluster: clinical expression and neuropharmacology. Neuropsychiatr Dis Treat. 2011;7(suppl 1):15-20. PubMed
7. Montgomery S, Briley M. Noradrenergic symptom cluster in depression. Neuropsychiatr Dis Treat. 2011;7(suppl 1):1-2. PubMed
8. Kielbasa W, Quinlan T, Jin L, et al. Pharmacokinetics and pharmacodynamics of edivoxetine (LY2216684), a norepinephrine reuptake inhibitor, in pediatric patients with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22(4):269-276. PubMed doi:10.1089/cap.2011.0151
9. Ball S, Dellva MA, D’ Souza DN, et al. A double-blind, placebo-controlled study of edivoxetine as an adjunctive treatment for patients with major depressive disorder who are partial responders to selective serotonin reuptake inhibitor treatment. J Affect Disord. 2014;167:215-223. PubMed doi:10.1016/j.jad.2014.06.006
10. Ball S, Ferguson MB, Martinez JM, et al. Efficacy outcomes from 3 clinical trials of edivoxetine as adjunctive treatment for patients with major depressive disorder who are partial responders to selective serotonin reuptake inhibitor treatment. J Clin Psychiatry. 2016;77(5):635-642. PubMed doi:10.4088/JCP.14m09619
11. Dubé S, Dellva MA, Jones M, et al. A study of the effects of LY2216684, a selective norepinephrine reuptake inhibitor, in the treatment of major depression. J Psychiatr Res. 2010;44(6):356-363. PubMed doi:10.1016/j.jpsychires.2009.09.013
12. Pangallo B, Dellva MA, D’ Souza DN, et al. A randomized, double-blind study comparing LY2216684 and placebo in the treatment of major depressive disorder. J Psychiatr Res. 2011;45(6):748-755. PubMed doi:10.1016/j.jpsychires.2011.03.014
13. Chandler GM, Iosifescu DV, Pollack MH, et al. RESEARCH: Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325. PubMed doi:10.1111/j.1755-5949.2009.00102.x
14. Fava M, Iosifescu DV, Pedrelli P, et al. Reliability and validity of the Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire. Psychother Psychosom. 2009;78(2):91-97. PubMed doi:10.1159/000201934
15. Phillips GA, Matza LS, Murray L, et al. Reliability and validity of the Fatigue Associated with Depression questionnaire (FAsD): an interim analysis. Eur J Neurol. 2009;16(suppl 3):460.
16. Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. PubMed doi:10.1192/bjp.134.4.382
17. Fava M, Lophaven S, Olsen CK. Effects of vortioxetine on cognitive symptoms of major depressive disorder. 25th ECNP Congress, October 5-9, 2013, Barcelona, Spain.
18. Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(suppl 3):89-95. PubMed doi:10.1097/00004850-199606003-00015
19. Sheehan KH, Sheehan DV. Assessing treatment effects in clinical trials with the discan metric of the Sheehan Disability Scale. Int Clin Psychopharmacol. 2008;23(2):70-83. PubMed doi:10.1097/YIC.0b013e3282f2b4d6
20. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression scale. Acta Psychiatr Scand. 1983;67(6):361-370. PubMed doi:10.1111/j.1600-0447.1983.tb09716.x
21. World Health Organization. Arterial Hypertension: Report of a WHO Expert Committee. Technical Report Series No 628. Geneva, Switzerland: World Health Organization; 1978.
22. Battioui S, Shen L, Ruberg SA. A resampling-based ensemble tree method to identify patient subgroups with enhanced treatment effect. Proceedings to 2014 Joint Statistical Meetings, August 2-7, 2014. 2014:4613-4623.
23. Shen D. Ding Y, Battioui C.. A framework of statistical methods for identification of subgroups with differential treatment effects in randomized trials. In: Chen Z, Liu A, Qu Y, et al, eds. Applied Statistics in Biomedicine and Clinical Trials Design: Selected Papers from 2013 ICSA/ISBS Joint Statistical Meetings. New York, NY: Springer International Publishing; 2015:411-425. doi:10.1007/978-3-319-12694-4_25
24. Breiman L, Friedman JH, Olshen RA, et al. Classification and Regression Trees. Belmont, CA: Wadsworth; 1984.
25. Song C, Zhang H. Comments on fifty years of classification and regression trees. Int Stat Rev. 2014;82(3):359-361. PubMed doi:10.1111/insr.12060
26. Nelson JC, Portera L, Leon AC. Are there differences in the symptoms that respond to a selective serotonin or norepinephrine reuptake inhibitor? Biol Psychiatry. 2005;57(12):1535-1542. PubMed doi:10.1016/j.biopsych.2005.03.030
27. Blier P. Crosstalk between the norepinephrine and serotonin systems and its role in the antidepressant response. J Psychiatry Neurosci. 2001;(26 suppl):S3-S10. PubMed
28. Szabo ST, de Montigny C, Blier P. Modulation of noradrenergic neuronal firing by selective serotonin reuptake blockers. Br J Pharmacol. 1999;126(3):568-571. PubMed doi:10.1038/sj.bjp.0702343
29. Szabo ST, Blier P. Effect of the selective noradrenergic reuptake inhibitor reboxetine on the firing activity of noradrenaline and serotonin neurons. Eur J Neurosci. 2001;13(11):2077-2087. PubMed doi:10.1046/j.0953-816x.2001.01583.x
30. Ressler KJ, Nemeroff CB. Role of norepinephrine in the pathophysiology and treatment of mood disorders. Biol Psychiatry. 1999;46(9):1219-1233. PubMed doi:10.1016/S0006-3223(99)00127-4
31. Papakostas GI, Thase ME, Fava M, et al. Are antidepressant drugs that combine serotonergic and noradrenergic mechanisms of action more effective than the selective serotonin reuptake inhibitors in treating major depressive disorder? a meta-analysis of studies of newer agents. Biol Psychiatry. 2007;62(11):1217-1227. PubMed doi:10.1016/j.biopsych.2007.03.027
32. Nelson JC, Mazure CM, Jatlow PI, et al. Combining norepinephrine and serotonin reuptake inhibition mechanisms for treatment of depression: a double-blind, randomized study. Biol Psychiatry. 2004;55(3):296-300. PubMed doi:10.1016/j.biopsych.2003.08.007
33. Tranter R, Healy H, Cattell D, et al. Functional effects of agents differentially selective to noradrenergic or serotonergic systems. Psychol Med. 2002;32(3):517-524. PubMed doi:10.1017/S0033291701005086
34. Keller M. Role of serotonin and noradrenaline in social dysfunction: a review of data on reboxetine and the Social Adaptation Self-evaluation Scale (SASS). Gen Hosp Psychiatry. 2001;23(1):15-19. PubMed doi:10.1016/S0163-8343(00)00115-8
35. Dubini A, Bosc M, Polin V. Noradrenaline-selective versus serotonin-selective antidepressant therapy: differential effects on social functioning. J Psychopharmacol. 1997;11(4 suppl):S17-S23. PubMed
Enjoy this premium PDF as part of your membership benefits!